2. 青岛海洋科学与技术国家实验室 海洋渔业科学与食物产出过程功能实验室 青岛 266071;
3. 上海海洋大学水产与生命学院 上海 201306
2. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071;
3. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306
在鱼类中,黑色素富集激素(Melanin concentration hormone,MCH)基因最初是从大马哈鱼(Oncorhynchus keta)中分离鉴定出来的(Kawauchi et al, 1983)。在体色调控方面,MCH的主要功能是弱化或抑制鲆鲽类无眼侧黑化发生。Takeshi等(2007)对牙鲆(Paralichthys olivaceus)的研究发现,白色养殖环境下无眼侧黑化比例显著低于黑色养殖环境,而且白色养殖环境下脑MCH mRNA表达水平显著高于黑色养殖环境,表明MCH在抑制牙鲆无眼侧黑化方面具有重要调控作用。外源MCH处理可令条斑星鲽(Verasper moseri)有眼侧皮肤黑化程度大为弱化,同时脑中的MCH mRNA的表达水平升高(Takahashi et al, 2004)。20世纪80年代以来,从大马哈鱼(Minth et al, 1989)、罗非鱼(Oreochromis niloticus)(Gröneveld et al, 1993)、虹鳟(Oncorhynchus mykiss)(Baker et al, 1995)、金鱼(Carassius auratus)(Mizusawa et al,2009)、斑马鱼(Danio rerio)(Berman et al, 2009)、美洲拟鲽(Pseudopleuronectes americanus)(Tuziak et al, 2012)和星斑川鲽(Platichthys stellatus)(Kang et al, 2013)等多种鱼类的脑中相继得到了MCH类神经肽,特别是由斑马鱼、美洲拟鲽和星斑川鲽等中均鉴定出MCH的2种亚型基因(MCH1和MCH2)。Kang等(2013)研究发现,在星斑川鲽对白色与暗色背景适应过程中,其无眼侧体色和丘脑中的MCH1、MCH2 mRNA水平都发生了明显的差异表达变化,另有研究证实,MCH1对无眼侧黑化有抑制作用(Amiya et al, 2005),MCH2表达量的增加导致鱼类皮肤颜色变白(Mizusawa et al, 2015),表明MCH1和MCH2均参与了鲆鲽类无眼侧体色的变化调控。
半滑舌鳎(Cynoglossus semilaevis)为暖温性近海大型底层鱼类,我国近岸海域均有分布,以渤海、黄海为多,是一种理想的增殖放流和人工养殖种类,现已成为我国鲆鲽类三大主导养殖种类之一(邓景耀等, 1988; 柳学周等,2006)。随着养殖业的发展,养殖半滑舌鳎无眼侧体色黑化的问题日益凸显,这种无眼侧黑化现象严重影响了其市场价格(比正常鱼低20%-30%),成为阻碍半滑舌鳎养殖产业持续发展的瓶颈之一。为深入认识半滑舌鳎无眼侧黑化发生及其抑制调控机制,作者研究了MCH基因的结构及组织表达特性,分析了其表达调控与无眼侧黑化程度的关系,以期为探究MCH调控半滑舌鳎无眼侧黑化的作用机制提供理论依据。
1 材料与方法 1.1 实验材料实验用半滑舌鳎取自山东省日照市海洋资源增殖站。取样实验鱼3尾,全长为25-35 cm,体重为250-450 g,用MS-222 (260 mg/L)麻醉致死后,快速置于冰上,取性腺、肝脏、心脏、胃、肠、脾、肾、头肾、垂体、脑、鳃、肌肉、有眼侧皮肤、无眼侧黑化皮肤(黑皮)、无眼侧正常皮肤(白皮),共15个组织,投入液氮中速冻,后转入-80℃超低温冰箱保存,提取总RNA,进行基因克隆和组织表达分析。
取同一产卵批次的全长为10-15 cm的半滑舌鳎12尾,按照其无眼侧黑化面积的不同划分为4组(0、10%、50%、80%),每组3尾,取其脑垂体(脑与垂体合并)和皮肤组织,用于分析MCH mRNA表达与无眼侧黑化程度的关系。
1.2 总RNA提取和cDNA第1链的合成利用RNAiso Plus (TaKaRa,日本)分别提取1.1中15个组织的总RNA,以1%琼脂糖凝胶电泳和Nanodrop2000 (Thermo,美国)检测RNA的质量和浓度。
取1 μg脑组织RNA,以PrimeScript RT reagent Kit反转录试剂盒(TaKaRa,日本)合成cDNA第1链,于-20℃保存,用于中间片段克隆。以SMARTerTM RACE cDNA Amplification Kit (Clontech,美国)合成5'-RACE及3'-RACE cDNA第1链,用于RACE实验。取各组织的总RNA,用反转录合成cDNA第1链,用于MCH mRNA的定量表达分析。各操作步骤均严格按照试剂盒说明书进行。
1.3 MCH2基因cDNA克隆pMCH2核心片段序列从NCBI下载(GenBank序列号:XM_008315412.1),设计MCH2的RACE引物MCH2-GSP5、MCH2-GSP3、MCH2-NGSP5、MCH2-NGSP3(表 1),应用SMARTTMRACE cDNA扩增试剂盒(TaKaRa,日本)扩增pMCH2的cDNA全长序列。以脑第1链cDNA为模板,用Smart RACE Advantage 2 PCR试剂盒(Clontech,美国)进行PCR扩增。第1次梯度PCR使用引物MCH2-5'OUTER和MCH2-3'OUTER,反应体系(25 μl):17.25 μl ddH2O、2.5 μl Buffer、0.5 μl 50×dNTP Mix、0.5 μl 50×Advantage 2 Polymerase Mix、1.25 μl cDNA、2.5 μl UPM引物和0.5 μl OUTER引物;PCR反应条件:94℃ 30 s、67℃ 30 s (每个循环递减0.5℃)、72℃ 1 min,15个循环;94℃ 30 s、58℃ 30 s、72℃ 1 min,共28个循环。
|
|
表 1 半滑舌鳎MCH2 cDNA序列克隆引物 Table 1 Primers used for cloning MCH2 of C. semilaevis |
以pMCH2第1次PCR产物为模板,使用引物MCH2-5'INNER和MCH2-3'INNER分别进行巢式PCR,反应体系(25 μl)为:1.25 μl第1次PCR产物稀释液、19.25 μl ddH2O、2.5 μl Buffer、0.5 μl 50×dNTP Mix、0.5 μl 50×Advantage 2 Polymerase Mix、0.5 μl NUP引物、0.5 μl INNER引物,PCR扩增条件同第1次PCR。PCR产物经1%琼脂糖凝胶电泳后,切胶回收、连接、转化,接种至LB固体培养基,37℃培养过夜。以通用引物M13F和M13R进行菌落PCR扩增,反应条件为94℃预变性5 min,(94℃ 30 s,55℃ 30 s,72℃ 50 s) 30个循环,72℃延伸10 min。挑选阳性克隆送生工生物工程(上海)股份有限公司测序。
1.4 MCH mRNA的定量表达分析根据从NCBI下载的pMCH1(GenBank序列号: XP_008322511.1)的cDNA序列和本研究获得的半滑舌鳎pMCH2的cDNA序列全长,分别设计2对定量引物MCH1-DF和MCH1-DR、MCH2-DF和MCH2-DR,以β-actin为内参设计定量引物β-actin-F和β-actin-R,定量PCR所用各引物序列见表 2。
|
|
表 2 半滑舌鳎MCH1、MCH2和β-actin实时定量PCR用引物序列 Table 2 Primers used in real-time PCR assays for MCH1, MCH2 and β-actin of C. semilaevis |
β-actin、pMCH1和pMCH2定量PCR扩增条件:95℃预变性30 s,95℃ 5 s,58℃ 20 s,共40个循环。标准曲线制作以脑cDNA为模板(同1.3)进行5倍梯度稀释成6个标准品进行。每一组织样品的分析设置3个重复,每次实验均设置空白对照,PCR特异性扩增效率为95%-106%。
1.5 结果分析pMCH基因的序列拼接、氨基酸序列推导、分子量预测、等电点预测及氨基酸同源性分析均使用DNAstar 5.0.1,信号肽预测使用SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/)。氨基酸序列比对和系统进化分析使用ClustalX 2.0.12 (http://www.clustal.org/download/current/)和MEGA 5.1 (http://www.megasoftware.net/mega51.html)。系统进化树构建使用MEGA 5.1软件中Neighbor-joining法(自展值为1000)。
定量PCR分析利用2-ΔΔCt方法计算目的基因的相对表达量(Livak et al, 2001)。实验数据采用SPSS (17.0版本)统计进行单因素方差分析(One-way ANOVA)和Duncan’s多重比较分析,当P < 0.05时为差异显著。
2 结果 2.1 pMCH cDNA序列结构分析半滑舌鳎pMCH1 cDNA序列长为476 bp,包括405 bp的开放阅读框(ORF)、71 bp的3'非编码区(3'-UTR),编码134个氨基酸,信号肽长为24个氨基酸(图 1),成熟肽分子量为14.8 kDa,等电点为6.46。pMCH2 cDNA序列全长为626 bp,包括444 bp的ORF、32 bp 5'-UTR和150 bp的3'-UTR,编码147个氨基酸,信号肽长为18个氨基酸(图 1),成熟肽的分子量为16.2 kDa,等电点为5.89。

|
图 1 半滑舌鳎pMCH1和pMCH2基因的cDNA序列及其推导的氨基酸序列 Figure 1 Nucleotides and deduced amino acid sequences of pMCH1 and pMCH2 cDNA of C. semilaevis 虚线部分为信肽序列,实线部分为MCH成熟肽序列,3'-UTR端AATAA序列以方框标注,终止密码子(TGA)以星号标注,圆圈部分为组成二硫键的半胱氨酸残基 Signal peptide was indicated by a broken underline; the mature peptide of MCH was indicated by an underline; the nucleotides corresponding to the polyadenylation signal in the 3'-untranslated region (AATAA) were marked with box; the asterisk indicated the stop codon (TGA); the cysteine residues to form disulfide linkage were circled |
半滑舌鳎MCH 2个亚基序列的C端区域均有1个RR裂解位点,其成熟肽序列中都包含有2个保守的环状结构,由2个半胱氨酸残基相互间形成二硫键形成。MCH1成熟肽含有17个氨基酸(DNMRCMVGRVYRPCWEV),而MCH2成熟肽则由21个氨基酸(ELDMLRCMIGRVYRPCWGTSN)组成,这种氨基酸组成的差异提示其可能存在功能上的差异。
2.2 氨基酸序列同源性分析半滑舌鳎MCH的氨基酸序列与鲽形目、鲈形目鱼类MCH的氨基酸序列的相似度最高为57.3%-66.9%。其中,pMCH1与牙鲆、条斑星鲽、星突江鲽、罗非鱼等具有较高的氨基酸相似度(58.4%-66.9%),与软骨鱼类的相似度为37.3%,而与哺乳类的相似度降至30%以下;pMCH2基因的氨基酸序列与条斑星鲽、星突江鲽、牙鲆和布氏新亮丽鲷(Neolamprologus brichardi)具有较高相似度(56.0%-61.3%),与哺乳动物的相似度降低到25%以下,但与大鼠(Rattus norvegicus)的同源性却高达53.7%(表 2)。MCH 2个亚基间氨基酸相似度仅为22.3%,但其成熟肽序列高度保守,C端区域都具有保守的RR裂解位点,且都有2个保守的环状结构。
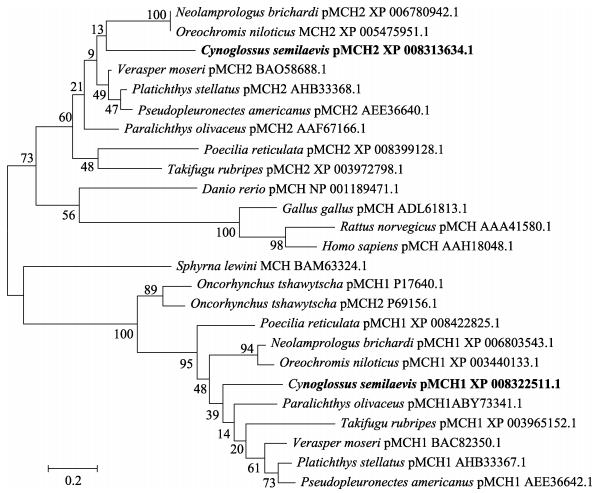
2.3 系统进化分析半滑舌鳎pMCH1和pMCH2亚基分别处于2个不同的进化分支。pMCH1与鲽形目、鲤形目和鲈形目鱼类的pMCH1处于同1个小分支,并与其他鱼类的pMCH1组成1个大分支;而pMCH2则与鲽形目、魨形目鱼类的pMCH2组成1个小分支,并且与其他鱼类的pMCH2以及鸟类、哺乳类和人的MCH组成1个大分支(图 3)。表明MCH的2个亚基的进化分化时间可能比较早。

|
图 2 半滑舌鳎MCH氨基酸与其他脊椎动物MCH氨基酸序列比对 Figure 2 Comparison of the amino acid sequences of C. semilaevis MCH gene and other vertebrates 灰色为二元精氨酸残基组成的蛋白质水解切割位点;MCH氨基酸序列号见表 3;“*”表示一致的氨基酸;“:”表示高度保守度的氨基酸;“.”表示低保守度的氨基酸;在部分序列引入空白(连字符表示)来最大限度进行比对分析;NB1、NB2:布氏新亮丽鲷;ON1、ON2:罗非鱼;PS1、PS2:星斑川鲽;PA1、PA2:美洲拟鲽;VM1、VM2:条斑星鲽;PO1、PO2:牙鲆;CS1、CS2:半滑舌鳎;TR1、TR2:红鳍东方鲀;PR1、PR2:网纹鳉;OT1、OT2:大马哈鱼;DR:斑马鱼;RAT:褐鼠;Homo:人类;Gallus:鸡;SL:路氏双髻鲨 The dibasic (RR) cleavages of various MCHs were shown in shadow; GenBank accession numbers are shown in Tab. 3; Asterisks (*) indicated identical amino acid sequences; Dot (:) indicated highly conserved amino acid sequences; Dot (.) indicated amino acid sequences of low degree conserved; Gaps (indicated by hyphens) were introduced in some sequences to maximize alignment; NB1, NB2: N. brichardi; ON1, ON2: O. niloticus; PS1, PS2: P. stellatus; PA1, PA2: Pseudo-pleuronectes americanus; VM1, VM2: V. moseri; PO1, PO2: P. olivaceus; CS1, CS2: C. semilaevis; TR1, TR2: T. rubripes; PR1, PR2: P. reticulata; OT1, OT2: O. keta; DR: D. rerio; RAT: R. norvegicus; Homo: H. sapiens; Gallus: Gallus; SL: S. lewini |
|
|
表 3 半滑舌鳎MCH氨基酸序列与其他脊椎动物的相似度比较 Table 3 Homology comparison of the precursor peptide sequences of MCH genes between C. semilaevis and other vertebrates |
|
图 3 基于MCH氨基酸序列的NJ系统进化树 Figure 3 NJ phylogenetic tree based on the MCH amino acid sequences |
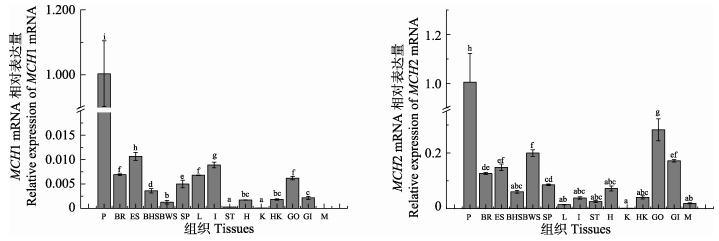
MCH1和MCH2都在垂体中的表达量最高。另外,MCH1 mRNA在脑、有眼侧皮肤、性腺、脾脏、肝脏和肠中检测到较高表达,而无眼侧黑化皮肤、无眼侧正常皮肤、心脏、头肾和鳃中表达量较低,肌肉中未检测到表达;MCH2 mRNA在脑、有眼侧皮肤、无眼侧正常皮肤、性腺和鳃中检测到较高表达,而无眼侧黑化皮肤、脾脏、肠、胃、心脏和头肾中有少量表达。有眼侧皮肤中MCH1 mRNA表达显著高于无眼侧黑化皮肤和无眼侧正常皮肤,无眼侧黑化皮肤中MCH1 mRNA表达显著高于无眼侧正常皮肤;有眼侧皮肤和无眼侧正常皮肤中MCH2 mRNA表达显著高于无眼侧黑化皮肤(图 4)。
|
图 4 半滑舌鳎MCH mRNA的组织表达分析 Figure 4 Spatial expression pattern of MCH mRNA in C. semilaevis P:垂体;BR:脑;ES:有眼侧皮肤;BHS:无眼侧黑化皮肤;BWS:无眼侧正常皮肤;SP:脾;L:肝;I:肠;ST:胃;H:心脏;K:肾;HK:头肾;GO:性腺;GI:鳃;M:肌肉。不同字母代表差异显著(P < 0.05),下同 P: Pituitary; B: Brain; ES: Eye-side skin; BHS: Blind-side skin with hypermelanosis; BWS: Normal blind-side white skin; SP: Spleen; L: Liver; I: Intestine; ST: Stomach; H: Heart; K: Kidney; HK: Head kidney; GO: Gonad; GI: Gill; M: Muscle. Bars with different letters differed significantly (P < 0.05), the same as below |
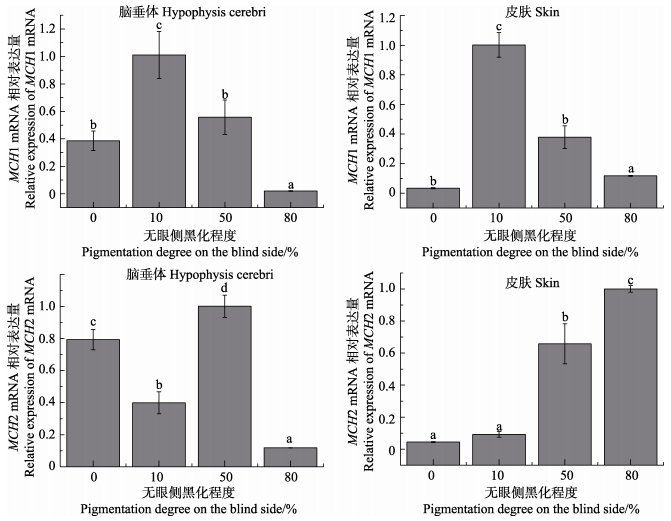
检测了无眼侧黑化程度不同的半滑舌鳎脑垂体和皮肤中MCH mRNA的表达情况(图 5)。MCH1mRNA在脑垂体和皮肤中表现出类似的表达变化趋势,都在10%黑化组表达量达最高值,而后随着黑化程度的增加显著降低。对于MCH2而言,无眼侧正常鱼和无眼侧50%黑化鱼的脑垂体中都具有较高mRNA表达水平,但在无眼侧10%黑化组和无眼侧80%黑化组其表达水平显著降低。皮肤中MCH2的表达量表现出随黑化程度加大而显著增强的趋势,在无眼侧80%黑化组则显著升高至最高值。
|
图 5 半滑舌鳎MCH mRNA表达与无眼侧黑化程度的关系 Figure 5 The relationship between expression levels of MCH mRNA and pigmentation degree on the blind side of C. semilaevis |
本研究获得了半滑舌鳎pMCH2的cDNA序列全长。结合NCBI数据库已有pMCH1序列,比对分析了半滑舌鳎2个MCH亚基的结构,发现其氨基酸同源性仅为22.3%,特别是在成熟肽序列上差异明显,表明其进化保守性较低,其生理功能可能存在显著差异。系统进化分析表明,半滑舌鳎MCH的2个亚基分别属于2个不同的进化分支,表明其祖先基因复制分化的时间可能较早,且朝两个不同方向进化,其具体功能的差异尚需进一步研究确证。
本研究发现,半滑舌鳎MCH mRNA在垂体中表达量最高,这与美洲拟鲽(Tuziak et al, 2012)、星斑川鲽(Kang et al, 2013)和牙鲆(Jeon et al, 2003) MCH mRNA主要在脑和垂体中表达的研究结果相似。半滑舌鳎MCH1 mRNA在除肌肉外的其他组织中均可检测到表达;MCH2 mRNA在脑、有眼侧皮肤、无眼侧正常皮肤、性腺和鳃也可检测到较高表达,其他外围组织中微量表达,这与牙鲆MCH mRNA的组织表达特性不同,牙鲆MCH mRNA在除垂体外的其他组织中没有检测到表达(Jeon et al, 2003)。而Kang等(2013)对星斑川鲽MCH的研究表明,除垂体外,只在鳃和精巢中检测到MCH1 mRNA微量表达,鳃、心、精巢中MCH2 mRNA微量表达。在美洲拟鲽中则发现,MCH1 mRNA在除心和肝脏外的其他组织中均可检测到表达,MCH2 mRNA在所有组织中均检测到表达,而且生殖腺和内脏中表达量较高(Tuziak et al, 2012),这与本研究结果相似,可能是由种的差异性及其生理功能的不同决定的。另外,半滑舌鳎MCH的组织表达的广泛性,也说明MCH除了内分泌外,可能还以自分泌或旁分泌的形式参与半滑舌鳎其他生理活动的过程调控。近年来,MCH被视为一种重要的生理调控因子而进行了许多的功能研究,如摄食活动与体重(Kawano et al, 2002)、睡眠与觉醒(Peyron et al, 2009)和体内能量平衡(MacNeil et al, 2013)等调节功能。
对牙鲆、星斑川鲽和星突江鲽的研究都表明,MCH具有抑制无眼侧黑化发生和发育的生理调控作用(Kang et al, 2013; Takanishi et al, 2007; Takeshi et al, 2007)。本研究分析了半滑舌鳎2种MCH亚基与无眼侧黑化程度的关系,发现垂体和皮肤中MCH1 mRNA在无眼侧黑化发生过程中表现出类似的变化趋势,其表达水平都在黑化发生早期(无眼侧10%黑化)达峰值,但随着黑化程度的增加却显著降低,表明无论垂体还是皮肤中的MCH1都主要参与了黑化的早期发生过程调控,而随着黑化程度的增加其调控作用减弱,其可能的机制值得今后深入解析。研究还发现,半滑舌鳎无眼侧正常鱼和无眼侧50%黑化鱼的脑垂体中都具有较高的MCH2 mRNA表达水平,但在无眼侧10%黑化组和无眼侧80%黑化组表达水平却显著降低,表明垂体MCH2可能不是通过直接的内分泌途径参与调控无眼侧黑化过程,可能有其他体色因子通过不同的信号途径参与了黑化的调控过程。而在皮肤组织中,发现MCH2 mRNA表达水平随黑化程度增加而显著升高,并在无眼侧80%黑化组显著升高至最高值,表明皮肤MCH2对无眼侧黑化现象的发生和发展过程起到了关键的调控作用,今后应深入开展其表达调控的相关机制研究,进一步解析MCH2的生理功能。
| Amiya N, Amano M, Takahashi A, et al. Effects of tank color on melanin-concentrating hormone levels in the brain, pituitary gland, and plasma of the barfin flounder as revealed by a newly developed time-resolved fluoroimmunoassay. General and Comparative Endocrinology , 2005, 143 (3) : 251-256 DOI:10.1016/j.ygcen.2005.04.012 | |
| Baker B, Levy A, Hall L, et al. Cloning and expression of melanin-concentrating hormone genes in the rainbow trout brain. Neuroendocrinology , 1995, 61 (1) : 67-76 | |
| Berman JR, Skariah G, Maro GS, et al. Characterization of two melanin-concentrating hormone genes in zebrafish reveals evolutionary and physiological links with the mammalian mch system. Journal of Comparative Neurology , 2009, 517 (5) : 695-710 DOI:10.1002/cne.v517:5 | |
| Deng JY, Meng TX, Ren SM, et al. Species composition, abundance and distribution of fishes in the Bohai Sea. Marine Fisheries Research , 1988, 9 : 11-89 [邓景耀, 孟田湘, 任胜民, 等. 渤海鱼类种类组成及数量分布. 海洋水产研究 , 1988, 9 : 11-89] | |
| Gröneveld D, Hut MJ, Balm PHM, et al. Cloning and sequence analysis of hypothalamus cDNA encoding tilapia melanin-concentrating hormone. Fish Physiology and Biochemistry , 1993, 11 (1-6) : 117-124 DOI:10.1007/BF00004557 | |
| Jeon JM, Song YH. Cloning of melanin-concentrating hormone cDNA gene from olive flounder (Paralichthys olivaceus). Journal of the Korean Fisheries Society , 2003, 36 (5) : 442-448 | |
| Kang DY, Kim HC. Functional characterization of two melanin-concentrating hormone genes in the color camouflage, hypermelanosis, and appetite of starry flounder. General and Comparative Endocrinology , 2013, 189 : 74-83 DOI:10.1016/j.ygcen.2013.04.025 | |
| Kawano H, Honma S, Hayashi A, et al. Melanin-concentrating hormone neuron system: The wide web that controls the feeding. Anatomical Science International , 2002, 77 (3) : 149-160 DOI:10.1046/j.0022-7722.2002.00027.x | |
| Kawauchi H, Kawazoe I, Tsubokawa M, et al. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature , 1983, 305 (5932) : 321-323 DOI:10.1038/305321a0 | |
| Liu XZ, Zhuang ZM, Ma AJ, et al. Operative technologies for seedling rearing of Cynoglossus semilaevis Günther. Marine Fisheries Research , 2006, 27 (2) : 17-24 [柳学周, 庄志猛, 马爱军, 等. 半滑舌鳎苗种生产技术的开发研究. 海洋水产研究 , 2006, 27 (2) : 17-24] | |
| Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods , 2001, 25 (4) : 402-408 DOI:10.1006/meth.2001.1262 | |
| MacNeil DJ. The role of melanin-concentrating hormone and its receptors in energy homeostasis. Frontiers in Endocrinology , 2013, 4 : 49 | |
| Minth CD, Qiu H, Akil H, et al. Two precursors of melanin-concentrating hormone: DNA sequence analysis and in situ and immunochemical localization. Proceedings of the National Academy of Sciences , 1989, 86 (11) : 4292-4296 DOI:10.1073/pnas.86.11.4292 | |
| Mizusawa K, Kawashima Y, Sumuma T, et al. Involvement of melanin-concentrating hormone 2 in background color adaptation of barfin flounder Verasper moseri. General and Comparative Endocrinology , 2015, 214 : 140-148 DOI:10.1016/j.ygcen.2014.07.008 | |
| Mizusawa KL, Saito Y, Wang Z, et al. Molecular cloning and expression of two melanin-concentrating hormone receptors in goldfish. Peptides , 2009, 30 (11) : 1990-1996 DOI:10.1016/j.peptides.2009.04.010 | |
| Peyron C, Sapin E, Leger L, et al. Role of the melanin-concentrating hormone neuropeptide in sleep regulation. Peptides , 2009, 30 (11) : 2052-2059 DOI:10.1016/j.peptides.2009.07.022 | |
| Takahashi A, Itoh T, Nakanishi A, et al. Molecular cloning of proopiomelanocortin cDNA in the ratfish, a holocephalan. General and Comparative Endocrinology , 2004, 135 (1) : 159-165 DOI:10.1016/j.ygcen.2003.08.007 | |
| Takahashi A, Kosugi T, Kobayashi Y, et al. The melanin-concentrating hormone receptor 2 (MCH-R2) mediates the effect of MCH to control body color for background adaptation in the barfin flounder. General and Comparative Endocrinology , 2007, 151 (2) : 210-219 DOI:10.1016/j.ygcen.2007.01.011 | |
| Takeshi Y, Masafumi A, Noriko A, et al. Hypermelanosis on the blind side of Japanese flounder Paralichthys olivaceusis diminished by rearing in a white tank. Fisheries Science , 2007, 73 (2) : 466-468 DOI:10.1111/fis.2007.73.issue-2 | |
| Tuziak SM, Volkoff H. A preliminary investigation of the role of melanin-concentrating hormone (MCH) and its receptors in appetite regulation of winter flounder (Pseudopleuronectes americanus). Molecular and Cellular Endocrinology , 2012, 348 (1) : 281-296 DOI:10.1016/j.mce.2011.09.015 |



