2. 青岛海洋科学与技术国家实验室 海洋渔业科学与食物产出过程功能实验室 青岛 266071;
3. 上海海洋大学水产与生命学院 上海 201306
2. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071;
3. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306
硬骨鱼类的性腺成熟主要受到脑-垂体-性腺轴(HPG轴)的调控。下丘脑神经肽促性腺激素释放激素(Gonadotropin-releasing hormone, GnRH)促进垂体促性腺激素(Gonadotropins, GTHs)即促卵泡激素(Follicle-stimulating hormone, FSH)和黄体生成素(Luteinizing hormone, LH)的合成与分泌,GTHs进而作用于性腺促进类固醇激素的分泌。到目前为止,在脊椎动物中已经鉴定出15种GnRH亚型,其中,8种存在于硬骨鱼类中;每种硬骨鱼类中存在至少2种形式的GnRH多肽(Ohkubo et al, 2010)。进化和功能分析表明,GnRH分为3大支:GnRH1 (之前被称作seabream GnRH)、GnRH2 (之前被称作chicken GnRH-Ⅱ)及GnRH3 (之前被称作salmon GnRH)。GnRH1是一种物种特异性的GnRH多肽,主要在嗅球、端脑腹侧及视前区表达,通过促进垂体GTHs合成与分泌进而调控性腺成熟;GnRH2从鱼类到哺乳类动物高度保守,GnRH2神经元位于中脑盖,并且其神经元轴突广泛分布于整个中枢神经系统,可能参与了性行为和摄食行为;GnRH3是鱼类所特有的一种亚型,位于前脑腹侧,在嗅球、端脑腹侧及视前区与GnRH1有部分重叠分布,可能参与神经调节功能(Hildahl et al, 2011; Shahjahan et al, 2010)。
目前,已在多种鱼类中鉴定出了GnRH2同源基因,从鱼类到哺乳类GnRH2前体氨基酸序列高度保守,均由信号肽、GnRH2十肽、酶切位点及GnRH相关肽组成(Guilgur et al, 2006)。尽管GnRH2高度保守,然而其生理学功能研究较少,尤其是GnRH2在鱼类生殖调控中的生理作用仍不清楚。与其他性腺时期相比,黑鲷(Acanthopagrus schlegeli)和小丑鱼(Amphiprion melanopus)性腺gnrh2 mRNA在成熟精巢和成熟卵巢中表达量较高,说明GnRH2可能参与了二者性腺发育和性逆转(An et al, 2008; Kim et al, 2012)。然而,美洲黑石斑(Centropristis striata)脑gnrh2 mRNA水平在性逆转过程中保持稳定(Breton et al, 2015)。此外,在银汉鱼(Odontesthes bonariensis)、星点东方鲀(Takifugu niphobles)、大西洋鳕(Gadus morhua)以及圆斑星鲽(Verasper variegatus)卵巢成熟过程中脑gnrh2 mRNA水平基本保持不变(Guilgur et al, 2009; Hildahl et al, 2011; Shahjahan et al, 2010; Xu et al, 2012)。鲐鱼(Scomber japonicus)脑和垂体gnrh2 mRNA表达量以及脑中GnRH2多肽含量在卵巢成熟过程中也没有发生显著性变化(Selvaraj et al, 2012)。上述结果说明,GnRH2可能没有直接参与上述鱼类性腺发育成熟过程。然而,鲐鱼脑gnrh2 mRNA表达量在性腺分化过程中显著性减少(Selvaraj et al, 2015)。鲻鱼(Mugil cephalus)脑gnrh2 mRNA表达量在卵巢成熟过程中也显著性降低(Nocillado et al, 2007)。相反,大菱鲆(Scophthalmus maximus)脑中GnRH2含量随着卵母细胞直径增加而升高,并且一直持续到排卵后期仍保持较高水平(Andersson et al, 2001)。综上所述,GnRH2在鱼类性腺成熟过程中的生理作用尚未完全阐明,需要进一步深入研究。
半滑舌鳎(Cynoglossus semilaevis)是一种重要的海水养殖经济鱼类。近年来,对于半滑舌鳎生殖相关功能基因研究已有过一些报道,例如FSHβ、LHβ、GTHα、mPR-like等(Shi et al, 2015; 柳学周等, 2015)。为了进一步研究GnRH2在半滑舌鳎卵巢成熟过程中的生理作用,本研究通过RT-PCR及RACE方法获得GnRH2全长cDNA序列,并通过实时荧光定量PCR (qPCR)方法研究了gnrh2 mRNA的组织分布和在卵巢成熟过程中的时空表达特性,旨在为半滑舌鳎生殖内分泌研究提供基础资料。
1 材料与方法 1.1 实验鱼及试剂耗材实验所用半滑舌鳎购自山东海阳市黄海水产有限公司,其中,克隆及组织分布所用半滑舌鳎为2龄雌鱼,体重为553.0-844.1 g,体长为48.0-53.0 cm;卵巢发育不同时期所用半滑舌鳎为性成熟3龄雌鱼,体重为1183.9-1349.2 g,体长为52.0-59.0 cm。
RNA提取试剂RNAiso Plus、反转录试剂盒PrimeScriptTM RT Reagent Kit with gDNA Eraser (Perfect Real-time)及荧光定量PCR试剂盒SYBR®Premix Ex TaqTM Ⅱ (Tli RNaseH Plus)购自TaKaRa公司;RACE试剂盒SMARTerTM RACE cDNA Amplification Kit购自Clontech公司;胶回收试剂盒Zymoclean Gel DNA Recovery Kit购自ZYMO公司;pEASY-T1载体以及Trans1-T1 Phage Resistant感受态细胞购自全式金公司;其余为国产分析纯。引物和测序均由生工生物工程(上海)有限公司完成。无RNA酶EP管及PCR管购自Axygen公司;NanoDrop2000C分光光度计购自Thermo公司;VeritiTM 96-孔热循环仪购自Applied Biosystems公司;荧光定量Mastercycler ep realplex Real-time PCR仪购自Eppendorf公司。
1.2 GnRH2全长cDNA克隆及序列分析半滑舌鳎用0.05% MS-222麻醉后,用剪刀沿侧线剪开头部处死,迅速取出全脑,置于液氮中。按照RNAiso-Plus操作说明提取总RNA,通过NanoDrop2000C分光光度计测定RNA的纯度和浓度,通过1%琼脂糖凝胶电泳检验RNA的完整性。纯度高且完整的RNA按照RACE cDNA Amplification Kit进行反转录,得到3'-RACE-Ready cDNA和5'-RACE-Ready cDNA。根据已知其他鱼类GnRH2的保守序列设计引物(表 1),以上述cDNA为模板,分别进行3'/5'RACE。第1轮PCR反应体系:10×PCR Buffer 2.5 μl,dNTP Mixture (10 mmol/L) 2 μl,GnRH2-F1/GnRH2-R1 (10 μmol/L) 0.5 μl,UPM (long) 2.5 μl,Taq聚合酶0.2 μl,cDNA 1 μl,ddH2O补至25 μl。第1轮PCR程序:94℃ 2 min;94℃ 30 s,68℃ 30 s,72℃ 1 min,共10个循环,每个循环退火温度下降1℃;94℃ 30 s,58℃ 30 s,72℃ 1 min,共20个循环,72℃ 10 min。第2轮(巢式) PCR扩增体系中以第1轮PCR产物为模板,UPM (short)替代UPM (long),GnRH2-F2/GnRH2-R2(10 μmol/L)替代GnRH2-F1/ GnRH2-R1(10 μmol/L),其余组分均不变。巢式PCR程序:94℃ 3 min;94℃ 30 s,58℃ 30 s,72℃ 1 min,共30个循环;72℃ 10 min。以1.5%的琼脂糖凝胶电泳对PCR产物进行检测,将符合目的基因大小的条带按照Gel DNA Recovery Kit说明书进行胶回收。将胶回收产物与pEASY-T1载体25℃连接10 min后转化Trans1-T1 Phage Resistant感受态细胞,进行蓝白斑筛选。挑取阳性单克隆,经菌液PCR验证后条带大小合适的菌液样品送至生工生物工程(上海)有限公司测序。
|
|
表 1 本研究所用引物 Table 1 Primers used in this study |
将测序所得的片段进行拼接,获得半滑舌鳎GnRH2全长cDNA序列。通过DNAman软件推导其氨基酸序列;利用在线软件SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/)预测信号肽;利用在线软件Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/)进行同源性序列比对;利用软件Mega 6.06通过邻接法(NJ)构建进化树,1000次自举(bootstrap)重复检验进化树的置信度;利用在线软件ExPASy (http://web.expasy.org/compute_pi/)推断前体多肽的分子量及等电点。
1.3 gnrh2 mRNA组织分布及卵巢成熟过程中表达分析为了检测gnrh2 mRNA在半滑舌鳎中的组织分布,取3条雌性半滑舌鳎用0.05% MS-222麻醉后,用剪刀沿侧线剪开头部处死,迅速取出脑、垂体、鳃、心、肝、脾、肾、胃、肠、肌肉及卵巢置于液氮中保存至RNA提取。
将处于卵巢不同发育阶段的半滑舌鳎用0.05% MS-222麻醉后,用剪刀沿侧线剪开头部处死,迅速取出性腺置于Davis固定液中固定24 h后,转入70%酒精保存,用于组织切片分析;同时,将脑和垂体置于液氮中保存至RNA提取。参考柳学周等(2009)半滑舌鳎卵巢发育组织学研究中的分类标准,对卵巢组织切片的HE染色结果进行分析并确定卵巢发育时期。将各个时期对应的脑和垂体样品经RNA提取、反转录后,通过荧光定量PCR检测gnrh2 mRNA在卵巢成熟过程中的表达特性。
1.4 总RNA提取、反转录与荧光定量检测按照RNAiso Plus操作说明提取组织总RNA,通过NanoDrop2000C分光光度计测定RNA的纯度和浓度,通过1%琼脂糖凝胶电泳检验RNA的完整性。纯度高且完整的RNA按照PrimeScriptTM RT Reagent Kit with gDNA Eraser (Perfect Real-time)反转录试剂盒说明书进行反转录实验。反转录实验体系为20 μl,先取1 μg总RNA与2 μl 5×g DNA Eraser Buffer、1 μl gDNA Eraser,用无RNase水补充至10 μl,缓慢混匀、短暂离心,按以下反应程序进行:42℃ 2 min;将这一步的反应液与1 μl RT Primer Mix、1 μl PrimeScriptTM RT Enzyme Mix Ⅰ、4 μl 5×PrimeScript Buffer、4 μl无RNase水,缓慢混匀、短暂离心,按以下反应程序进行:37℃ 15 min,85℃ 5 s;将反转录产物10倍稀释后-20℃保存备用。
按照Wang等(2016)所述方法通过荧光定量PCR检测相关基因的表达。荧光定量扩增体系为20 μl,包括10 μl 2×SYBR®Premix Ex TaqTMⅡ、0.8 μl上下游引物(每种引物浓度均为10 μmol/L)、2 μl稀释的cDNA模板以及7.2 μl无菌水,缓慢混匀、短暂离心后,通过Mastercycler ep realplex Real-time PCR仪(Eppendorf)进行荧光定量检测,每个样品3个平行,PCR程序采用两步法:95℃ 30 s;95℃ 5 s,60℃ 20 s,共40个循环。反应结束后进行熔解曲线分析以验证产物特异性。18S作为内参基因,本研究所用引物信息见表 1。基因相对表达量参照2-ΔΔCt法计算,结果以平均值±标准误(Mean±SE)表示。采用SPSS 17进行单因素方差分析(One-way ANOVA)与Ducan多重比较,P<0.05认为有显著性统计差异。
2 结果 2.1 GnRH2全长cDNA克隆及序列分析如图 1所示,半滑舌鳎GnRH2全长cDNA为538 bp (不包括poly A尾),其中,5'非编码区(Untranslated region, UTR)为154 bp,3'UTR为126 bp,开放阅读框(Open reading frame, ORF)为258 bp,编码85个氨基酸(GenBank登录号:KX090947)。GnRH2前体多肽由4部分组成:信号肽(23个氨基酸)、GnRH2十肽(QHWSHGWYPG)、酶切位点(GKR)以及GnRH相关肽(49个氨基酸)。

|
图 1 半滑舌鳎GnRH2前体cDNA核苷酸序列与推测的氨基酸序列 Figure 1 The nucleotide sequence and the deduced amino acid sequence of cDNA encoding the GnRH2 precursor of C. semilaevis 开放阅读框用大写字母表示,5'和3'非编码区用小写字母表示。起始密码子和终止密码子分别用黑框和星号表示;下划实线为信号肽序列;下划实线且粗体字母为GnRH2十肽;下划虚线为GnRH相关肽 The ORF was denoted by capital letters, and the 5'-and 3'-UTRs were denoted by lower-case letters. The initiation codon was boxed, and the stop codon was denoted by an asterisk. The signal peptide was underlined with a solid line. GnRH2 decapeptide was highlighted in bold letters and underlined with a solid line. The GnRH-associated peptide was underlined with a dotted line |
如图 2所示,将半滑舌鳎GnRH2前体与其他硬骨鱼类进行同源性比对分析,发现GnRH2在鱼类中同源性极高,尤其是十肽(QHWSHGWYPG)在所有硬骨鱼类中完全相同。半滑舌鳎GnRH2与鲈形目同源性最高(89.41%-90.59%),其次为鲽形目、鲑形目和鲀形目(78.82%-85.88%),与鲤形目同源性最低(61.18%-71.76%)。

|
图 2 不同硬骨鱼类GnRH2前体氨基酸序列比对 Figure 2 Multiple amino acid sequence alignments of GnRH2 precursors from different fish species 相同的氨基酸用(*)标识;高度保守的氨基酸用(:)标识;低度保守的氨基酸用(.)标识。GenBank登录号分别为:半滑舌鳎(Cynoglossus semilaevis; ANW83256)、星点东方鲀(Takifugu niphobles; BAJ07189)、美洲拟鲽(Pseudopleuronectes americanus; AER93392)、斜带石斑鱼(Epinephelus coioides; ACZ51152)、尼罗罗非鱼(Oreochromis niloticus; BAC65155)、鲐鱼(Scomber japonicus; ADP89592)、黑鲷(Acanthopagrus schlegeli; ABU92552)、欧洲海鲈(Dicentrarchus labrax; AAF62900)、金鱼(Carassius auratus; AAB86989)、斑马鱼(Danio rerio; AAM43951)以及虹鳟(Oncorhynchus mykiss; AAF08687) The identical amino acids were labeled with "*", the highly conserved amino acids were labeled with ":", and the less conserved amino acids were labeled with ".". The GenBank accession numbers of the GnRH2 precursor sequences of teleosts were as follows: half-smooth tongue sole (Cynoglossus semilaevis; ANW83256), grass puffer (Takifugu niphobles; BAJ07189), winter flounder (Pseudopleuronectes americanus; AER93392), orange-spotted grouper (Epinephelus coioides; ACZ51152), Nile tilapia (Oreochromis niloticus; BAC65155), chub mackerel (Scomber japonicus; ADP89592), black porgy (Acanthopagrus schlegeli; ABU92552), European sea bass (Dicentrarchus labrax; AAF62900), goldfish (Carassius auratus; AAB86989), zebrafish (Danio rerio; AAM43951), and rainbow trout (Oncorhynchus mykiss; AAF08687) |
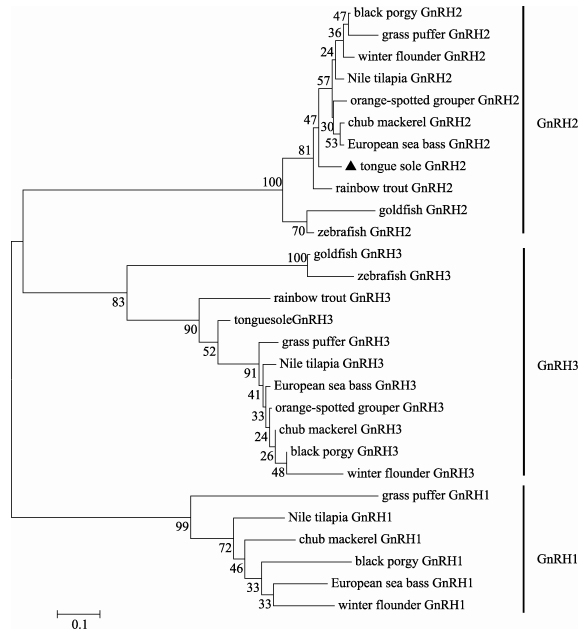
采用Mega 6.06软件,基于GnRH前体氨基酸序列比对分析结果,以邻近法构建鱼类GnRH进化树。如图 3所示,进化树共分为3大支:GnRH1分支、GnRH2分支以及GnRH3分支。本研究克隆所得半滑舌鳎GnRH2与其他鱼类GnRH2聚为一支。
|
图 3 不同硬骨鱼类GnRH前体进化树分析 Figure 3 Phylogenetic analysis of GnRH precursors from different teleosts 鱼类GnRH1、GnRH2以及GnRH3的GenBank登录号分别为:半滑舌鳎(Cynoglossus semilaevis; ANW83256/AFP23141)、星点东方鲀(Takifugu niphobles; BAJ07188/BAJ07189/ BAJ07190)、美洲拟鲽(Pseudopleuronectes americanus; AER93394/AER93392/AER93395)、斜带石斑鱼(Epinephelus coioides; ACZ51152/ ACZ51151)、尼罗罗非鱼(Oreochromis niloticus; BAC65154/BAC65155/ BAC65156)、鲐鱼(Scomber japonicus; ADP89591/ADP89592/ ADP89593)、黑鲷(Acanthopagrus schlegeli; ABU92553/ABU92552/ ABV03808)、欧洲海鲈(Dicentrarchus labrax; AAF62898/AAF62900/ AAF62899)、金鱼(Carassius auratus; AAB86989/BAB18904)、斑马鱼(Danio rerio; AAM43951/AAL99294)以及虹鳟(Oncorhynchus mykiss; AAF08687/AAF91280) The GenBank accession numbers of GnRH1, GnRH2 and GnRH3 precursor sequences of teleosts were as follows: half-smooth tongue sole (Cynoglossus semilaevis; ANW83256/AFP23141), grass puffer (Takifugu niphobles; BAJ07188/BAJ07189/BAJ07190), winter flounder (Pseudopleuronectes americanus; AER93394/AER93392/AER93395), orange-spotted grouper (Epinephelus coioides; ACZ51152/ACZ51151), Nile tilapia (Oreochromis niloticus; BAC65154/BAC65155/BAC65156)、chub mackerel (Scomber japonicus; ADP89591/ADP89592/ADP89593), black porgy (Acanthopagrus schlegeli; ABU92553/ABU92552/ABV03808), European sea bass (Dicentrarchus labrax; AAF62898/AAF62900/AAF62899), goldfish (Carassius auratus; AAB86989/BAB18904), zebrafish (Danio rerio; AAM43951/AAL99294) and rainbow trout (Oncorhynchus mykiss; AAF08687/AAF91280) |
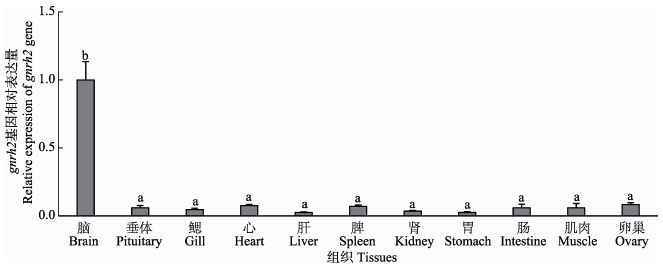
如图 4所示,gnrh2 mRNA主要在脑中表达,在垂体及其他外周组织中表达量很低,只有脑中表达量的2.5%-8.3%。
|
图 4 半滑舌鳎gnrh2 mRNA组织分布 Figure 4 Relative mRNA levels of gnrh2 in various tissues of C. semilaevis 图中不同字母表示不同组之间有显著性差异,下同 Groups with different letters are significantly different from each other, the same as below |
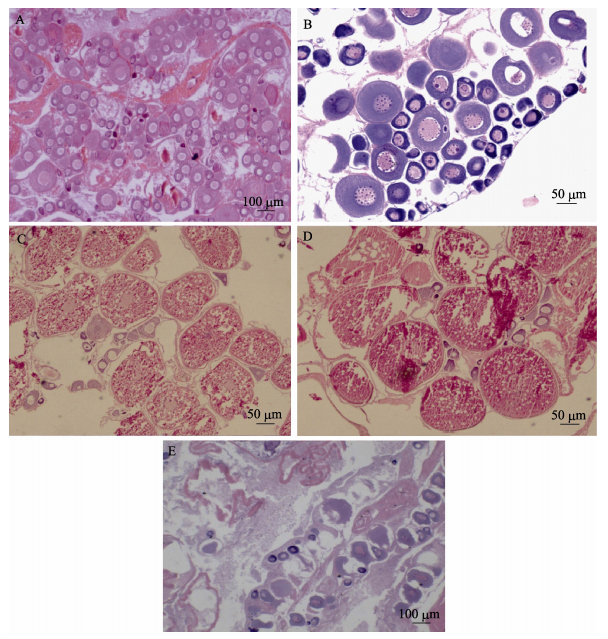
如图 5所示,根据卵母细胞不同发育时相,以各期切片视野中数量或者面积占优势的卵母细胞类型作为卵巢划分依据,卵巢发育周期共分为5个时期:时期Ⅱ,卵黄生成前期;时期Ⅲ,卵黄生成期;时期Ⅳ,卵黄生成后期;时期Ⅴ,成熟期;时期Ⅵ,排卵后期。
|
图 5 半滑舌鳎卵巢发育时期组织切片 Figure 5 Histological sections of C. semilaevis ovaries at different stages of development A. Ⅱ期卵巢; B. Ⅲ期卵巢; C. Ⅳ期卵巢; D. Ⅴ期卵巢; E. Ⅵ期卵巢.比例尺: 100 μm (A和E); 50 μm (B、C和D) A. Ovaries at stage Ⅱ; B. Ovaries at stage Ⅲ; C. Ovaries at stage Ⅳ; D. Ovaries at stage Ⅴ; E. Ovaries at stage Ⅵ. Scale bar: 100 μm (A, E); 50 μm (B, C, and D) |
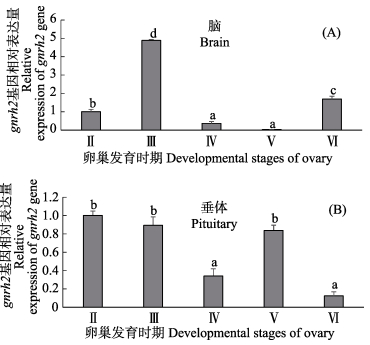
如图 6-A所示,以Ⅱ期作为参照,在卵巢成熟过程中,脑gnrh2 mRNA表达量在卵黄生成期(Ⅲ期)显著性增加,达到峰值;随后,脑gnrh2 mRNA表达量急剧下降,在成熟期(Ⅴ期)达到最小值;然而,在排卵后期(Ⅵ期)又显著性增加。如图 6-B所示,以Ⅱ期作为参照,在卵巢成熟过程中垂体gnrh2 mRNA表达量在卵黄生成期(Ⅲ期)略微降低,与Ⅱ期相比无显著性差异;然而,垂体gnrh2 mRNA表达量在卵黄生成后期(Ⅳ期)显著性降低;随后,垂体gnrh2 mRNA表达量在成熟期(Ⅴ期)较Ⅳ期显著性增加,在排卵后期(Ⅵ期)又急剧下降。
|
图 6 半滑舌鳎卵巢不同发育时期脑(A)和垂体(B)中gnrh2 mRNA表达分析 Figure 6 Expression profiles of gnrh2 mRNA in the brain (A) and pituitary (B) during ovarian maturation of C. semilaevis |
本研究首先通过RT-PCR及RACE方法获得了半滑舌鳎GnRH2全长cDNA序列。进化分析表明,半滑舌鳎GnRH2与其他硬骨鱼类GnRH2聚为一支,不同于GnRH1和GnRH3分支。多重序列比对发现,鱼类GnRH2前体序列高度保守,由信号肽、成熟十肽、酶切位点以及GnRH相关肽组成,尤其是成熟十肽在所有硬骨鱼类中完全相同。此外,半滑舌鳎gnrh2 mRNA主要在脑中表达,以及在卵巢成熟过程中脑中gnrh2 mRNA表达量的动态变化,表明脑GnRH2可能参与了半滑舌鳎性腺发育。该结果增加了对GnRH2参与鱼类生殖调控作用的认识。
半滑舌鳎GnRH2 ORF为258 bp,编码85个氨基酸的前体多肽(图 1),这与黑鲷(An et al, 2008)、小丑鱼(Kim et al, 2012)、星点东方鲀(Shahjahan et al, 2010)、鲐鱼(Selvaraj et al, 2012)、三刺鱼(Gasterosteus aculeatus) (Shao et al, 2015)、美洲拟鲽(Tuziak et al, 2013a)、美洲黑石斑(Morin et al, 2015)等结果是一致的。不同鱼类GnRH2前体多肽氨基酸长度略有差异,例如底鳉(Fundulus heteroclitus)、中华鲟(Acipenser sinensis)、大西洋鳕GnRH2前体分别为83个氨基酸、86个氨基酸、86个氨基酸(Hildahl et al, 2011; Ohkubo et al, 2010; Yue et al, 2013)。不同硬骨鱼类GnRH2前体同源性比对分析显示,GnRH2在进化中高度保守,半滑舌鳎GnRH2与鲈形目同源性最高,其次为鲽形目、鲑形目和鲀形目,与鲤形目同源性最低(图 2)。进化树分析进一步证实,本研究克隆所得序列确实为半滑舌鳎GnRH2序列。此外,GnRH进化树分为3大支也表明3种GnRH序列在硬骨鱼类进化中高度保守(图 3),暗示3种GnRH多肽可能发挥了重要的生理功能。
为了研究GnRH2潜在的生理功能,本研究对其组织表达模式进行了分析。荧光定量PCR结果显示,半滑舌鳎gnrh2 mRNA主要在脑中表达,在垂体及其他外周组织中表达量极低,只有脑中表达量的2.5%-8.3% (图 4)。与之类似,圆斑星鲽gnrh2 mRNA只在脑中表达(Xu et al, 2012)。除了主要在脑中表达外,gnrh2 mRNA在不同鱼类外周组织中表达有所差异,在底鳉肾(Ohkubo et al, 2010)、中华鲟垂体(Yue et al, 2013)、美洲拟鲽精巢(Tuziak et al, 2013a)、大西洋鳕卵巢(Hildahl et al, 2011)、精巢和脾(Tuziak et al, 2013b)等组织中也有少量表达。综上所述,GnRH2主要由脑分泌进而作用于垂体发挥其生理功能。此外,通过免疫细胞化学方法在金鱼及尼罗尖吻鲈(Lates niloticus)脑和垂体中均检测到了GnRH2的阳性信号(Kim et al, 1995; Mousa et al, 2003)。
尽管GnRH2在进化过程中高度保守,但其生理学功能研究较少。在金鱼中,侧脑室注射GnRH2直接影响了雌鱼生殖行为(Volkoff et al, 1999),抑制了金鱼的摄食行为(Matsuda et al, 2008)。此外,GnRH2促进了金鱼垂体LH及GH分泌(Chang et al, 2012、2009)。然而,GnRH2在鱼类生殖调控中的作用仍不清楚,需要进一步深入研究。本研究中,半滑舌鳎脑gnrh2 mRNA在卵黄生成期(Ⅲ期)显著性增加,达到峰值;随后表达量急剧下降,在成熟期(Ⅴ期)达到最小值;在排卵后期(Ⅵ期)又显著性增加。与此同时,垂体FSHβ和LHβ也在Ⅲ期显著性增加,分别在Ⅳ期和Ⅴ期达到峰值(Shi et al, 2015)。因为FSH在早期性腺复苏和配子发育过程中起了主要作用,而LH在最后性成熟过程中发挥了重要作用(Shahjahan et al, 2010),因此,GnRH2可能通过促进垂体FSH和LH合成与分泌的方式在半滑舌鳎卵巢发育中发挥了重要作用。此外,半滑舌鳎为非同步分批多次产卵型鱼类,脑gnrh2 mRNA水平在排卵后期(Ⅵ期)显著性增加,有可能是为下一次卵巢发育做准备。与之类似,星点东方鲀脑gnrh2 mRNA表达量也在排卵后期有所增加(Shahjahan et al, 2010)。
为了进一步研究垂体GnRH2是否参与半滑舌鳎卵巢发育,对半滑舌鳎卵巢成熟过程中垂体gnrh2 mRNA表达差异也进行了分析。在半滑舌鳎卵巢成熟过程中,垂体gnrh2 mRNA表达量除在成熟期(Ⅴ期)相对有所增加外,随着卵巢发育垂体gnrh2 mRNA表达量显著降低,这说明垂体GnRH2多肽可能没有参与卵巢发育。与之类似,鲐鱼垂体GnRH2多肽水平在卵巢成熟过程中没有发生显著性变化(Selvaraj et al, 2012)。在条纹鲈(Morone saxatilis)、金头鲷(Sparus aurata)及真鲷(Pagrus major)垂体中不表达gnrh2或者GnRH2含量不随着生殖状况的变化而变化,进一步证实了上述结论(Xu et al, 2012)。此外,半滑舌鳎垂体gnrh2 mRNA表达量随卵巢发育降低,可能是由于受到垂体FSH/LH负反馈作用导致的,然而这一推断需要进一步深入研究。
4 小结本研究获得了半滑舌鳎GnRH2的全长cDNA序列,并对其系统进化、基因组织分布及卵巢成熟过程中的时空表达特性进行了分析。进化分析表明,半滑舌鳎GnRH2与其他硬骨鱼类GnRH2聚为一支,不同于GnRH1和GnRH3分支。多重序列比对发现,鱼类GnRH2前体序列高度保守,荧光定量PCR结果表明,gnrh2 mRNA主要在脑中表达。此外,卵巢成熟过程中脑gnrh2 mRNA表达量的动态变化,暗示脑GnRH2可能参与了半滑舌鳎性腺发育。该结果增加了对GnRH2参与鱼类生殖调控作用的认识。
| An KW, Nelson ER, Habibi HR, et al. Molecular characterization and expression of three GnRH forms mRNA during gonad sex-change process, and effect of GnRHa on GTH subunits mRNA in the protandrous black porgy (Acanthopagrus schlegeli). General and Comparative Endocrinology , 2008, 159 (1) : 38-45 DOI:10.1016/j.ygcen.2008.07.012 | |
| Andersson E, Fjelldal PG, Klenke U, et al. Three forms of GnRH in the brain and pituitary of the turbot, Scophthalmus maximus: Immunological characterization and seasonal variation. Comparative Biochemistry and Physiology, Part B, Biochemistry and Molecular Biology , 2001, 129 (2-3) : 551-558 DOI:10.1016/S1096-4959(01)00363-3 | |
| Breton TS, DiMaggio MA, Sower SA, et al. Brain aromatase (cyp19a1b) and gonadotropin releasing hormone (gnrh2 and gnrh3) expression during reproductive development and sex change in black sea bass (Centropristis striata). Comparative Biochemistry and Physiology, Part A, Molecular and Integrative Physiology , 2015, 181 : 45-53 DOI:10.1016/j.cbpa.2014.11.020 | |
| Chang JP, Habibi HR, Yu Y, et al. Calcium and other signalling pathways in neuroendocrine regulation of somatotroph functions. Cell Calcium , 2012, 51 (3-4) : 240-252 DOI:10.1016/j.ceca.2011.11.001 | |
| Chang JP, Johnson JD, Sawisky GR, et al. Signal transduction in multifactorial neuroendocrine control of gonadotropin secretion and synthesis in teleosts-studies on the goldfish model. General and Comparative Endocrinology , 2009, 161 (1) : 42-52 DOI:10.1016/j.ygcen.2008.09.005 | |
| Guilgur L, Strüssmann C, Somoza G. mRNA expression of GnRH variants and receptors in the brain, pituitary and ovaries of pejerrey (Odontesthes bonariensis) in relation to the reproductive status. Fish Physiology and Biochemistry , 2009, 35 (1) : 157-166 DOI:10.1007/s10695-008-9215-4 | |
| Guilgur LG, Moncaut NP, Canario AV, et al. Evolution of GnRH ligands and receptors in gnathostomata. Comparative Biochemistry and Physiology, Part A: Molecular and Integrative Physiology , 2006, 144 (3) : 272-283 DOI:10.1016/j.cbpa.2006.02.016 | |
| Hildahl J, Sandvik GK, Edvardsen RB, et al. Identification and gene expression analysis of three GnRH genes in female Atlantic cod during puberty provides insight into GnRH variant gene loss in fish. General and Comparative Endocrinology , 2011, 172 (3) : 458-467 DOI:10.1016/j.ygcen.2011.04.010 | |
| Kim MH, Oka Y, Amano M, et al. Immunocytochemical localization of sGnRH and cGnRH-Ⅱ in the brain of goldfish, Carassius auratus. Journal of Comparative Neurology , 1995, 356 (1) : 72-82 DOI:10.1002/(ISSN)1096-9861 | |
| Kim NN, Shin HS, Habibi HR, et al. Expression profiles of three types of GnRH during sex-change in the protandrous cinnamon clownfish, Amphiprion melanopus: Effects of exogenous GnRHs. Comparative Biochemistry and Physiology, Part B: Biochemistry and Molecular Biology , 2012, 161 (2) : 124-133 DOI:10.1016/j.cbpb.2011.10.003 | |
| Liu XZ, Shi B, Li XX, et al. Molecular characterization of the novel membrane progestin receptor gene and its role during ovarian development in the half smooth tongue sole Cynoglossus semilaevis Günther. Journal of Fishery Sciences of China , 2015, 22 (4) : 608-619 [柳学周, 史宝, 李晓晓, 等. 半滑舌鳎新型膜孕激素受体基因分子特征及其在卵巢发育过程的作用. 中国水产科学 , 2015, 22 (4) : 608-619] | |
| Liu XZ, Xu YJ, Liu NZ, et al. Study on histological and morphometric characters of gonad development of Cynoglossus semilaevis Günther. Progress in Fishery Sciences , 2009, 30 (6) : 25-35 [柳学周, 徐永江, 刘乃真, 等. 半滑舌鳎卵巢发育的组织学和形态数量特征研究. 渔业科学进展 , 2009, 30 (6) : 25-35] | |
| Mousa MA, Mousa SA. Immunohistochemical localization of gonadotropin releasing hormones in the brain and pituitary gland of the Nile perch, Lates niloticus (Teleostei, Centropomidae). General and Comparative Endocrinology , 2003, 130 (3) : 245-255 DOI:10.1016/S0016-6480(02)00611-1 | |
| Matsuda K, Nakamura K, Shimakura S, et al. Inhibitory effect of chicken gonadotropin-releasing hormone Ⅱ on food intake in the goldfish, Carassius auratus. Hormones and Behavior , 2008, 54 (1) : 83-89 DOI:10.1016/j.yhbeh.2008.01.011 | |
| Morin SJ, Decatur WA, Breton TS, et al. Identification and expression of GnRH2 and GnRH3 in the black sea bass (Centropristis striata), a hermaphroditic teleost. Fish Physiology and Biochemistry , 2015, 41 (2) : 383-395 DOI:10.1007/s10695-014-9990-z | |
| Nocillado JN, Levavi-Sivan B, Carrick F, et al. Temporal expression of G-protein-coupled receptor 54 (GPR54), gonadotropin-releasing hormones (GnRH), and dopamine receptor D2 (drd2) in pubertal female grey mullet, Mugil cephalus. General and Comparative Endocrinology , 2007, 150 (2) : 278-287 DOI:10.1016/j.ygcen.2006.09.008 | |
| Ohkubo M, Aranishi F, Shimizu A. Molecular cloning and brain distribution of three types of gonadotropin-releasing hormone from mummichog Fundulus heteroclitus. Journal of Fish Biology , 2010, 76 (2) : 379-394 DOI:10.1111/jfb.2010.76.issue-2 | |
| Selvaraj S, Kitano H, Amano M, et al. Molecular characterization and expression profiles of three GnRH forms in the brain and pituitary of adult chub mackerel (Scomber japonicus) maintained in captivity. Aquaculture , 2012, 356-357 (4) : 200-210 | |
| Selvaraj S, Kitano H, Ohga H, et al. Expression changes of mRNAs encoding kisspeptins and their receptors and gonadotropin-releasing hormones during early development and gonadal sex differentiation periods in the brain of chub mackerel (Scomber japonicus). General and Comparative Endocrinology , 2015, 222 : 20-32 DOI:10.1016/j.ygcen.2014.09.019 | |
| Shahjahan M, Hamabata T, Motohashi E, et al. Differential expression of three types of gonadotropin-releasing hormone genes during the spawning season in grass puffer, Takifugu niphobles. General and Comparative Endocrinology , 2010, 167 (1) : 153-163 DOI:10.1016/j.ygcen.2010.01.018 | |
| Shao YT, Tseng YC, Chang CH, et al. GnRH mRNA levels in male three-spined sticklebacks, Gasterosteus aculeatus, under different reproductive conditions. Comparative Biochemistry and Physiology, Part A: Molecular and Integrative Physiology , 2015, 180 : 6-17 DOI:10.1016/j.cbpa.2014.10.008 | |
| Shi B, Liu X, Xu Y, et al. Molecular characterization of three gonadotropin subunits and their expression patterns during ovarian maturation in Cynoglossus semilaevis. International Journal of Molecular Sciences , 2015, 16 (2) : 2767-2793 DOI:10.3390/ijms16022767 | |
| Tuziak SM, Volkoff H. Gonadotrophin-releasing hormone in winter flounder (Pseudopleuronectes americanus): Molecular characterization, distribution and effects of fasting. General and Comparative Endocrinology , 2013a, 184 (3) : 9-21 | |
| Tuziak SM, Volkoff H. Melanin-concentrating hormone (MCH) and gonadotropin-releasing hormones (GnRH) in Atlantic cod, Gadus morhua: Tissue distributions, early ontogeny and effects of fasting. Peptides , 2013b, 50 (3) : 109-118 | |
| Volkoff H, Peter RE. Actions of two forms of gonadotropin releasing hormone and a GnRH antagonist on spawning behavior of the goldfish Carassius auratus. General and Comparative Endocrinology , 1999, 116 (3) : 347-355 DOI:10.1006/gcen.1999.7377 | |
| Wang B, Jia J, Yang G, et al. In vitro effects of somatostatin on the growth hormone-insulin-like growth factor axis in orange-spotted grouper (Epinephelus coioides). General and Comparative Endocrinology , 2016, 237 : 1-9 DOI:10.1016/j.ygcen.2015.10.014 | |
| Xu YJ, Liu XZ, Liao MJ, et al. Molecular cloning and differential expression of three GnRH genes during ovarian maturation of spotted halibut, Verasper variegatus. Journal of Experimental Zoology, Part A, Ecological Genetics and Physiology , 2012, 317 (7) : 434-446 DOI:10.1002/jez.1736 | |
| Yue H, Ye H, Chen X, et al. Molecular cloning of cDNA of gonadotropin-releasing hormones in the Chinese sturgeon (Acipenser sinensis) and the effect of 17 beta-estradiol on gene expression. Comparative Biochemistry and Physiology, Part A, Molecular and Integrative Physiology , 2013, 166 (4) : 529-537 DOI:10.1016/j.cbpa.2013.08.011 |



