2. 上海海洋大学水产与生命学院 上海 201306
2. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306
包括硬骨鱼类在内的脊椎动物生殖神经内分泌活动主要受到下丘脑-垂体-性腺轴(Hypothalamo-pituitary-gonadal axis)的调控。下丘脑神经肽促性腺激素释放激素(Gonadotropin-releasing hormone, GnRH)曾被认为是促性腺激素(Gonadotropin, GTH)神经内分泌调控的主要促进因子,但没有一种下丘脑因子能够抑制GTH分泌。直到Tsutsui等(2000)从日本鹌鹑(Coturnix japonica)脑中分离出一种下丘脑十二肽(SIKPSAYLPLRFa),因其能够抑制鹌鹑垂体GTH的分泌,所以命名为促性腺激素抑制激素(Gonadotropin-inhibitory hormone, GnIH),这是第1次在脊椎动物中鉴定出的具有抑制生殖功能的下丘脑多肽。随后,从鱼类到哺乳类中都鉴定出了GnIH的同源基因,并对其结构与功能的多样性开展了大量的研究(Ogawa et al, 2014; Osugi et al, 2014; Ubuka et al, 2013; 王滨等, 2016)。
不同物种GnIH基因编码的前体多肽经过酶切加工可产生不同种类的成熟肽(Ogawa et al, 2014; Osugi et al, 2014; Tsutsui et al, 2012)。这些多肽的C末端都有一个特征性基序-LPXRFa (X=L或者Q),因此,从结构看应该被称作LPXRFa多肽。
现在普遍认为,在鸟类和哺乳类中GnIH作用于下丘脑GnRH神经元或者直接作用于垂体来抑制GTH的合成与分泌(Bentley et al, 2009; Clarke et al, 2014; Kriegsfeld et al, 2010)。然而,GnIH对鱼类垂体激素合成与分泌的表达调控作用仍存在争议。金鱼(Carassius auratus) GnIH多肽gfGnIH-1促进了星点东方鲀(Takifugu niphobles)垂体LHβ、FSHβ、GH以及PRL的表达(Shahjahan et al, 2011、2016)。腹腔注射金鱼GnIH多肽gfGnIH-2和gfGnIH-3显著降低了金鱼垂体LHβ/FSHβ的表达水平(Qi et al, 2013)。同样,腹腔注射斜带石斑鱼(Epinephelus coioides) gGnIH-2也降低了垂体LHβ的表达水平(Wang et al, 2015)。此外,侧脑室注射欧洲海鲈(Dicentrarchus labrax) sbGnIH-1和sbGnIH-2的结果显示,sbGnIH-1不影响垂体LHβ、FSHβ和GH的表达,但sbGnIH-2显著降低了三者的表达(Paullada-Salmeron et al, 2016)。综上所述,GnIH多肽参与了脊椎动物垂体功能,但GnIH在不同物种间,尤其是硬骨鱼类中,对垂体激素合成与分泌的作用是不同的。
除了直接作用于垂体外,GnIH也能够通过作用于下丘脑GnRH神经元进而调控GTH的合成与分泌。然而,GnIH对鱼类下丘脑生殖相关基因表达调控的研究较少,尚无定论。
半滑舌鳎(Cynoglossus semilaevis)是一种重要的海水养殖经济鱼类。近年来,对半滑舌鳎生殖相关功能基因已有一些报道,例如FSHβ、LHβ、GTHα和mPR-Like等(Shi et al, 2015; 柳学周等, 2015),但鲆鲽鱼类中尚未见有关GnIH的研究报道。为研究GnIH在鱼类生殖调控中的作用机制,本研究首次通过下丘脑离体孵育实验,结合荧光定量PCR技术,研究GnIH多肽对下丘脑生殖相关基因(包括GnRH、kiss2和GnIH)的表达调控影响,以期阐明GnIH对半滑舌鳎生殖轴的调控作用机制。
1 材料与方法 1.1 试剂半滑舌鳎成熟肽tsGnIH-1 (SLDLERLNMRVTPTASKSSLPTIIKLYPPTVNPHIHANMPMRF-NH2)和tsGnIH-2 (EVEPEDDQSHNTPNMPQRF-NH2)均由上海强耀生物科技有限公司合成,纯度>95%。将多肽溶于灭菌超纯水配成1 mmol/L母液,分装于PCR管(5 μl/管),-80℃保存备用。L15培养基干粉(Leibovitz’s L15 Medium powder)及双抗(Penicillin-Streptomycin-Glutamine)均购自Life Technologies公司;牛血清白蛋白BSA (Albumin Bovine Serum, Fraction V,Fatty Acid-Poor,Endotoxin-Free)购自Calbiochem公司;HEPES购自Solabio公司;RNA提取试剂RNAiso Plus、反转录试剂盒PrimeScriptTM RT Reagent Kit with gDNA Eraser (Perfect Real-time)以及荧光定量PCR试剂盒SYBR®Premix Ex TaqTMⅡ (Tli RNaseH Plus)均购自TaKaRa公司,其余为国产分析纯。
1.2 实验用鱼实验用半滑舌鳎购自山东青岛钧予水产有限公司,2龄,雌性,体重为(573.8±26.6) g,性腺指数(Gonadosomatic index, GSI=性腺重/体重×100)为(1.54±0.08)%。半滑舌鳎用0.05%的MS-222麻醉后,用剪子沿侧线的延长线剪开头部处死,迅速取出下丘脑,置于冰预冷的新鲜L15培养基中。L15培养基配方(表 1)参照Wang等(2014)。取样工具在使用前均在180℃烘烤6 h,取样结束后,立刻将样品转移到细胞房内进一步处理。
|
|
表 1 Leibovitz’s L15培养基配方 Table 1 The formula of Leibovitz's L15 medium |
将表 1组分溶于800 ml超纯水中,用5 mol/L NaOH调pH至7.2,用超纯水定容至1 L,用磁力搅拌器搅拌,待充分溶解后,用0.2 μm滤膜过滤除菌,分装至50 ml离心管,用封口膜密封管盖,4℃保存备用。
1.3 离体孵育实验所有操作均在无菌超净工作台中进行。将下丘脑转移到培养皿中,用新鲜的L15培养液清洗2次。用巴氏吸管将下丘脑逐个转移至24孔培养板中,每孔加入1 ml L15培养液,然后放在25℃的CO2培养箱(HF 151 UV,Heal Force公司)预孵育1 h。预孵育结束后,去除孵育液,按以下方法处理:对照组,每孔加入1 ml的L15培养液;tsGnIH-1实验组,每孔加入1 ml终浓度为1 μmol/L tsGnIH-1的L15培养液;tsGnIH-2实验组,每孔加入1 ml终浓度为1 μmol/L tsGnIH-2的L15培养液,然后将培养板放在25℃细胞培养箱中孵育24 h。GnIH多肽浓度选择参考之前已发表文献(Biran et al, 2014; Di Yorio et al, 2016)。孵育结束后,将下丘脑转移到含有1 ml RNAiso Plus的Eppendorf管中。每个下丘脑用注射器抽打数次,使之与RNAiso Plus匀浆完全,在-80℃冰箱中保存,直至RNA提取。该实验重复2次,每次实验每种处理4-6个重复。
1.4 总RNA提取、反转录与荧光定量检测按照RNAiso Plus操作说明提取下丘脑总RNA,通过NanoDrop2000C分光光度计(Thermo公司)测定RNA的纯度和浓度,取1 μg总RNA非变性电泳(0.8%琼脂糖凝胶)检验其完整性。纯度高且完整的RNA按照PrimeScriptTM RT Reagent Kit with gDNA Eraser (Perfect Real-time)反转录试剂盒说明书进行反转录实验。反转录实验体系为20 μl,先取1 μg总RNA与2 μl 5×gDNA Eraser Buffer、1 μl gDNA Eraser,用无RNase水补充至10 μl,充分混匀、短暂离心后按以下反应程序进行:42℃ 2 min;将这一步的反应液与1 μl RT Primer Mix、1 μl PrimeScriptTM RT Enzyme Mix Ⅰ、4 μl 5×PrimeScript Buffer、4 μl无RNase水,充分混匀、短暂离心后按以下反应程序进行:37℃ 15 min,85℃ 5 s;将反转录产物10倍稀释后-20℃保存备用。
按照Wang等(2016)所述方法通过荧光定量PCR检测相关基因表达分析。荧光定量扩增体系为20 μl,包括10 μl 2×SYBR®Premix Ex TaqTMⅡ、0.8 μl上下游引物(每种引物浓度均为10 μmol/L)、2 μl稀释的cDNA模板和7.2 μl无菌水,充分混匀、短暂离心后通过Mastercycler ep realplex Real-time PCR仪(Eppendorf公司)进行荧光定量检测,每个样品3个平行,PCR程序采用两步法:95℃ 30 s;95℃ 5 s,60℃ 20 s,共40个循环。反应结束后进行熔解曲线分析以及PCR产物测序,进而验证产物特异性。18S作为内参基因,定量PCR检测所使用的引物(利用Primer premier 5.0设计)以及它们的扩增片段长度与扩增效率等信息见表 2。基因相对表达量参照2-ΔΔCt法计算(Livak et al, 2001),结果以平均值±标准误(Mean±SE)表示。采用SPSS 17进行单因素方差分析(One-way ANOVA),与Ducan多重比较,P<0.05认为有显著性统计差异。
|
|
表 2 定量PCR引物信息 Table 2 Primer information for quantitative Real-Time PCR |
以对照组作为参照,研究比较tsGnIH-1与tsGnIH-2对半滑舌鳎下丘脑gnrh2 mRNA表达量的影响。如图 1所示,tsGnIH-1多肽显著促进了gnrh2 mRNA的表达水平(P<0.05),tsGnIH-1处理组gnrh2 mRNA的表达量是对照组的1.85倍;而tsGnIH-2多肽有所降低了gnrh2 mRNA表达量,与对照组相比无显著差异。

|
图 1 半滑舌鳎GnIH多肽对离体孵育下丘脑gnrh2 mRNA表达量的影响 Figure 1 in vitro effects of GnIH peptides on gnrh2 mRNA levels in the hypothalamus of C. semilaevis 不同字母表示不同组之间有显著性差异,下同 Groups with different letters are significantly different from each other, the same as below |
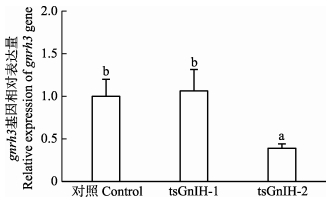
以对照组作为参照,研究比较tsGnIH-1与tsGnIH-2对半滑舌鳎下丘脑gnrh3 mRNA表达量的影响。如图 2所示,tsGnIH-2多肽显著抑制了gnrh3 mRNA的表达水平(P<0.05),tsGnIH-2处理组gnrh3 mRNA表达量为对照组的39%;然而,tsGnIH-1多肽不影响gnrh3 mRNA的表达水平,tsGnIH-1处理组gnrh3 mRNA表达量较对照组增加6%。
|
图 2 半滑舌鳎GnIH多肽对离体孵育下丘脑gnrh3 mRNA表达量的影响 Figure 2 in vitro effects of GnIH peptides on gnrh3 mRNA levels in the hypothalamus of C. semilaevis |
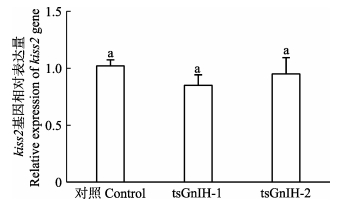
为了研究GnIH多肽是否通过Kiss系统调控半滑舌鳎生殖,以对照组作为参照,分析比较了tsGnIH-1与tsGnIH-2对半滑舌鳎下丘脑kiss2 mRNA表达量的影响。如图 3所示,tsGnIH-1处理组kiss2 mRNA的表达量为对照组的85%,而tsGnIH-2处理组kiss2 mRNA的表达量为对照组的95%。结果表明,tsGnIH-1与tsGnIH-2多肽均不影响kiss2 mRNA的表达水平。
|
图 3 半滑舌鳎GnIH多肽对离体孵育下丘脑kiss2 mRNA表达量的影响 Figure 3 in vitro effects of GnIH peptides on kiss2 mRNA levels in the hypothalamus of C. semilaevis |
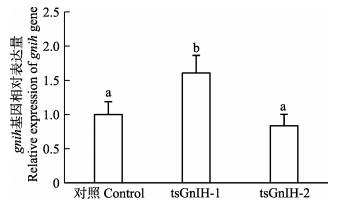
为了研究GnIH多肽对GnIH系统的自调控,以对照组作为参照,分析比较了tsGnIH-1与tsGnIH-2对半滑舌鳎下丘脑gnih mRNA表达量的影响。如图 4所示,tsGnIH-1多肽显著促进了gnih mRNA的表达水平(P<0.05),tsGnIH-1处理组gnih mRNA表达量为对照组的1.61倍;然而,tsGnIH-2多肽不影响gnih mRNA的表达水平,tsGnIH-2处理组gnih mRNA表达量为对照组的83.53%。
|
图 4 半滑舌鳎GnIH多肽对离体孵育下丘脑gnih mRNA表达量的影响 Figure 4 in vitro effects of GnIH peptides on gnih mRNA levels in the hypothalamus of C. semilaevis |
GnIH是最近发现的一种新型下丘脑神经肽,也是目前脊椎动物中唯一发现的生殖抑制因子,其在鸟类和哺乳类生殖调控中的作用受到了广泛研究,然而,有关GnIH对鱼类生殖调控的研究相对较少,尤其是GnIH在鱼类生殖调控中的精确作用及其分子机制尚未阐明(Kriegsfeld et al, 2015; Tsutsui et al, 2015; Ubuka et al, 2016)。目前,在金鱼、斑马鱼(Danio rerio)、星点东方鲀、尼罗罗非鱼(Oreochromis niloticus)、斜带石斑鱼和欧洲海鲈等鉴定出了GnIH同源基因,并对其调控鱼类生殖活动的功能进行了初步研究(Kriegsfeld et al, 2015; Tsutsui et al, 2015; Ubuka et al, 2016)。为了进一步研究GnIH在鱼类生殖调控中的作用机制,本研究首次通过下丘脑离体孵育的方法研究了GnIH多肽对半滑舌鳎下丘脑生殖相关基因的表达调控。
在之前的研究中,通常采用在体手段(腹腔注射或者侧脑室注射)研究GnIH对下丘脑生殖相关基因的表达调控。然而,多种内源性因子参与或者互相作用,导致在体实验结果经常很难解释。因此,本研究采用下丘脑离体孵育方法研究GnIH多肽对半滑舌鳎下丘脑生殖相关基因的表达调控,避免了内源性因子的影响。GnRH是垂体GTH合成与分泌的主要促进因子,在脊椎动物中已经鉴定出了15种GnRH亚型,其中8种存在于硬骨鱼类中;每种硬骨鱼类中存在至少2种形式的GnRH多肽(Ohkubo et al, 2010)。目前,在半滑舌鳎中鉴定出2种gnrh基因,即gnrh2 (GenBank登录号:KX090947)和gnrh3 (GenBank登录号:JQ028869)。在本研究中,tsGnIH-1促进了半滑舌鳎下丘脑gnrh2的表达,但不影响gnrh3的表达。侧脑室注射欧洲海鲈sbGnIH-1和腹腔注射斜带石斑鱼gGnIH-1均不影响gnrh3的表达(Paullada-Salmeron et al, 2016; Wang et al, 2015)。然而,侧脑室注射欧洲海鲈sbGnIH-1也不影响gnrh2的表达(Paullada-Salmeron et al, 2016)。不同于tsGnIH-1,tsGnIH-2显著降低了半滑舌鳎下丘脑gnrh3的表达,但不影响gnrh2的表达。腹腔注射金鱼gfGnIH-2显著降低了gnrh3的表达,但不影响gnrh2的表达(Qi et al, 2013)。相反,侧脑室注射欧洲海鲈sbGnIH-2显著降低了gnrh2的表达,但不影响gnrh3的表达(Paullada-Salmeron et al, 2016);腹腔注射斜带石斑鱼gGnIH-2也不影响gnrh3的表达(Wang et al, 2015)。半滑舌鳎和欧洲海鲈中只存在2种GnIH多肽,然而,在金鱼和斜带石斑鱼中存在3种GnIH多肽。腹腔注射金鱼gfGnIH-3不影响gnrh2的表达,但抑制了gnrh3的表达(Qi et al, 2013)。相反,腹腔注射斜带石斑鱼gGnIH-3却促进了gnrh3的表达(Wang et al, 2015)。综上所述,GnIH对下丘脑GnRH亚型的不同调控具有物种特异性,甚至同一前体蛋白编码的不同GnIH多肽具有不同的功能。
Kiss也是生殖调控的一种新型下丘脑神经肽,通过直接作用于GnRH神经元促进GnRH分泌进而上调生殖轴,是青春期启动的一个关键因子(Tsutsui et al, 2010)。在斑马鱼、金鱼、青鳉(Oryzias latipes)、欧洲海鲈和鲐鱼(Scomber japonicus)中存在2种kiss基因,即kiss1和kiss2;然而,在塞内加尔鳎(Solea senegalensis)、星点东方鲀和斜带石斑鱼中只存在kiss2基因(Mechaly et al, 2013)。目前,在半滑舌鳎中也只鉴定出了部分kiss2序列(GenBank登录号:KX090946)。在本研究中,tsGnIH-1和tsGnIH-2均不影响kiss2表达。同样,腹腔注射斜带石斑鱼3种GnIH多肽均不影响kiss1及kiss2的表达(Wang et al, 2015)。哺乳类GnIH同源多肽RFRP3也不影响大鼠kiss1表达(Johnson et al, 2008)。上述结果说明,GnIH多肽对GnRH亚型的表达调控可能不需要Kiss系统介导。此外,侧脑室注射欧洲海鲈sbGnIH-1也不影响kiss1和kiss2的表达,但sbGnIH-2均降低了kiss1及kiss2的表达,这说明在欧洲海鲈中,sbGnIH-2主要发挥生殖调控的抑制作用(Paullada-Salmeron et al, 2016)。
本研究也探索了GnIH对其自身前体基因的表达调控。结果表明,tsGnIH-1上调了半滑舌鳎下丘脑gnih表达,然而,tsGnIH-2对gnih表达无影响。同样,腹腔注射金鱼gfGnIH-3也促进了小丑鱼gnih表达(Choi et al, 2016)。然而,侧脑室注射欧洲海鲈sbGnIH-1不影响gnih的表达,sbGnIH-2却抑制了gnih的表达(Paullada-Salmeron et al, 2016)。在哺乳类和鸟类中已证实多种因子(例如光照、压力等)影响了gnih的表达,进而影响季节性生殖调控(Tsutsui, 2009; Tsutsui et al, 2015; Ubuka et al, 2008)。鱼类生殖活动也受到光照等环境因子的影响,GnIH是否参与了鱼类季节性生殖活动需要进一步深入研究。
4 小结GnIH是一个多功能的神经肽,在下丘脑-垂体-性腺轴多个水平参与了生殖调控。GnIH对鱼类生殖调控的作用仍存在争议,需要进一步深入研究;GnIH调控鱼类垂体激素合成与分泌的信号转导机制网络需要进一步完善;GnIH与其他因子之间如何互相作用、在垂体水平将多种信号整合进而调控生殖等生理过程仍不清楚(王滨等, 2016)。本研究首次通过下丘脑离体孵育的方法研究了GnIH多肽对半滑舌鳎下丘脑生殖相关基因的表达调控。结果表明,tsGnIH-1促进了gnrh2和gnih的表达,对gnrh3和kiss2的表达无影响;tsGnIH-2抑制了gnrh3的表达,对gnrh2、kiss2和gnih的表达无影响。GnIH多肽对生殖相关基因的不同调控表明了生殖调控的复杂性,即使同一前体蛋白编码的不同GnIH多肽在生殖调控中的作用也不尽相同。该研究结果增加了对GnIH参与鱼类生殖调控机制的认识,为下一步研究奠定了基础。
| Bentley GE, Ubuka T, McGuire NL, et al. Gonadotrophin-inhibitory hormone: A multifunctional neuropeptide. Journal of Neuroendocrinology , 2009, 21 (4) : 276-281 DOI:10.1111/jne.2009.21.issue-4 | |
| Choi YJ, Kim NN, Habibi HR, et al. Effects of gonadotropin inhibitory hormone or gonadotropin-releasing hormone on reproduction-related genes in the protandrous cinnamon clownfish, Amphiprion melanopus. General and Comparative Endocrinology , 2016, 235 : 89-99 DOI:10.1016/j.ygcen.2016.06.010 | |
| Clarke IJ, Parkington HC. Gonadotropin inhibitory hormone (GnIH) as a regulator of gonadotropes. Molecular and Cellular Endocrinology , 2014, 385 (1-2) : 36-44 DOI:10.1016/j.mce.2013.08.017 | |
| Di Yorio MP, Perez Sirkin DI, Delgadin TH, et al. Gonadotrophin-inhibitory hormone in the cichlid fish Cichlasoma dimerus: Structure, brain distribution and differential effects on the secretion of gonadotrophins and growth hormone. Journal of Neuroendocrinology , 2016, 28 (5) DOI:10.1111/jne.12377 | |
| Johnson MA, Fraley GS. Rat RFRP-3 alters hypothalamic GHRH expression and growth hormone secretion but does not affect KiSS-1 gene expression or the onset of puberty in male rats. Neuroendocrinology , 2008, 88 (4) : 305-315 DOI:10.1159/000145718 | |
| Kriegsfeld LJ, Gibson EM, Williams WP 3rd, et al. The roles of RFamide-related peptide-3 in mammalian reproductive function and behaviour. Journal of Neuroendocrinology , 2010, 22 (7) : 692-700 DOI:10.1111/j.1365-2826.2010.02031.x | |
| Kriegsfeld LJ, Ubuka T, Bentley GE, et al. Seasonal control of gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Frontiers in Neuroendocrinology , 2015, 37 : 65-75 DOI:10.1016/j.yfrne.2014.12.001 | |
| Liu XZ, Shi B, Li XX, et al. Molecular characterization of the novel membrane progestin receptor gene and its role during ovarian development in the half smooth tongue sole Cynoglossus semilaevis Günther. Journal of Fishery Sciences of China , 2015, 22 (4) : 608-619 [柳学周, 史宝, 李晓晓, 等. 半滑舌鳎新型膜孕激素受体基因分子特征及其在卵巢发育过程的作用. 中国水产科学 , 2015, 22 (4) : 608-619] | |
| Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods , 2001, 25 (4) : 402-408 DOI:10.1006/meth.2001.1262 | |
| Mechaly AS, Vinas J, Piferrer F. The kisspeptin system genes in teleost fish, their structure and regulation, with particular attention to the situation in Pleuronectiformes. General and Comparative Endocrinology , 2013, 188 : 258-268 DOI:10.1016/j.ygcen.2013.04.010 | |
| Ogawa S, Parhar IS. Structural and functional divergence of gonadotropin-inhibitory hormone from jawless fish to mammals. Frontiers in Endocrinology (Lausanne) , 2014, 5 : 177 | |
| Ohkubo M, Aranishi F, Shimizu A. Molecular cloning and brain distribution of three types of gonadotropin-releasing hormone from mummichog Fundulus heteroclitus. Journal of Fish Biology , 2010, 76 (2) : 379-394 DOI:10.1111/jfb.2010.76.issue-2 | |
| Osugi T, Ubuka T, Tsutsui K. Review: evolution of GnIH and related peptides structure and function in the chordates. Frontiers in Neuroscience , 2014, 8 : 255 | |
| Paullada-Salmeron JA, Cowan M, Aliaga-Guerrero M, et al. Gonadotropin inhibitory hormone down-regulates the brain-pituitary reproductive axis of male European sea bass (Dicentrarchus labrax). Biology of Reproduction , 2016, 94 (6) : 121 DOI:10.1095/biolreprod.116.139022 | |
| Qi X, Zhou W, Li S, et al. Evidences for the regulation of GnRH and GTH expression by GnIH in the goldfish, Carassius auratus. Molecular and Cellular Endocrinology , 2013, 366 (1) : 9-20 DOI:10.1016/j.mce.2012.11.001 | |
| Shahjahan M, Doi H, Ando H. LPXRFamide peptide stimulates growth hormone and prolactin gene expression during the spawning period in the grass puffer, a semi-lunar synchronized spawner. General and Comparative Endocrinology , 2016, 227 : 77-83 DOI:10.1016/j.ygcen.2015.09.008 | |
| Shahjahan M, Ikegami T, Osugi T, et al. Synchronised expressions of LPXRFamide peptide and its receptor genes: seasonal, diurnal and circadian changes during spawning period in grass puffer. Journal of Neuroendocrinology , 2011, 23 (1) : 39-51 DOI:10.1111/jne.2010.23.issue-1 | |
| Shi B, Liu X, Xu Y, et al. Molecular characterization of three gonadotropin subunits and their expression patterns during ovarian maturation in Cynoglossus semilaevis. International Journal of Molecular Sciences , 2015, 16 (2) : 2767-2793 DOI:10.3390/ijms16022767 | |
| Tsutsui KA. New key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Progress in Neurobiology , 2009, 88 (1) : 76-88 DOI:10.1016/j.pneurobio.2009.02.003 | |
| Tsutsui K, Bentley GE, Kriegsfeld LJ, et al. Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: New key neuropeptides controlling reproduction. Journal of Neuroendocrinology , 2010, 22 (7) : 716-727 | |
| Tsutsui K, Saigoh E, Ukena K, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochemical and Biophysical Research Communications , 2000, 275 (2) : 661-667 DOI:10.1006/bbrc.2000.3350 | |
| Tsutsui K, Ubuka T, Bentley GE, et al. Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect. General and Comparative Endocrinology , 2012, 177 (3) : 305-314 DOI:10.1016/j.ygcen.2012.02.013 | |
| Tsutsui K, Ubuka T, Son YL, et al. Contribution of GnIH research to the progress of reproductive neuroendocrinology. Frontiers in Endocrinology (Lausanne) , 2015, 6 : 179 | |
| Ubuka T, McGuire NL, Calisi RM, et al. The control of reproductive physiology and behavior by gonadotropin-inhibitory hormone. Integrative and Comparative Biology , 2008, 48 (5) : 560-569 DOI:10.1093/icb/icn019 | |
| Ubuka T, Son YL, Bentley GE, et al. Gonadotropin-inhibitory hormone (GnIH), GnIH receptor and cell signaling. General and Comparative Endocrinology , 2013, 190 (9) : 10-17 | |
| Ubuka T, Son YL, Tsutsui K. Molecular, cellular, morphological, physiological and behavioral aspects of gonadotropin-inhibitory hormone. General and Comparative Endocrinology , 2016, 227 : 27-50 DOI:10.1016/j.ygcen.2015.09.009 | |
| Wang B, Jia J, Yang G, et al. In vitro effects of somatostatin on the growth hormone-insulin-like growth factor axis in orange-spotted grouper (Epinephelus coioides). General and Comparative Endocrinology , 2016, 237 : 1-9 DOI:10.1016/j.ygcen.2015.10.014 | |
| Wang B, Liu XZ, Xu YJ, et al. Progress of research on gonadotropin-inhibitory hormone and its receptors in fish. Journal of Fisheries of China , 2016, 40 (2) : 278-287 [王滨, 柳学周, 徐永江, 等. 鱼类促性腺激素抑制激素及其受体的研究进展. 水产学报 , 2016, 40 (2) : 278-287] | |
| Wang B, Qin C, Zhang C, et al. Differential involvement of signaling pathways in the regulation of growth hormone release by somatostatin and growth hormone-releasing hormone in orange-spotted grouper (Epinephelus coioides). Molecular and Cellular Endocrinology , 2014, 382 (2) : 851-859 DOI:10.1016/j.mce.2013.10.025 | |
| Wang Q, Qi X, Guo Y, et al. Molecular identification of GnIH/GnIHR signal and its reproductive function in protogynous hermaphroditic orange-spotted grouper (Epinephelus coioides). General and Comparative Endocrinology , 2015, 216 : 9-23 DOI:10.1016/j.ygcen.2015.04.016 |



