盐度是决定海洋生物分布并影响其生长、存活的重要环境因子。沿海近岸地区,尤其是河口附近,因为潮汐、降雨、高温干旱或人类活动等影响,经常发生不同程度的海水盐度波动。目前,大多数海洋经济贝类分布或养殖于潮间带、滩涂或河口地区,盐度变化可能会对贝类造成不同程度的胁迫 (Gagnaire et al, 2006; Carregosa et al, 2014)。
盐度胁迫会影响贝类的免疫功能,增加贝类感染疾病的风险。Reid等 (2003)研究发现,菲律宾蛤仔 (Ruditapes philippinarum) 血细胞THC随盐度升高而升高。高盐胁迫下,鸡帘蛤 (Chamelea gallina) 的细胞吞噬活性降低 (Matozzo et al, 2007)。随盐度上升,牡蛎 (Crassostrea virginica) 对寄生虫Perkinsus marinus和Haplosporidium nelsoni的感染率和死亡率也上升 (Ford et al, 1988; Chu et al, 1993)。盐度胁迫还会影响海水贝类的呼吸、摄食以及生长 (Navarro et al, 1998; Nakamura et al, 2005),贝类需要调整生理活动以适应盐度的改变。研究表明,免疫反应需要消耗能量,免疫功能的变化也会导致机体能量代谢的改变 (Roch, 1999; Rolff et al, 2003; Wang et al, 2012)。因此,生理代谢变化可以在一定程度上反应海水贝类所受到的胁迫。
在复杂多变的近海环境中,贝类进化产生了独特的逆境适应机制 (Zhang et al, 2012),了解这些机制对于海水贝类资源保护、新品种选育具有重要意义。黄边糙鸟蛤 (Trachycardium flavum) 分布于我国广东、广西和海南潮间带,是一种重要的经济贝类 (唐保军等, 2010)。受海表蒸发等因素影响,广东、海南沿海海水盐度会出现异常升高,有时达34以上 (叶龙飞, 1988; 袁淑尧等, 1998),此时,黄边糙鸟蛤会遭遇高盐胁迫。本研究通过测定高盐胁迫下代谢率和免疫相关酶活性的变化,探讨黄边糙鸟蛤的盐度胁迫适应机制,为该贝类的养殖和资源保护提供参考资料。
1 材料与方法 1.1 实验动物黄边糙鸟蛤采自海南文昌沿海,取回实验室后挑选大小一致的个体[壳高为 (50.35±2.64) mm,体重为 (46.51±6.91) g],清除表面附着物后放入2000 L玻璃钢桶暂养14 d,桶底铺10 cm厚细砂,暂养期间,海水盐度为31.0±0.5,通过室内空调控制水温为 (26.0±0.5)℃,每天换水1/2,早晚各投喂1次小球藻 (Chlorella pyrenoidsa) 和金藻 (Isochrysis galbana) 的混合藻液。
1.2 盐度处理取暂养的黄边糙鸟蛤150只,平均分为2组,一组仍在盐度为31的海水中作为对照,另外一组直接转入盐度为37的海水中。将海水晶溶于水配制成高浓度盐水,经脱脂棉过滤后调节海水盐度至37,盐度用比重计和温度计测定。实验持续72 h,期间每天换水1/2。于实验开始后2、12、24、48和72 h测定耗氧率和排氨率。每个时间点取3只黄边糙鸟蛤,分别取肝胰腺、鳃、外套膜和斧足肌肉样品,于–80℃保存,用于组织酶活分析。
1.3 耗氧率和排氨率的测定用静水法测定耗氧率和排氨率。每个1.5 L塑料呼吸瓶内放1只黄边糙鸟蛤,虹吸式注入实验用水后密封,26℃水浴,每个时间点分别设3个平行和1个空白对照。因每次实验持续2 h,故测得的耗氧率和排氨率分别为处理后1–3、11–13、23–25、47–49和71–73 h内的均值。实验结束后用虹吸法取水样分析,解剖实验用蛤,取软体部于65℃烘干24 h,称量组织干重。
(1) 采用Winkler法 (Stickland et al, 1968) 测定水中的溶解氧含量,根据下式计算耗氧率:
| $ R = V \times \left( {{O_0}-{O_{\rm{t}}}} \right)/\left( {W \times t} \right) $ |
式中,R为单位体重耗氧率,O0和Ot分别为实验结束时空白对照组和实验组水中的溶解氧浓度 (mg/L), V为呼吸瓶体积 (L),W为蛤组织干重 (g),t为实验持续时间 (h)。
(2) 采用次溴酸盐氧化法 (Solorzano, 1969) 测定水中的氨氮含量,根据下式计算排氨率:
| $ E = V \times \left( {{N_{\rm{t}}}-{N_0}} \right)/\left( {W \times t} \right) $ |
式中,E为单位体重排氨率,N0和Nt分别为实验结束时空白对照组和实验组水中的氨氮浓度 (µg/L), V为呼吸瓶体积 (L),W为蛤组织干重 (g),t为实验持续时间 (h)。
1.4 酶活测定 1.4.1 样品制备各组织样品于4℃解冻后,分别按质量体积比加入9倍预冷的生理盐水 (150 mmol/L NaCl, pH=7.4) 匀浆。制备好的匀浆液以2500 r/min、4℃离心15 min,取上清液用于酶活分析。
1.4.2 酶活力测定分析测定上清液中Na+/K+-ATP酶、超氧化物歧化酶 (SOD)、酸性磷酸酶 (ACP) 和碱性磷酸酶 (ALP) 的活力,各酶活力均采用试剂盒 (南京建成生物工程研究所) 测定,具体测定方法按照试剂盒说明书进行。采用考马斯亮蓝法测定上清液蛋白浓度。Na+/K+-ATP酶活力定义为每小时每毫克组织蛋白中ATP酶分解ATP产生1 µmol无机磷 (Pi) 的量为1个活力单位。SOD活力定义为每毫克组织蛋白在1 ml反应液中SOD抑制率达50%时所对应的SOD量为1个活力单位。ACP活力定义为30 min内产生1 mg p-硝基酚为1个活力单位。ALP活力定义为15 min内产生1 mg p-硝基酚为1个活力单位。
1.5 数据处理实验数据采用平均值±标准差 (Mean±SD) 表示。采用SPSS 11.5统计软件对数据进行独立样本t检验,P < 0.05表示差异显著,P < 0.01表示差异极显著。
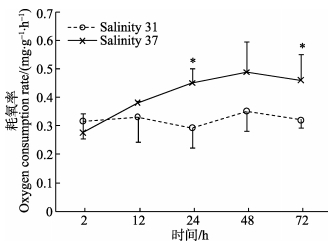
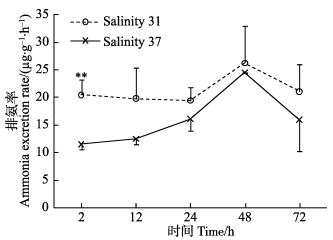
2 结果 2.1 高盐胁迫对呼吸排泄的影响盐度37处理后2 h,黄边糙鸟蛤的耗氧率低于对照组,其后逐渐上升,至48 h达到最大值 (图 1),在24和72 h显著高于对照组 (P < 0.05)。处理组黄边糙鸟蛤2 h时耗氧率显著低于24、48和72 h时的耗氧率 (P < 0.05)。排氨率在处理后2 h极显著低于对照组 (P < 0.01),其后逐渐升高。处理组2 h的排氨率显著高于其他时间点 (P < 0.05)。
|
图 1 高盐胁迫下黄边糙鸟蛤耗氧率变化 Figure 1 Variation of oxygen consumption rate of T. flavum under hypersaline stress *表示对照组与处理组有显著差异,下同 * indicated significant difference between the control and treatment, the same as below |
|
图 2 高盐胁迫下黄边糙鸟蛤排氨率变化 Figure 2 Variation of ammonia excretion rate of T. flavum under hypersaline stress |
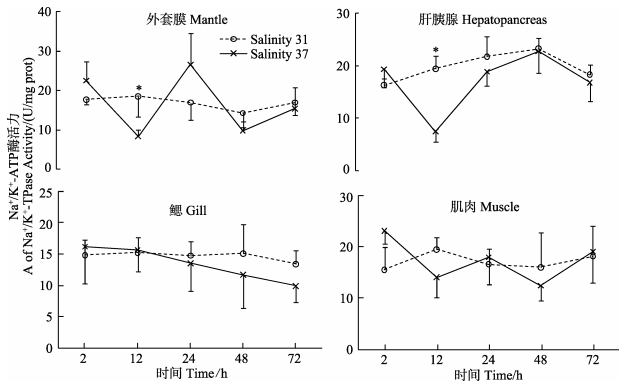
由图 3可知,盐度37处理后2 h,外套膜、肝胰腺中Na+/K+-ATP酶活力在12 h显著低于对照组 (P < 0.05),在其他各时间点与对照组无显著差异。肌肉和鳃中Na+/K+-ATP酶活力与对照组无显著差异。盐度胁迫后,外套膜、肝胰腺和肌肉中不同时间点之间Na+/K+-ATP酶活力有显著差异 (P < 0.05)。
|
图 3 高盐胁迫下黄边糙鸟蛤不同组织Na+/K+-ATP酶活力变化 Figure 3 Variation of Na+/K+-ATPase activity in different tissues of T. flavum under hypersaline stress |
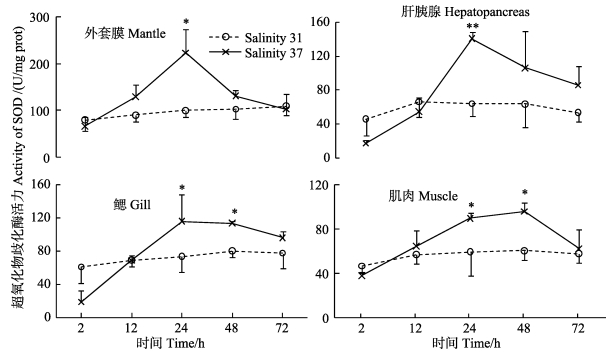
盐度37处理后,各组织中SOD活力呈先升高后下降的趋势 (图 4)。处理后2 h,与对照组相比,各组织中SOD活力下降,其后逐渐升高;24 h,肝胰腺中SOD活力极显著高于对照组 (P < 0.01);至72 h,各组织中SOD活力降至对照组水平。盐度处理后,各组织中不同时间点之间SOD活力存在显著差异 (P < 0.05)。
|
图 4 高盐胁迫下黄边糙鸟蛤不同组织超氧化物歧化酶活力变化 Figure 4 Variation of SOD activity in different tissues of T. flavum under hypersaline stress |
高盐度胁迫下,黄边糙鸟蛤外套膜和鳃中ACP活力呈先升高后降低的趋势,在24 h显著高于对照组 (P < 0.05),在48和72 h下降 (图 5)。肝胰腺中ACP活力在12 h显著低于对照组 (P < 0.05),随后逐渐升高,在72 h显著高于对照组 (P < 0.05)。与对照组相比,肌肉中ACP活力无显著差异。肝胰腺中ACP活力显著高于其他各组织 (P < 0.01)。高盐胁迫下,各组织中不同时间点之间ACP活力出现显著变化 (P < 0.05)。
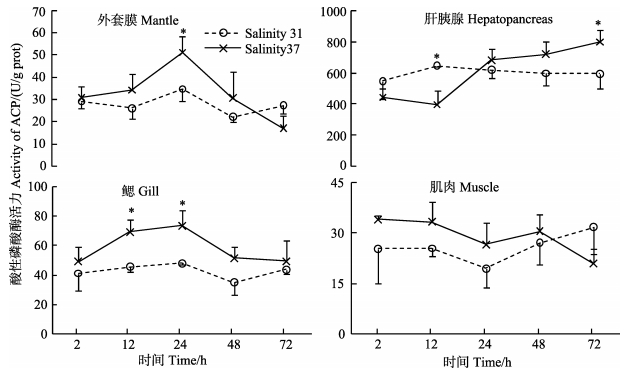
|
图 5 高盐胁迫下黄边糙鸟蛤不同组织酸性磷酸酶活力变化 Figure 5 Variation of ACP activity in different tissues of T. flavum under hypersaline stress |
盐度37处理后,与对照组相比,黄边糙鸟蛤外套膜和肌肉中ALP活力无显著差异 (图 6)。鳃中ALP活力在2 h和12 h极显著低于对照组 (P < 0.01),其后升高,在48 h显著高于对照组 (P < 0.01)。肝胰腺中ALP活力变化趋势与鳃相似,在24 h和72 h显著高于对照组 (P < 0.05)。肝胰腺中ALP活力显著高于其他各组织 (P < 0.01)。盐度处理后,各组织中不同时间点之间ALP活力出现显著变化 (P < 0.05)。
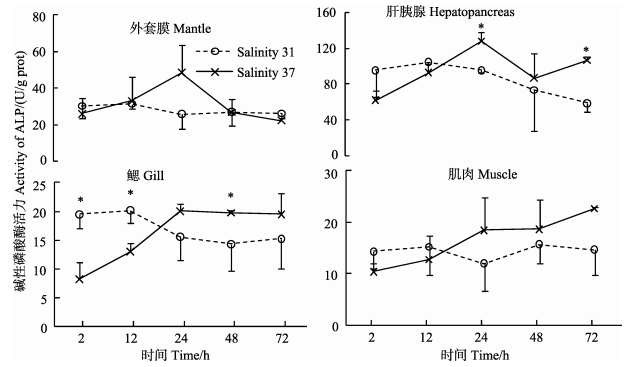
|
图 6 高盐胁迫下黄边糙鸟蛤不同组织碱性磷酸酶活力变化 Figure 6 Variation of ALP activity in different tissues of T. flavum under hypersaline stress |
当外界盐度发生突然改变时,海水贝类通过关闭贝壳保护内部组织免受伤害 (Pierce, 1971)。Brown等 (1981)报道,Bullia digitalis在盐度升高或降低时会关闭水管。本研究中,盐度处理初期,黄边糙鸟蛤贝壳开放程度明显降低,这可能是导致2 h耗氧率下降的原因。盐度胁迫条件下,呼吸率的降低也是贝类在不利环境中保存能量的一种方式 (Brown et al, 1990)。为了维持内环境的稳定,当环境改变时,海水无脊椎动物会进行生理补偿 (Bayne, 1973、1998; McMahon et al, 1991)。补偿后,如果新的稳定态与环境改变之前一致,说明补偿是完全的;如果新的稳定态与之前的不一致,说明补偿不完全,仍然存在环境胁迫 (Bayne, 1973)。本研究中,耗氧率12 h后逐渐升高,可能是贝类对盐度改变的生理补偿所致。唐保军等 (2010)的研究中,盐度缓慢升至36、40并暂养7 d后,黄边糙鸟蛤耗氧率与对照组 (盐度32) 无显著差异,表明达到完全补偿需要的时间与盐度胁迫程度有关,具体机制需要进一步研究。
作为主要的氮代谢终产物,氨离子参与海水动物渗透调节 (Silvia et al, 2004)。本研究中,高盐度处理后,排氨率下降,这与之前慢性盐度胁迫下的研究报道一致 (唐保军等, 2010)。其他贝类中也有类似报道:织纹螺 (Nassarius festivus) 从盐度25转入30、35后72 h,排氨率下降 (Cheung, 1997)。在高盐度胁迫下,双壳贝类组织细胞体积缩小并快速合成游离氨基酸,以应对高渗压力 (Baginski et al, 1977),首先合成丙氨酸,其后为甘氨酸、牛磺酸等 (Baginski et al, 1977; Henry et al, 1980; Deaton et al, 1985)。丙氨酸的合成需要将游离铵离子固定转化为丙酮酸酯或2-酮戊二酸,游离铵离子来自尿酸代谢、蛋白降解以及代谢氨等 (Zurburg et al, 1981)。本研究中,在2 h,排氨率显著低于对照组,可能因为黄边糙鸟蛤通过对代谢氨氮的重新利用以应对高渗胁迫;随着内环境逐渐达到新的平衡状态,排氨率也逐渐上升;至72 h排氨率又下降,表明机体尚未达到稳定态。对照组黄边糙鸟蛤的排氨率在48 h出现上升,可能为个体差异所致,多重比较显示,各时间点之间排氨率无显著差异。
Na+/K+-ATP酶对维持细胞中Na+、K+稳态发挥着重要作用,可有效调节和维持机体渗透压 (Cheng et al, 2002; 沈永龙等, 2013)。本研究中,外套膜、肝胰腺中Na+/K+-ATP酶活力在12 h显著低于对照组,表明Na+/K+-ATP酶参与黄边糙鸟蛤渗透稳态 (Osmolaity homeostasis) 调节。高盐度下Na+/K+-ATP酶活力降低的现象在其他贝类中也有报道。盐度自23骤升至26后,青蛤 (Cyclina sinensis) 鳃中Na+/K+-ATP酶活力下降,在第15天达到最小值后逐渐回升趋于平稳,但仍低于初始值 (林听听等, 2012)。盐度自30升至35、40后,魁蚶 (Anadara broughtonii) 稚贝鳃中Na+/K+-ATP酶活力下降,分别在24 h和12 h出现最小值后回升,但均低于对照组 (蔡星媛等, 2015)。
3.2 免疫酶活性变化环境突然变化会影响海水无脊椎动物的免疫功能,激活机体的NADPH氧化酶系统,导致耗氧率上升 (呼吸暴发),产生大量活性氧 (ROS)。活性氧能够杀伤异物,但也会损害机体的膜组织、DNA和酶等 (Roch, 1999; Finkel et al, 2000)。SOD是机体抗氧化防御系统的重要组成部分,可清除体内活性氧自由基 (Hermes-Lima et al, 1998)。Monari等 (2007)报道,盐度自34升至40后,鸡帘蛤血清中Cu/Zn-SOD活力升高。将仿刺参 (Apostichopus japonicas) 自盐度30转入盐度35海水中,其体腔液中SOD活力在3 h极显著升高 (P < 0.01),其后下降至对照组水平 (Wang et al, 2008)。本研究中,各组织中SOD活力均呈现“先降低再回升,然后下降”的变化规律,说明胁迫初期黄边糙鸟蛤体内自由基浓度降低,进而黄边糙鸟蛤产生应激反应,体内生成大量自由基,SOD等抗氧化酶被诱导激活以清除过多的自由基。
ACP和ALP是重要的水解酶,具有促进血细胞吞噬、清除异物的作用 (Cheng, 1978)。本研究中,胁迫初期黄边糙鸟蛤肝胰腺、鳃中ACP和ALP活力先降低,表明高盐度胁迫影响了黄边糙鸟蛤的免疫酶活性。这与盐度骤升后近江牡蛎 (C. hongkongensis) 血淋巴ALP活力的变化一致 (时少坤等, 2013)。酸性磷酸酶还是检测溶酶体的标志酶 (Cajaraville et al, 2000),ACP活力的变化,表明高盐度胁迫影响了溶酶体的稳定性。
本研究还发现,黄边糙鸟蛤的免疫反应具有组织特异性。对照组黄边糙鸟蛤外套膜SOD活力显著高于其他组织 (P < 0.05),肝胰腺中ACP和ALP活力显著高于其他组织 (P < 0.05)。这种组织差异性在其他贝类中也有报道。紫贻贝 (Mytilus edulis) 鳃中SOD活力较高 (Manduzio et al, 2004),而褐贻贝 (Perna perna) 鳃和肝胰腺中SOD活力相当 (Almeida et al, 2005)。浅沟蛤 (Scrobicularia plana) 肝胰腺中ACP和ALP活力较高,其次为鳃、足、水管和外套膜 (Mazorra et al, 2002)。这种组织差异性可能与蛋白的合成、转运有关,具体的机制需要借助免疫细胞化学、原位杂交等技术进一步开展研究。
| Almeida EA, Bainy ACD, Dafre AL, et al. Oxidative stress in digestive gland and gill of the brown mussel (Perna perna) exposed to air and re-submersed. Journal of Experimental Marine Biology and Ecology, 2005, 318(1): 21-30 DOI:10.1016/j.jembe.2004.12.007 | |
| Baginski RM, Pierce SK. The time course of intracellular free amino acid accumulation in tissues of Modiolus demissus during high salinity adaptations. Comparative Biochemistry and Physiology Part A: Physiology, 1977, 57(4): 407-412 DOI:10.1016/0300-9629(77)90137-2 | |
| Bayne BL. The physiology of suspension feeding by bivalve molluscs: An introduction to the Plymouth "TROPHEE" workshop. Journal of Experimental Marine Biology and Ecology, 1998, 219(1): 1-19 | |
| Bayne BL. The responses of three species of bivalve mollusc to declining oxygen tension at reduced salinity. Comparative Biochemistry and Physiology, Part A: Physiology, 1973, 45(3): 793-806 DOI:10.1016/0300-9629(73)90082-0 | |
| Brown AC, McLachlan A. Ecology of sandy shores. Amsterdam, the Netherlands: Elsevier, 1990 | |
| Brown AC, Meredith FL. The effects of salinity changes on respiration in the sandy-beach whelk Bullia digitalis (Dillwyn). Comparative Biochemistry and Physiology, Part A: Physiology, 1981, 69(3): 599-601 | |
| Cai XY, Zhang XM, Tian L, et al. Effect of salinity stress on hemolymph osmolality and gill Na+/K+-ATPase activity of juvenile ark shell (Anadara broughtonii). South China Fisheries Science, 2015, 11(2): 12-19 [蔡星媛, 张秀梅, 田璐, 等. 盐度胁迫对魁蚶稚贝血淋巴渗透压及鳃Na+/K+-ATP酶活性的影响. 南方水产科学, 2015, 11(2): 12-19] | |
| Cajaraville MP, Bebianno MJ, Blasco J, et al. The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: A practical approach. Science of the Total Environment, 2000, 247(2): 295-311 | |
| Carregosa V, Figueira E, Gil AM. Tolerance of Venerupis philippinarum to salinity: Osmotic and metabolic aspects. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology, 2014, 171(3): 36-43 | |
| Cheng TC. The role of lysosomal hydrolases in molluscan cellular response to immunologic challenge. Invertebrate Models for Biomedical Research. Springer US, 1978, 59-71 | |
| Cheng W, Yeh SP, Wang CS, et al. Osmotic and ionic changes in Taiwan abalone Haliotis diversicolor supertexta at different salinity levels. Aquaculture, 2002, 203(3): 349-357 | |
| Cheung SG. Physiological and behavioural responses of the intertidal scavenging gastropod Nassarius festivus to salinity changes. Marine Biology, 1997, 129(2): 301-307 DOI:10.1007/s002270050170 | |
| Chu FLE, La Peyre JF, Burreson CS. Perkinsus marinus infection and potential defense-related activities in eastern oysters, Crassostrea virginica: Salinity effects. Journal of Invertebrate Pathology, 1993, 62(3): 226-232 DOI:10.1006/jipa.1993.1104 | |
| Deaton LE, Hilbish TJ, Koehn RK. Hyper-osmotic volume regulation in the tissues of the mussel Mytilus edulis. Comparative Biochemistry and Physiology, Part A: Physiology, 1985, 80(4): 571-574 DOI:10.1016/0300-9629(85)90414-1 | |
| Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature, 2000, 408(6809): 239-247 DOI:10.1038/35041687 | |
| Ford SE, Haskin HH. Comparison of in vitro salinity tolerance of the oyster parasite, Haplosporidium nelsoni (MSX) and hemocytes from the host, Crassostrea virginica. Comparative Biochemistry and Physiology, Part A: Physiology, 1988, 90(1): 183-187 DOI:10.1016/0300-9629(88)91025-0 | |
| Gagnaire B, Frouin H, Moreau K, et al. Effects of temperature and salinity on haemocyte activities of the Pacific oyster, Crassostrea gigas (Thunberg). Fish and Shellfish Immunology, 2006, 20(4): 536-547 DOI:10.1016/j.fsi.2005.07.003 | |
| Henry RP, Mangum CP, Webb KL. Salt and water balance in the oligohaline clam, Rangia cuneata Ⅱ. Accumulation of intracellular free amino acids during high salinity adaptation. Journal of Experimental Zoology, 1980, 211(1): 11-24 DOI:10.1002/(ISSN)1097-010X | |
| Hermes-Lima M, Storey JM, Storey KB. Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails. Comparative Biochemistry and Physiology, Part B: Biochemistry and Molecular Biology, 1998, 120(3): 437-448 DOI:10.1016/S0305-0491(98)10053-6 | |
| Lin TT, Lai QF, Lu JX, et al. Effects of abrupt variations in salinity on the Na+/K+-ATPase activity in gills of Cyclina sinensis. Journal of Guangdong Ocean University, 2012, 32(1): 54-58 | |
| 林听听, 来琦芳, 陆建学, 等. 盐度突变对青蛤 (Cyclina sinensis) 鳃Na+/K+-ATPase活性的影响. 广东海洋大学学报, 2012, 32(1): 54-58 | |
| Manduzio H, Monsinjon T, Galap C, et al. Seasonal variations in antioxidant defences in blue mussels Mytilus edulis collected from a polluted area: major contributions in gills of an inducible isoform of Cu/Zn-superoxide dismutase and of glutathione S-transferase. Aquatic Toxicology, 2004, 70(1): 83-93 DOI:10.1016/j.aquatox.2004.07.003 | |
| Matozzo V, Monari M, Foschi J. Effects of salinity on the clam Chamelea gallina. Part Ⅰ. alterations in immune responses. Marine Biology, 2007, 151(3): 1051-1058 DOI:10.1007/s00227-006-0543-6 | |
| Mazorra MT, Rubio JA, Blasco J. Acid and alkaline phosphatase activities in the clam Scrobicularia plana: kinetic characteristics and effects of heavy metals. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2002, 131(2): 241-249 DOI:10.1016/S1096-4959(01)00502-4 | |
| McMahon BR, Burggren WW, Pinder AW, et al. Air exposure and physiological compensation in a tropical intertidal chiton, Chiton stokesii (Mollusca: Polyplacophora). Physiological Zoology, 1991, 64(3): 728-747 DOI:10.1086/physzool.64.3.30158204 | |
| Monari M, Serrazanetti GP, Foschi J, et al. Effects of salinity on the clam Chamelea gallina haemocytes. Part Ⅱ: Superoxide dismutase response. Marine Biology, 2007, 151(3): 1059-1068 DOI:10.1007/s00227-006-0544-5 | |
| Nakamura Y, Hashizume K, Koyama K. Effects of salinity on sand burrowing activity, feeding and growth of the clams Mactra veneriformis, Ruditapes philippinarum and Meretrix lusoria. Journal of Shellfish Research, 2005, 24(4): 1053-1059 DOI:10.2983/0730-8000(2005)24[1053:EOSOSB]2.0.CO;2 | |
| Navarro JM, Gonzalez CM. Physiological responses of the Chilean scallop Argopecten purpuratus to decreasing salinities. Aquaculture, 1998, 167(3): 315-327 | |
| Pierce SK. Volume regulation and valve movements by marine mussels. Comparative Biochemistry and Physiology, Part A: Physiology, 1971, 39(1): 103-117 DOI:10.1016/0300-9629(71)90350-1 | |
| Reid HI, Soudant P, Lambert C. Salinity effects on immune parameters of Ruditapes philippinarum challenged with Vibrio tapetis. Diseases of Aquatic Organisms, 2003, 56(3): 249-258 | |
| Roch P. Defense mechanisms and disease prevention in farmed marine invertebrates. Aquaculture, 1999, 172(1): 125-145 | |
| Rolff J, Siva-Jothy M. Invertebrate ecological immunology. Science, 2003, 301(5632): 472-475 DOI:10.1126/science.1080623 | |
| Shen YL, Ge XP, Huang JT, et al. Effects of salinity on Na+/K+-ATPase activity, the osmolality of pericardial cavity fluid and peritoneal fluid and ion content in Onchidium struma. Journal of Fisheries of China, 2013, 37(6): 851-857 [沈永龙, 戈贤平, 黄金田, 等. 盐度对瘤背石磺不同部位Na+/K+-ATP酶活性、围心腔液和腹腔液渗透压及离子含量的影响. 水产学报, 2013, 37(6): 851-857] | |
| Shi SK, Wang RX, Wang JY, et al. Effects of salinity stress on immune factors of Crassostrea hongkongensis. South China Fisheries Science, 2013, 9(3): 26-30 [时少坤, 王瑞璇, 王江勇, 等. 盐度胁迫对近江牡蛎几种免疫因子的影响. 南方水产科学, 2013, 9(3): 26-30] | |
| Silvia GJ, Antonio URA, Francisco VO, et al. Ammonia efflux rates and free amino acid levels in Litopenaeus vannamei postlarvae during sudden salinity changes. Aquaculture, 2004, 233(1): 573-581 | |
| Solorzano L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnology and Oceanography, 1969, 14: 799-801 DOI:10.4319/lo.1969.14.5.0799 | |
| Stickland JDH, Parsons TR. A practical handbook of seawater analysis. Bulletin of the Fisheries Research Board of Canada, 1968, 67: 1-11 | |
| Tang BJ, Yan WG, Wang H, et al. Effects of salinity on oxygen consumption and ammonia-N excretion rate of different-size of Trachycardium flavum. Marine Fisheries, 2010, 32(1): 30-34 [唐保军, 闫文罡, 王慧, 等. 盐度对不同规格黄边糙鸟蛤呼吸排泄的影响. 海洋渔业, 2010, 32(1): 30-34] | |
| Wang FY, Yang HS, Gao F, et al. Effects of acute temperature or salinity stress on the immune response in sea cucumber, Apostichopus japonicus. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology, 2008, 151(4): 491-498 | |
| Wang XQ, Wang LL, Zhang H, et al. Immune response and energy metabolism of Chlamys farreri under Vibrio anguillarum challenge and high temperature exposure. Fish and Shellfish Immunology, 2012, 33(4): 1016-1026 DOI:10.1016/j.fsi.2012.08.026 | |
| Ye LF. Standing tidal waves and salinity mixing in the Qinglan Estuary, Hainan Island. Transactions of Oceanology and Limnology, 1988(3): 9-14 [叶龙飞. 海南岛清澜河口的驻波型潮波和盐度混和. 海洋湖沼通报, 1988(3): 9-14] | |
| Yuan SY, Deng JZ. Temperature and salinity thermal structures in the northern South China Sea-Ⅲ. spatial and temporal salinity distribution characteristics in the northern South China Sea. South China Sea Research and Development, 1998(2): 28-36 [袁淑尧, 邓九仔. 南海北部的温盐热结构——Ⅲ.南海北部盐度时空分布特征. 南海研究与开发, 1998(2): 28-36] | |
| Zhang GF, Fang XD, Guo XM, et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature, 2012, 490(7418): 49-54 DOI:10.1038/nature11413 | |
| Zurburg W, De Zwaan A. The role of amino acids in anaerobiosis and osmoregulation in bivalves. Journal of Experimental Zoology, 1981, 215(3): 315-325 DOI:10.1002/(ISSN)1097-010X |



