2. 青岛海洋科学与技术国家实验室 海洋渔业科学与食物产出过程功能实验室 青岛 266071;
3. 青岛市渔业技术推广站 青岛 266071;
4. 青岛贝宝海洋科技有限公司 青岛 266400
2. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071;
3. Qingdao Extension Station of Fisheries Technology, Qingdao 266071;
4. Qingdao Beibao Marine Science and Technology Co. Ltd., Qingdao 266400
鱼类的生殖是一个配子分裂、生长、发育、最终成熟并通过结合形成胚胎的过程,性腺是主要的生殖器官,其发育、成熟及生殖行为受到遗传特性和外部环境条件的双重调控,主要是通过生殖轴(下丘脑-垂体-性腺,HPG axis)来实现的(雷霁霖, 2005; 林浩然, 2011)。众所周知,鱼类的生长过程是受生长轴[生长激素(GH)/类胰岛素生长因子Ⅰ(IGF-Ⅰ)轴,GH/IGF-Ⅰaxis]调控的。其中,GH和IGF-Ⅰ是2个关键的生长因子,调控机体的生长、代谢和能量平衡内稳态(Reinecke et al, 2005)。鱼类的生殖功能与生长性能紧密联系、协同作用,它们同时受诸多神经内分泌调控因子的级联调控,生殖调控因子[如促性腺激素释放激素(GnRH)、促性腺激素(FSH,LH)、kisspeptin等]与生长因子[如GH、IGFs等]共同调控鱼类配子的生长发育(Campbell et al, 2006; 林浩然, 2000)。作为重要的生长调控因子,GH及其受体可通过与GnRH、FSH、雌激素受体(ER)等生殖因子作用,促进配子生长发育和成熟(Kajimura et al, 2004; Campbell et al, 2006; Benedet et al, 2010)。另外,类胰岛素生长因子(IGFs)在鱼类卵巢生长发育中同样起着重要的调控作用(Reinecke, 2010),特别是IGF3,只在卵巢中特异性表达(Berishvili et al, 2010),表明其是卵巢发育不可或缺的关键因子。可见,鱼类的性腺发育过程受到生殖功能因子和生长因子的协同调控作用,从而使得配子最终达到成熟和排放。
半滑舌鳎(Cynoglossus semilaevis)为我国鲆鲽类三大主导养殖种类之一,2003年伴随着其自然产卵调控和苗种培育关键技术的突破,半滑舌鳎养殖业迅速发展,目前已在全国形成较大的养殖产业规模(柳学周等, 2014)。随着养殖产业的快速发展,诸如亲鱼性腺成熟不良、产卵效率下降、人工亲鱼性早熟等问题不断凸显,制约了养殖业的可持续发展。针对这些现象,我国学者已经围绕半滑舌鳎生殖调控机制开展相关研究,在GnRH (Zhou et al, 2012)、FSH、LH、mPRα及其受体(陈晓燕等, 2011; 李晓晓等, 2013; 史宝等, 2013; Shi et al, 2015; 柳学周等, 2015)等生殖功能因子对性腺发育调控的生理功能及作用机制方面取得了诸多进展,但目前对半滑舌鳎生殖调控机制认识仍显不足。近年来,在养殖生产中,雌性亲鱼卵巢发育不佳、配子无法最终成熟、无法形成自然产卵等仍是制约苗种生产的关键因素,而亲鱼及配子生长调控的不足可能是导致配子无法最终成熟和自然产卵的重要原因之一。因此,为了探讨半滑舌鳎性腺生长发育与生殖的关系,作者研究了GH和IGF-Ⅰ对卵巢发育和成熟的调控作用,并对其可能的机制进行了探讨,以期为全面和深入揭示半滑舌鳎生殖调控机制提供新的思路和基础资料。
1 材料与方法 1.1 实验鱼与生殖调控实验用半滑舌鳎亲鱼来自青岛某养殖公司,为人工培育条件下达性成熟年龄(3龄以上)的雌性亲鱼。亲鱼全长为42-57 cm,体重为1450-2630 g。
亲鱼在室内水泥池(面积为25 m2)培育,使用砂滤海水,周年培育水温为10-25℃,盐度为27-31,pH为7.8-8.2,溶解氧 > 5 mg/L,日换水率为300%-500%。亲鱼饵料为活沙蚕、贝肉,日投喂2次,投喂量为鱼体重的2%-3%,及时清除残饵、排污。繁殖周期到来前2个月,开始对亲鱼进行水温和光周期调控(徐永江等, 2011),同时进行亲鱼营养强化,促进亲鱼性腺发育成熟。经过70 d的人工调控,亲鱼性腺发育成熟并最终自然产卵。
1.2 实验鱼取样亲鱼培育和人工调控过程中,在卵巢发育的不同阶段进行取样,每次取样3-4尾亲鱼。实验鱼以MS-222麻醉(300 mg/L)后取血,血液以15000 r/min离心5 min,分离血清,于-40℃保存,用于血清中雌二醇(E2)、睾酮(T)、GH及IGF-Ⅰ表达水平的测定。实验鱼逐尾采集血液后,取出脑、垂体、卵巢和肝脏组织,液氮速冻后转入-80℃保存,用于总RNA提取。
卵巢组织以Davison固定液固定后,通过组织切片观察判定发育期,按照柳学周等(2009)建立的半滑舌鳎卵巢发育期判别方法判定。称重卵巢,计算性腺发育指数(GSI):
性腺发育指数(GSI)=性腺重量/(体重-内脏重) (Xu et al, 2012)。
1.3 基因的实时荧光定量PCR检测取不同卵巢发育时期的组织样品(3尾亲鱼),用RNAiso Plus (TaKaRa, 日本)分别抽提脑、垂体、卵巢和肝脏的总RNA,测定浓度和质量。选择高质量总RNA以PrimeScript RT Reagent Kit with gDNA Eraser反转录试剂盒(TaKaRa, 日本)合成用于实时荧光定量PCR (Quantitative real-time PCR, qRT-PCR)的cDNA第1链,操作按照试剂盒说明书进行。
利用qRT-PCR方法检测不同卵巢发育期垂体GH mRNA以及脑、肝脏、卵巢中IGF-ⅠmRNA表达水平的变化。根据半滑舌鳎GH (GenBank Accession No.: FJ608663)和IGF-Ⅰ(GenBank Accession No.: HQ334201)序列和18S rRNA序列设计定量PCR引物(表 1)。
|
|
表 1 半滑舌鳎GH、IGF-Ⅰ和18S rRNA的qRT-PCR引物 Table 1 Primers used for quantitative real-time PCR analysis of GH, IGF-Ⅰand 18S rRNA of C. semilaevis |
qRT-PCR反应使用SYBR Premix Ex TaqTM Ⅱ(Perfect Real Time)(TaKaRa,日本)试剂,PCR体系(20 μl)为:SYBR Premix Ex TaqTM Ⅱ10 μl,正反向引物各0.8 μl,cDNA模板1 μl,ddH2O 7.4 μl。垂体GH扩增条件:95℃ 30 s,(95℃ 5 s,60℃ 20 s) 40个循环。肝脏、卵巢和脑IGF-Ⅰ扩增条件:95℃ 30 s,(95℃ 5 s,60℃ 25 s)40个循环。以18S rRNA作为内参基因,GH、IGF-Ⅰ和18S rRNA的基因扩增效率均达到0.99以上,表明扩增的特异性强。
每个qRT-PCR反应均设置阴性对照,每个样品设置3个平行,重复3次以确认结果数据的可靠性。以2-ΔΔCT法计算处理定量扩增数据(Livak et al, 2001)。
1.4 血清激素的测定取每个发育时期3尾亲鱼血清样品用于激素测定。血清中E2和T的测定采用放射免疫法(RIA)进行,使用125I标记的哺乳动物E2和T放射免疫测定试剂盒(天津九鼎医学生物工程有限公司),T的检测灵敏度为1.9 ng/dl,E2的检测灵敏度为2.1 pg/ml,具体的测定操作和数据处理方法同Xu等(2012)。
采用酶联免疫吸附法(ELISA)测定血清中GH和IGF-Ⅰ表达水平,利用鱼类GH ELISA Kit和IGF-ⅠELISA Kit (CUSABIO)进行测定。选取每个不同卵巢发育时期的血清样品3个,设置3个平行。操作步骤和数据处理参照试剂盒说明书进行,首先制作标准曲线(r > 0.99),根据标准曲线计算血清样品中激素的表达水平。
1.5 数据处理GSI、激素表达与基因表达数据表示为平均值±标准差(Mean±SD),对GSI、血清激素、GH mRNA、IGF-Ⅰ mRNA表达水平分别进行单因素方差分析(One-way ANOVA),统计不同发育时期各指标的差异显著性。利用相关性分析法分析指标之间的相关性,使用SPSS 21.0软件进行统计分析,当P < 0.05为差异显著,P < 0.01为差异极显著。
2 结果 2.1 卵巢发育分期通过组织切片将本研究所用的半滑舌鳎亲鱼卵巢发育过程划分为Ⅱ、Ⅲ、Ⅳ、Ⅴ、Ⅵ 5个时期,每个时期的亲鱼数量为3-4尾。计算GSI值得知,Ⅱ期和Ⅲ期卵巢的GSI值较低( < 5),Ⅳ期卵巢GSI值显著升高(14.2-15.5,P < 0.05),至Ⅴ期卵巢时达峰值(25.6-30.8,P < 0.05),Ⅵ期卵巢GSI值与Ⅳ期无显著差异(P > 0.05) (图 1)。
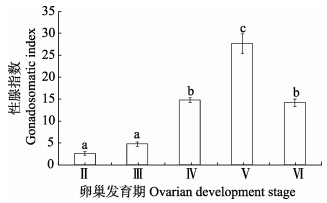
|
图 1 卵巢不同发育时期的性腺指数 Figure 1 The gonadosomatic index of ovary at different development stages 不同字母表示差异显著(P < 0.05),下同 Different letters indicated significant difference (P < 0.05), the same as below |
在卵巢的不同发育时期,垂体GH mRNA表达水平差异显著。Ⅱ期和Ⅲ期卵巢时,垂体GH mRNA表达水平较低(P < 0.01),Ⅳ期卵巢时GH mRNA表达水平显著升高,至Ⅴ期卵巢时表达水平达峰值(P < 0.01),但Ⅵ期卵巢时垂体,GH mRNA表达水平迅速下降至Ⅱ期和Ⅲ期卵巢水平(P < 0.01)(图 2)。
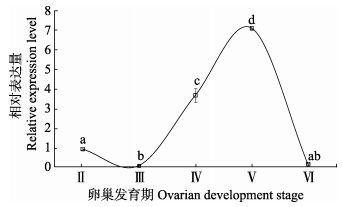
|
图 2 卵巢不同发育时期垂体中GH mRNA的表达水平变化 Figure 2 Pituitary GH mRNA levels at different ovarian developmental stages |
在卵巢不同发育时期,肝脏、卵巢和脑中IGF-ⅠmRNA表达水平变化见图 3。肝脏中IGF-Ⅰ mRNA表达水平相对较高,是脑和性腺中IGF-Ⅰ mRNA表达水平的10倍以上。肝脏IGF-Ⅰ mRNA表达水平自Ⅱ期卵巢开始下降(P < 0.01),在Ⅳ期和Ⅴ期卵巢时下降至最低水平(P < 0.01),但Ⅵ期卵巢时又显著升高(P < 0.01)。卵巢中IGF-Ⅰ mRNA表达水平自Ⅱ期开始显著升高(P < 0.05),并在Ⅳ期时达峰值(P < 0.05),但在Ⅴ期时明显下降(P < 0.05),并保持至Ⅵ期。脑中IGF-Ⅰ mRNA表达水平自Ⅱ期卵巢开始显著升高,至Ⅳ期卵巢时达峰值(P < 0.05)。
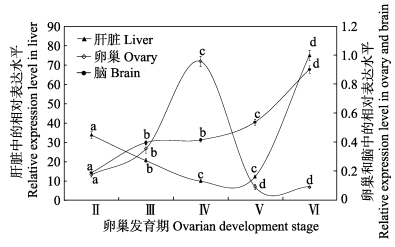
|
图 3 肝脏、卵巢和脑中IGF-ⅠmRNA在卵巢不同发育时期的表达水平变化 Figure 3 IGF-ⅠmRNA levels in liver, ovary and brain at different ovarian developmental stages |
血清GH表达水平与垂体GH mRNA表达水平表现出相同的变化趋势,在Ⅱ期和Ⅲ期卵巢时保持较低水平,最低值[(724.54±75.79) pg/ml)]出现在卵巢发育至Ⅲ期时。Ⅳ期卵巢时,血清GH水平显著升高并在Ⅴ期卵巢时达到峰值[(2485.59±130.63) pg/ml] (P < 0.05),其后在Ⅵ期卵巢时显著降低(P < 0.05)(图 4-A)。

|
图 4 卵巢不同发育时期血清中GH、IGF-Ⅰ、E2和T表达水平 Figure 4 The serum GH, IGF-Ⅰ, E2 and T expression levels at different ovarian development stages |
血清中IGF-Ⅰ表达水平在Ⅱ期和Ⅲ期卵巢时差异不显著(P > 0.05),而在Ⅳ期卵巢时显著降低[(165.53±26.71) pg/ml],其后在Ⅴ期时显著升高(P < 0.05),并在Ⅵ期卵巢达到峰值[(630.18±31.49) pg/ml] (P < 0.05) (图 4-B)。血清中IGF-Ⅰ浓度与肝脏IGF-Ⅰ mRNA表达水平表现出类似的变化规律。
血清中E2表达水平从Ⅱ期卵巢开始显著升高,并在Ⅳ期卵巢达峰值[(313.41±15.48) pg/ml] (P < 0.05),其后又在Ⅴ期卵巢显著降低(P < 0.05),并在Ⅵ期卵巢继续降低[(93.38±3.56) pg/ml] (P < 0.05)(图-C)。血清T表达水平随卵巢发育逐渐升高,并在Ⅴ期卵巢时达峰值[(45.26±3.14) ng/dl] (P < 0.05),但在Ⅵ期卵巢时显著下降至Ⅱ期卵巢水平[(5.56±0.39) ng/dl] (P < 0.05) (图 4-D)。
2.5 卵巢发育过程中生殖与生长因子的相关性分析卵巢发育过程中生殖与生长因子的相关性分析表明(表 2),半滑舌鳎卵巢发育过程中,垂体GH mRNA表达水平(PGH)与GSI (P < 0.01)、血清GH表达水平(SGH)(P < 0.01)、血清IGF-Ⅰ表达水平(SIGF) (P < 0.05)存在显著的正相关关系。肝脏IGF-Ⅰ mRNA表达水平(LIGF)与脑IGF-Ⅰ mRNA表达水平(BIGF)、SIGF呈显著正相关关系(P < 0.01),而与血清雌二醇表达水平(SE)(P < 0.05)、ST (P < 0.01)呈显著负相关关系。卵巢IGF-Ⅰ mRNA表达水平(OIGF)与SE呈显著正相关关系(P < 0.01),而与SIGF呈显著的负相关关系(P < 0.01)。BIGF与SIGF呈显著的负相关关系(P < 0.01)。ST与SGH呈显著正相关关系(P < 0.01)。
|
|
表 2 半滑舌鳎卵巢不同发育时期生长与生殖因子表达的关系 Table 2 The correlation between growth factors and reproductive factors at different ovarian development stages |
本研究揭示了生长因子GH和IGF-Ⅰ的mRNA与多肽在半滑舌鳎不同卵巢发育阶段的差异表达特性及其与GSI和性类固醇激素表达变化的互作关系,证明了GH/IGF-Ⅰ轴对卵巢发育起着重要的调控作用,为深入认识半滑舌鳎卵巢发育的调控机制提供了新的思路。
硬骨鱼类必须保持一定的生长潜力和速度才能为自身的生殖活动提供充足的能量储备(Berishvili et al, 2010)。鱼类的生长主要由GH/IGF-Ⅰ轴调控,垂体释放GH至血液循环系统,达到肝脏后通过与其受体结合作用刺激IGF-Ⅰ分泌和表达,从而进一步促进细胞分裂、增殖和生长(Reinecke et al, 2005)。GH是迄今研究最为深入的生长因子,除与其他生长因子互作共同调控动物生长与生产性状外,GH还受到GnRH、E2、FSH、LH等生殖因子的调控(Riley et al, 2002; Cannata et al, 2010; 周立斌等, 2004),表明GH在动物的生殖过程同样起着重要的调控作用。王德寿等(1999)对大鳍鳠(Mystus macropterus) GH的研究表明,血清GH含量的变化与生殖周期密切相关,并在产卵期急剧升高,至性腺退化期达最高,可能是对生殖消耗能量代谢的一种有效补充调控。周立斌等(2004)对长尾
半滑舌鳎IGF-Ⅰ具有广泛的组织表达特性,在包括肝脏、脑、卵巢等多个组织中均有较高水平的表达(季相山, 2009)1)。GH与IGF-Ⅰ在半滑舌鳎早期生长发育过程中起着同样重要的调控作用(Ma et al, 2011、2012)。IGF-Ⅰ可在体外促进鱼类卵巢滤泡细胞性类固醇激素分泌,并可通过直接或者间接的作用调控鱼类配子的发育和成熟(Kagawa et al, 2003)。在大马哈鱼(Oncorhynchus keta)中,卵巢成熟产卵前血清IGF-Ⅰ与血清E2、T都有正相关关系,表明其与性类固醇激素一起参与产卵调控过程(Onuma et al, 2009)。在虹鳟(Oncorhynchus mykiss)卵子的卵黄发生期,IGF-Ⅰ可通过调控芳香化酶基因cyp19a1a上调表达来促进卵子发育(Nakamura et al, 2016)。本研究发现,在转录水平上,肝脏IGF-Ⅰ mRNA表达水平在卵巢发育过程中下调表达,但在产卵结束后的Ⅵ期卵巢显著上调表达,这与血清IGF-Ⅰ表达水平变化趋势一致,Ⅳ期卵巢是卵子卵黄发生的关键时期,此时期E2因为参与到配子卵黄发生调控过程而大量分泌和合成(Zohar et al, 2010),而肝脏IGF-Ⅰ mRNA表达水平与SIGF呈显著正相关关系,而与血清雌二醇表达水平(SE)呈显著负相关关系,表明肝脏与血清IGF-Ⅰ与性类固醇激素共同参与了配子的卵黄发生和累积过程。脑中IGF-Ⅰ mRNA一直处于上调表达状态,直至Ⅵ期卵巢才达峰值,并与血清IGF-Ⅰ呈显著的负相关关系,表明脑IGF-Ⅰ与垂体GHmRNA和血清IGF-Ⅰ表达水平在调控卵巢发育过程中可能存在某种应答或协同作用,有待于深入研究确证。卵巢IGF-Ⅰ mRNA在Ⅳ期卵巢前一直处于显著上升表达,而在产卵期后(Ⅴ期)显著下调表达,与血清E2表达水平呈显著正相关关系,而与血清IGF-Ⅰ表达水平呈显著的负相关关系,表明卵巢IGF-Ⅰ直接参与配子的发生和发育过程调控,与Campbell等(2006)对鲑鱼的研究结果一致,具体的调控机制与雌二醇表达特性有关,尚需进一步研究。血清IGF-Ⅰ表达水平在产卵前一直较低,而在产卵期(Ⅴ期)和产卵后期(Ⅵ期)均处于较高表达水平,这种变化趋势与产卵期配子生长、卵黄累积及产卵后的卵巢恢复生长密切相关(Guzmán et al, 2008)。
1) JI XS. Study on the genes of male and female half smooth tongue sole, Cynoglossus semilaevis Günther differentially expressed in the procedure of gynogenesis induction, genetic analysis and growth. Doctoral Dissertation of Shandong Agricultural University, 2009, 68-129 [季相山.半滑舌鳎雌核发育诱导、遗传分析及生长相关基因雌雄差异表达研究.山东农业大学博士研究生学位论文, 2009, 68-129]
综上所述,本研究发现GH、IGF-Ⅰ从转录和血清学水平都以协同或者拮抗的方式共同参与了半滑舌鳎卵巢发育的过程,且其作用途径与性类固醇激素的合成与分泌有关,表明GH/IGF-Ⅰ轴对卵巢发育具有重要的调控作用,但其具体的机制有待于对其信号途径的深入研究。
| Benedet S, Andersson E, Mittelholzer C, et al. Pituitary and plasma growth hormone dynamics during sexual maturation of female Atlantic salmon. General and Comparative Endocrinology , 2010, 167 (1) : 77-85 DOI:10.1016/j.ygcen.2010.02.011 | |
| Berishvili G, Baroiller J, Eppler E, et al. Insulin-like growth factor-3 (IGF-3) in male and female gonads of the tilapia: Development and regulation of gene expression by growth hormone (GH) and 17α-ethinylestradiol (EE2). General and Comparative Endocrinology , 2010, 167 (1) : 128-134 DOI:10.1016/j.ygcen.2010.01.023 | |
| Campbell B, Dickey J, Beckman B, et al. Previtellogenic oocyte growth in salmon: Relationships among body growth, plasma insulin-like growth factor-1, estradiol-17 beta, follicle-stimulating hormone and expression of ovarian genes for insulin-like growth factors, steroidogenic-acute regulatory protein and receptors for gonadotropins, growth hormone, and somatolactin. Biology of Reproduction , 2006, 75 (1) : 34-44 DOI:10.1095/biolreprod.105.049494 | |
| Cannata D, Vijayakumar A, Fierz Y, et al. The GH/IGF-1 axis in growth and development: New insights derived from animal models. Advances in Pediatrics , 2010, 57 (1) : 331-351 DOI:10.1016/j.yapd.2010.09.003 | |
| Chen XY, Wen HS, He F, et al. Expression analysis of FSHR and LHR mRNAs during the reproductive cycle of males Cynoglossus semilaevis. Oceanologica et Limnologia Sinica , 2011, 42 (2) : 201-206 [陈晓燕, 温海深, 何峰, 等. 半滑舌鳎(Cynoglossus semilaevis)促性腺激素受体在雄性生殖周期中的表达. 海洋与湖沼 , 2011, 42 (2) : 201-206] | |
| Guzmán J, Norberg B, Ramos J, et al. Vitellogenin, steroid plasma levels and spawning performance of cultured female Senegalese sole (Solea senegalensis). General and Comparative Endocrinology , 2008, 156 (2) : 285-297 DOI:10.1016/j.ygcen.2008.02.002 | |
| Kagawa H, Gen K, Okuzawa K, et al. Effects of luteinizing hormone and follicle-stimulating hormone and insulin-like growth factor-I on aromatase activity and P450 aromatase gene expression in the ovarian follicles of red seabream, Pagrus major. Biology of Reproduction , 2003, 68 (5) : 1562-1568 | |
| Kajimura S, Kawaguchi N, Kaneko T, et al. Identification of the growth hormone receptor in an advanced teleost, the tilapia (Oreochromis mossambicus) with special reference to its distinct expression pattern in the ovary. Journal of Endocrinology , 2004, 181 (1) : 65-76 DOI:10.1677/joe.0.1810065 | |
| Lei JL. Marine fish culture theory and technology. Beijing: China Agricultural Press, 2005 . [雷霁霖. 海水鱼类养殖理论与技术. 北京: 中国农业出版社, 2005 .] | |
| Li XX, Liu XZ, Shi B, et al. Cloning and mRNA expression pattern of common glycoprotein α subunit of GtH in tongue sole Cynoglossus semilaevis Günther. Progress in Fishery Sciences , 2013, 34 (5) : 23-30 [李晓晓, 柳学周, 史宝, 等. 半滑舌鳎促性腺激素α亚基cDNA的克隆及组织表达特征. 渔业科学进展 , 2013, 34 (5) : 23-30] | |
| Lin HR. Fish Physiology. Guangzhou: Sun Yat-sen University Press, 2011 : 153 -264. [林浩然. 鱼类生理学. 广州: 中山大学出版社, 2011 : 153 -264.] | |
| Lin HR. The interactions of neuroendocrine regulation on reproduction and growth in fish. Zoological Research , 2000, 21 (1) : 12-16 [林浩然. 神经内分泌因子调控鱼类生殖和生长的相互作用. 动物学研究 , 2000, 21 (1) : 12-16] | |
| Liu XZ, Shi B, Li XX, et al. Molecular characterization of the novel membrane progestin receptor gene and its role during ovarian development in the half smooth tongue sole Cynoglossus semilaevis Günther. Journal of Fishery Sciences of China , 2015, 22 (4) : 608-619 [柳学周, 史宝, 李晓晓, 等. 半滑舌鳎新型膜孕激素受体基因分子特征及其在卵巢发育过程的作用. 中国水产科学 , 2015, 22 (4) : 608-619] | |
| Liu XZ, Xu YJ, Liu NZ, et al. Study on histological and morphometrical characters of gonadal development of Cynoglossus semilaevis Günther. Progress in Fishery Sciences , 2009, 30 (6) : 25-35 [柳学周, 徐永江, 刘乃真, 等. 半滑舌鳎卵巢发育的组织学和形态数量特征研究. 渔业科学进展 , 2009, 30 (6) : 25-35] | |
| Liu XZ, Zhuang ZM. Reproductive biology and culture technology of half-smooth tongue soleCynoglossus semilaevis. Beijing: China Agricultural Press, 2014 : 259 -394. [柳学周, 庄志猛. 半滑舌鳎繁育理论与养殖技术. 北京: 中国农业出版社, 2014 : 259 -394.] | |
| Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods , 2001, 25 (4) : 402-408 DOI:10.1006/meth.2001.1262 | |
| Ma Q, Liu SF, Zhuang ZM, et al. Genomic structure, polymorphism and expression analysis of the growth hormone (GH) gene in female and male Half-smooth tongue sole (Cynoglossus semilaevis). Gene , 2012, 493 (1) : 92-104 DOI:10.1016/j.gene.2011.11.015 | |
| Ma Q, Liu SF, Zhuang ZM, et al. Molecular cloning, expression analysis of insulin-like growth factor I (IGF-Ⅰ) gene and IGF-Ⅰserum concentration in female and male Tongue sole (Cynoglossus semilaevis). Comparative Biochemistry and Physiology Part B Biochemistry and Molecular Biology , 2011, 160 (4) : 208-214 DOI:10.1016/j.cbpb.2011.08.008 | |
| Nakamura I, Kusakabe M, Swanson P, et al. Regulation of sex steroid production and mRNAs encoding gonadotropin receptors and steroidogenic proteins by gonadotropins, cyclic AMP and insulin-like growth factor-I in ovarian follicles of rainbow trout (Oncorhynchus mykiss) at two stages of vitellogenesis. Comparative Biochemistry and Physiology Part A Molecular and Integrative Physiology , 2016, 201 : 132-140 DOI:10.1016/j.cbpa.2016.06.035 | |
| Onuma TA, Makino K, Ban M, et al. Elevation of the plasma level of insulin-like growth Factor-I with reproductive maturation prior to initiation of spawning migration of chum salmon. Annals of the New York Academy of Sciences , 2009, 1163 (3) : 497-500 | |
| Reinecke M, Björnsson BT, Dickhoff WW, et al. Growth hormone and insulin-like growth factors in fish: Where we are and where to go. General and Comparative Endocrinology , 2005, 142 (1-2) : 20-24 DOI:10.1016/j.ygcen.2005.01.016 | |
| Reinecke M. Insulin-like growth factors and fish reproduction. Biology of Reproduction , 2010, 82 (4) : 656-661 DOI:10.1095/biolreprod.109.080093 | |
| Riley LG, Richman NH Ⅲ, Hirano T, et al. Activation of the growth hormone/insulin-like growth factor axis by treatment with 17α-methyltestosterone and seawater rearing in the tilapia, Oreochromis mossambicus. General and Comparative Endocrinology , 2002, 127 (3) : 285-292 DOI:10.1016/S0016-6480(02)00051-5 | |
| Shi B, Li XX, Liu XZ, et al. Molecular cloning and tissue expression analysis of membrane progestin receptor alpha gene (mPRα) from half smooth tongue sole Cynoglossus semilaevis Günther. Progress in Fishery Sciences , 2013, 34 (3) : 61-67 [史宝, 李晓晓, 柳学周, 等. 半滑舌鳎膜孕激素受体基因克隆与组织表达分析. 渔业科学进展 , 2013, 34 (3) : 61-67] | |
| Shi B, Liu XZ, Xu YJ, et al. Molecular characterization of three gonadotropin subunits and their expression patterns during ovarian maturation inCynoglossus semilaevis. International Journal of Molecular Sciences , 2015, 16 (2) : 2767-2793 DOI:10.3390/ijms16022767 | |
| Wang DS, Lin HR, Zhang WM. Seasonal changes of the pituitary and serum basal growth hormone levels of Mystus macropterus. Journal of Fisheries of China , 1999, 23 (1) : 1-5 [王德寿, 林浩然, 张卫民. 大鳍鳠脑垂体和血清生长激素水平的季节变化. 水产学报 , 1999, 23 (1) : 1-5] | |
| Xu YJ, Liu XZ, Liao MJ, et al. Molecular cloning and differential expression of three GnRH genes during ovarian maturation of spotted halibut, Verasper variegatus. Journal of Experimental Zoology Part A Ecological Genetics and Physiology , 2012, 317 (7) : 434-446 DOI:10.1002/jez.1736 | |
| Xu YJ, Liu XZ, Wang QY, et al. Relationships between serum sex steroids levels and gonadal development and photothernal regulation during the annual maturation of captive Cynoglossus Semilaevis Günther. Oceanologica et Limnologia Sinica , 2011, 42 (1) : 67-74 [徐永江, 柳学周, 王清印, 等. 半滑舌鳎(Cynoglossus semilaevis)血浆性类固醇激素表达与卵巢发育及温光调控的关系研究. 海洋与湖沼 , 2011, 42 (1) : 67-74] | |
|
Zhou LB, Liu XC, Ye W, et al. Sexual cycle changes of growth hormone in Helmet catfish, Cranoglanis bouderius.
Acta Hydrobiologica Sinica , 2004, 28 (6) : 679-681 [周立斌, 刘晓春, 叶卫, 等. 长臀 |
|
| Zhou XS, Yi Q, Zhang QQ, et al. Molecular cloning, tissue distribution, and ontogeny of gonadotropin-releasing hormoneⅢgene (GnRH-Ⅲ) in half-smooth tongue sole (Cynoglossus semilaevis). Comparative Biochemistry and Physiology Part B Biochemistry abd Molecular Biology , 2012, 163 (1) : 59-64 DOI:10.1016/j.cbpb.2012.04.010 | |
| Zohar Y, Mu oz-Cueto JA, Elizur A, et al. Neuroendocrinology of reproduction in teleost fish. General and Comparative Endocrinology , 2010, 165 (3) : 438-455 DOI:10.1016/j.ygcen.2009.04.017 | |
| Zohar Y, Mylonas CC. Endocrine manipulations of spawning in cultured fish: From hormones to genes. Aquaculture , 2001, 197 : 99-136 DOI:10.1016/S0044-8486(01)00584-1 |



