2. 北京吉天仪器有限公司 北京 100015;
3. 山东省产品质量检验研究院 济南 272000;
4. 南京农业大学无锡渔业学院 无锡 214081
2. Beijing Titan Instruments Co. Ltd., Beijing 100015;
3. Shandong Product Quality Inspection Research Institute, Jinan 272000;
4. Wuxi Fisheries College, Nanjing Agricultural University, Wuxi 214081
镉(Cd)作为有毒有害重金属元素,一直是人们关注的重点。海洋生物对重金属有很强的富集作用,尤其是贝类体内Cd含量较高(吴春峰等, 2013;崔正国等, 2015),是人体摄入Cd的重要来源之一。因此,监测环境及水产品中Cd的含量,是预防和减少Cd对人体危害的重要举措。目前,国际上Cd的检测方法已较为成熟,如原子吸收法(AAS)(Miranda et al, 2012;李立等, 2011)、原子荧光法(AFS) (Cava-Montesinos et al, 2004;王志嘉等, 2012)、电感耦合等离子体发射光谱和质谱法(ICP-AES/MS) (O'Sullivan et al, 2013;陈国娟, 2012; Juranović et al, 2012)等,但均需繁琐的样品消解,难以实现现场及快速检测。直接进样分析是解决这一问题的有效手段,且已与多种检测方法结合并得到应用(Duarte et al, 2013;张英等, 2015; Bu et al, 2013),然而,直接进样引入复杂基体所产生的干扰则需要用加入基体改进剂的方法来进行消除(叶佩琳等, 2015; Trk et al, 2012)。此外,也可以利用捕获技术来消除基体干扰,如Feng等(2010) 利用钨丝“在线原子阱”实现了电热蒸出Cd的捕获,消除了基体干扰,并成功应用到了蔬菜、鸡肉等农产品中Cd的测定(Zhang et al, 2012)。目前,电热蒸发钨丝在线捕获技术与光谱技术联用直接测定食品中的Cd含量在粮食、蔬菜、水果、茶叶、猪肝及干粉贝类中已有报道(Zhang et al, 2016;冯礼等, 2014; Zhang et al, 2015;黄亚涛等, 2013;王昌钊等, 2012; Mao et al, 20131); 毛雪飞, 2015)。而水产品由于含水量高、粘度大、基质复杂等特点,在该方面的研究应用还较少(Wen et al, 2009; Yang et al, 2015)。
1) Mao XF. Study on Determination of cadmium and mercury in agri-foods by solid sampling electrothermal vaporization spectrometry using atomic traps. Doctoral Dissertation of Chinese Academy of Agricultural Sciences, 2015 [毛雪飞. 固体进样电热蒸发原子阱捕获光谱技术快速测定农产品中镉和汞的研究. 中国农业科学院博士研究生学位论文, 2015]
本研究应用集成多孔石墨管电热蒸发和钨丝捕获阱技术的固体进样测Cd装置串联原子荧光光谱分析系统,针对贝类样品特点,通过优化前处理方法,确定最佳测定程序和条件,建立了适用于现场快速检测贝类中Cd的测定方法,旨在为贝类中Cd的现场快速检测提供一种有效技术手段。
1 材料与方法 1.1 材料 1.1.1 实验材料扇贝、菲律宾蛤仔(Ruditapes philippinarum)、缢蛏(Sinonovacula constricta)等贝类样品(采集于山东青岛胶南地区);扇贝标准物质GBW10024(国家标准物质中心);贻贝(Mytilus edulis)标准物质GBW0857 (国家海洋局第二海洋研究所);牡蛎(Ostrea gigas)标准物质ESA-2(中国环境监测总站);其他验证试剂均为分析纯;所用标准溶液均来自国家标准物质中心;实验用水为超纯水;载气及屏蔽气为Ar-H2 (9:1, V/V)混合气。
1.1.2 仪器设备DCD-200固体进样装置、AFS-8230原子荧光光度计(北京吉天仪器有限公司);AA800石墨炉原子吸收光度计(美国Perkin-Elmer公司);Milli-Q超纯水(密理博公司);微量移液器(德国Eppendorf公司);T18均质机(德国IKA集团);旋涡混匀器(德国IKA集团)。
1.2 实验方法 1.2.1 样品处理取新鲜贝类可食部分用均质机处理,称20 mg左右样品于进样舟中直接上机测量(视样品含量高低,用1%硝酸稀释上机)。
称取1.00 g标准物质用1%硝酸稀释至10 ml,充分混匀,吸取20 μl于进样舟中上机测定。
1.2.2 标准曲线的制备分别取50 μg/L Cd标准溶液0、2、4、6、8、10 μl,于石墨进样舟中测定其荧光值,以进样Cd的绝对含量(ng)与测定荧光值绘制标准曲线。
1.2.3 标准加入法样品系列制备取等量待测样品1 ml分装至5个试管中,分别吸取5 μg/ml Cd标准溶液0、5、10、15、20 μl于相应管中(对应加标量分别为0、25、50、75、100 ng),用1%硝酸稀释至2 ml,混匀,制备加标样品系列。
1.2.4 测定方法实验以1%硝酸溶液作为空白,进样10 μl于样品舟中上机检测,每个样品平行测定3次,取平均值。
1.2.5 仪器条件固体进样测Cd装置与电热蒸发原子荧光光谱仪联用(王昌钊等, 2012;王金玉等, 2013),原子荧光光谱仪工作参数见表 1。
|
|
表 1 原子荧光光谱仪工作参数 Table 1 The operating parameters of atomic fluorescence spectrometry (AFS) |
用于方法验证的石墨炉原子吸收光谱仪(GF-AAS)仪器条件参考GB/T5009.12-2003。
1.3 统计分析采用SPSS 18统计软件进行t检验分析,比较电热蒸发原子荧光法(ETV-AFS)和湿法消解法(GF-AAS)测定贝类样品中Cd含量结果的差异性。在95%的置信度下,如P>0.05,则说明两种方法之间不存在显著性差异。
2 结果与讨论 2.1 固体进样装置参数优化根据设备操作相关信息,通过添加0.1 ng Cd标准溶液,对灰化功率、灰化时间、蒸发功率、蒸发时间进行四因素三水平的正交实验,研究灰化和蒸发功率及时间对测定的影响。结果以测定的荧光响应值面积为判定依据,重复测定3次的相对标准偏差RSD值作为参考。温控程序的正交实验设计和结果分别如表 2、表 3所示。
|
|
表 2 温度程序正交实验设计 Table 2 The orthogonal experimental design of temperature programs |
|
|
表 3 温度程序控制的正交实验结果 Table 3 Orthogonal experimental results of temperature programs |
由正交实验L9(34)(表 3) 可知,RC > RD > RA > RB,说明C因素影响最大,其次为D、A、B因素,影响逐渐变小,即固体进样温控程序中影响测定结果的程序因素依次为蒸发功率 > 蒸发时间 > 灰化功率 > 灰化时间,根据Cd因素指标趋势得出最佳温控程序组合为C3D3A3B2。因此,实验最终确定灰化功率为100 W,灰化时间为50 s,蒸发功率为80 W,蒸发时间为40 s。按照最佳条件进行验证实验(表 4),重复3次,测得荧光面积为5.8×105 mV·s,RSD为3.7%。
|
|
表 4 固体进样装置分析过程 Table 4 Analytical procedures of the solid sampling device |
实验在最优条件下添加不同Cd标准溶液的多谱图如图 1所示。仪器在最适条件下,8 h内每隔1 h测量1次,每次平行测定3次,其测量值的相对标准偏差(RSD)为7.41%。测量含Cd 0.1 ng的RSD小于10%,测定稳定性较好。
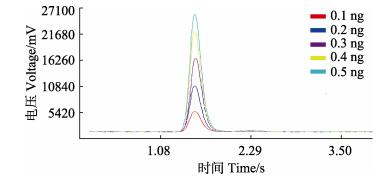
|
图 1 标准溶液光谱 Figure 1 The spectrogram of standard solutions |
基于鲜贝样品基体成分特点(李苹苹, 2014;俞佳锋等, 2014),实验以1 ng Cd为基准,通过添加一定量的有机成分(葡萄糖、蛋白胨、琼脂等用于验证糖类、蛋白等主要组分)和无机元素(Ca、Na、Zn、Se、Al、Pb、Hg、As等样品中的主要元素),验证基体干扰情况。实验结果如表 5所示,除NaCl干扰较大外,Cd回收率(n=3) 在99.3%–110.8%,干扰不显著。
|
|
表 5 干扰物对测量的影响 Table 5 The interferences of inorganic and organic materials |
贝类样品Cd含量较高且基体成分复杂,为保证实验测量精确度,需将样品稀释,同时为减少可能的干扰,通过稀释减少基体对测量的影响。研究发现,酸性条件有助于分解有机物、降低干扰。实验研究了常用的酸,包括硝酸、盐酸、高氯酸和硫酸,发现同浓度的硝酸介质在测定试样时荧光响应值及稳定性优于盐酸、硫酸和高氯酸(图 2)。进一步研究表明,样品加入1%硝酸效果较好,如图 3所示。因此,本方法选择1%硝酸作为样品稀释液。
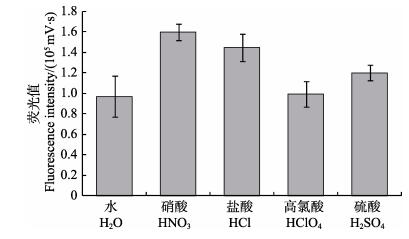
|
图 2 不同酸条件下的荧光值 Figure 2 Fluorescence intensities in different acid solutions |
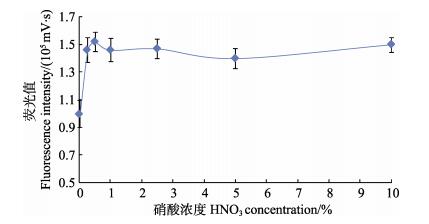
|
图 3 硝酸浓度对荧光值的影响 Figure 3 The effect of HNO3 concentration on fluorescence intensity |
Cd元素测定的常规方法是标准溶液曲线法(Shai et al, 1994),但对于固体进样方式,标准加入法是消除基质干扰的有效方法之一,因此应用更多(Pouny et al, 1992)。本研究采用扇贝标准物质作为基质添加标准溶液进行验证,两者无显著差异。图 4、图 5分别为标准溶液拟合标准曲线及扇贝标准物质的标准加入法拟合曲线,R2 > 0.995,相关性较好。
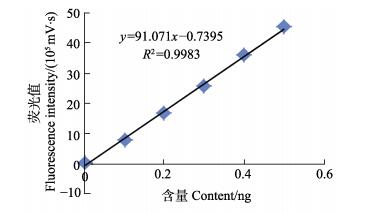
|
图 4 标准曲线法拟合曲线 Figure 4 The fitting curves of standard curve method |
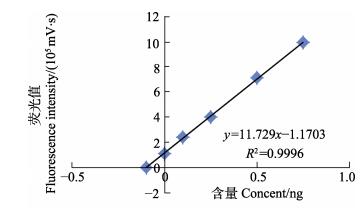
|
图 5 标准加入法拟合曲线 Figure 5 The fitting curves of standard addition method |
室温条件下,仪器在10 h内每隔1 h测量1次(n=3),其RSD为8.4%,说明仪器稳定性良好;对同一样品连续测定6次,其RSD在6.1%–12.1%之间。按IUPAC标准,以0.2 μg/L Cd标准溶液(n=20) 测得标准加入法和标准曲线法的方法检测限分别为0.6 pg和3 pg。
2.6.2 加标回收实验对于菲律宾蛤仔和扇贝样品分别添加一定量的Cd进行加标回收实验(n=3),结果显示(表 6),标准加入法加标回收率在96.4%–105.5%之间,RSD在6.5%–7.7%之间;标准曲线法加标回收率在98.1%–110.6%之间,RSD在3.3%–8.6%之间。说明标准加入法的准确度、精密度要优于标准曲线法。
|
|
表 6 不同测定方法的加标回收结果 Table 6 Recoveries of spiked Cd using different methods |
由表 7(n=3) 可以看出,标准曲线法测定标准物质中Cd含量与标准参考值相差较大,准确度低;而用标准加入法测定的标准物质Cd含量在标准参考值范围内,准确度较高。
|
|
表 7 不同方法标准物质验证结果 Table 7 Validation of Cd measurements using different methods |
对于扇贝、菲律宾蛤仔、缢蛏等贝类样品,应用标准加入法ETV-AFS与GF-AAS法进行比较(表 8),方差分析显示,检测结果无显著性差异(P > 0.05)。
|
|
表 8 ETV-AFS与GF-AAS测定值对比 Table 8 Comparison of ETV-AFSA and GF-AAS determinations (n=3) |
本研究确定了利用钨丝在线捕获的电热蒸发原子荧光光谱法测定贝类中Cd的最佳仪器条件,建立了适用于现场快速检测的Cd检测方法,可快速准确测定不同贝类样品中的Cd含量。研究发现,标准加入法和标准曲线法均能满足现场小批量检测的要求,样品前处理的分析时间可以控制在5 min之内,方法检出限分别为0.6 pg和3 pg。相对于标准曲线法,标准加入法具有更好的测定准确度和重现性,因此,建议采用标准加入法进行贝类中Cd的快速检测。
本方法无需消解,操作简便,检验时间短,可实现小批量样品的现场快速检测,为产地准出和市场准入提供了一种有效手段。
| Bu K, Cizdziel JV, Reidy L. Analysis of herbal supplements for selected dietary minerals and trace elements by laser ablation-and solution-based ICPMS. Microchemical Journal, 2013, 106: 244-249 DOI:10.1016/j.microc.2012.07.011 | |
| Cava-Montesinos P, Cervera ML, Pastor A, et al. Determination of As, Sb, Se, Te and Bi in milk by slurry sampling hydride generation atomic fluorescence spectrometry. Talanta, 2004, 62(1): 175-184 | |
| Chen GJ. Determination of 5 kinds of heavy metal elements in carbonated drinks by inductively coupled plasma mass spectrometry (ICP-MS). Agricultural Technology and Equipment, 2012, 11061106(11): 58-60 DOI:10.3969/j.issn.1674-1161.2012.11.024 [陈国娟. 电感耦合等离子体质谱法(ICP-MS)测定碳酸饮料中的5种重金属元素. 农业科技与装备, 2012, 11061106(11): 58-60] | |
| Cui ZG, Yu XZ, Cui Y, et al. Study on the accumulation and elimination of Pb and Cd in Patinopecten yessoensis. Progress in Fishery Sciences, 2015, 36(3): 116-124 DOI:10.11758/yykxjz.20150318 [崔正国, 苑旭洲, 崔毅, 等. 虾夷扇贝(Patinopecten yessoensis)对铅和镉的生物富集与释放规律. 渔业科学进展, 2015, 36(3): 116-124] | |
| Duarte AT, Dessuy MB, Vale MG, et al. Sequential determination of Cd and Cr in biomass samples and their ashes using high-resolution continuum source graphite furnace atomic absorption spectrometry and direct solid sample analysis. Talanta, 2013, 115(17): 55-60 | |
| Feng L, Cao XD, Chen HX, et al. Introduction of novel solid sampling atomic fluorescence spectrometry and in-field cadmium determination in rice sample. Modern Scientific Instruments, 2014, 11061106(1): 92-95 [冯礼, 曹小丹, 陈慧霞, 等. 固体进样原子荧光光度计现场、快速、准确测定大米中镉. 现代科学仪器, 2014, 11061106(1): 92-95] | |
| Feng L, Liu JX. Solid sampling graphite fibre felt electrothermal atomic fluorescence spectrometry with tungsten coil atomic trap for the determination of cadmium in food samples. Journal of Analytical Atomic Spectrometry, 2010, 25(7): 1072-1078 DOI:10.1039/b924270h | |
| GB/T 5009.12-2003.Determination of cadmium in foods.Beijing: National Standards of the People's Republic of China | |
| GB/T 5009. 12-2003. 食品中铅的测定. 北京: 中国标准出版社 | |
| Huang YT, Mao XF, Liu JX, et al. Direct determination of ultratrace cadmium in spinach by electrothermal vaporization atomic fluorescence spectrometry using on-line atom trap of tungsten coil. Chinese Journal of Analytical Chemistry, 2013, 41(10): 1587-1591 [黄亚涛, 毛雪飞, 刘霁欣, 等. 电热蒸发钨丝在线捕获原子荧光光谱法直接测定菠菜中痕量镉. 分析化学, 2013, 41(10): 1587-1591] | |
| Juranović CI, Krizman I, Zeiner M, et al. ICP-AES determination of minor-and major elements in apples after microwave assisted digestion. Food Chemistry, 2012, 135(4): 2675-2680 DOI:10.1016/j.foodchem.2012.07.051 | |
| Li L, Lei C, Wang FQ, et al. Determination of trace cadmium in drinking water by flame atomic absorption spectrometry. Food Industry Technology, 2011, 32(6): 401-403: 406 [李立, 雷超, 王芳权, 等. 碳纳米管富集-火焰原子吸收法测定饮用水中的微量镉. 食品工业科技, 2011, 32(6): 401-403: 406] | |
| Li PP. Nutrient components and protein quality analysis of five kinds of economic shellfish. Food Research and Development, 2014, 11061106(15): 99-101 DOI:10.3969/j.issn.1005-6521.2014.15.028 [李苹苹. 五种经济贝类的营养成分及蛋白质质量分析. 食品研究与开发, 2014, 11061106(15): 99-101] | |
| Mao XF, Liu JX, Huang YT, et al. Assessment of homogeneity and minimum sample mass for cadmium analysis in powdered certified reference materials and real rice samples by solid sampling electrothermal vaporization atomic fluorescence spectrometry. Journal of Agricultural and Food Chemistry, 2013, 61(4): 848-853 DOI:10.1021/jf3045473 | |
| Miranda K, Dionísio AGG, Neto ODP, et al. Determination of Cd levels in smoke condensate of Brazilian and Paraguayan cigarettes by thermospray flame furnace atomic absorption spectrometry (TS-FF-AAS). Microchemical Journal, 2012, 100(1): 27-30 | |
| O'Sullivan JE, Watson RJ, Butler EC. An ICP-MS procedure to determine Cd, Co, Cu, Ni, Pb and Zn in oceanic waters using in-line flow-injection with solid-phase extraction for preconcentration. Talanta, 2013, 115: 999-1010 DOI:10.1016/j.talanta.2013.06.054 | |
| Pouny Y, Rapaport D, Mor A, et al. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry, 1992, 31(49): 12416-12423 DOI:10.1021/bi00164a017 | |
| Shai Y. Pardaxin: Channel formation by a shark repellant peptide from fish. Toxicology, 1994, 87(13): 109-129 | |
| Trk P, Žemberyová M. Comparison of chemical modifiers for direct determination of Cd, Cu and Zn in food stuffs by solid-sampling-ETAAS. Food Chemistry, 2012, 132(1): 554-560 DOI:10.1016/j.foodchem.2011.10.068 | |
| Wang CZ, Feng L, Zheng FX, et al. Solid sampling atomic fluorescence spectrometry for measuring cadmium in apple and apple. Modern Scientific Instruments, 2012, 11061106(6): 144-146, 150 | |
| 王昌钊, 冯礼, 郑逢喜, 等. 固体进样原子荧光用于测量苹果及苹果粒中的镉. 现代科学仪器, 2012, 11061106(6): 144-146, 150 | |
| Wang JY, Huang YT, Mao XF, et al. Direct determination of trace cadmium in beverages by atomic fluorescence spectrometry of tungsten capture-electrothermal vaporization. Food Science, 2013, 34(24): 131-134 DOI:10.7506/spkx1002-6630-201324027 [王金玉, 黄亚涛, 毛雪飞, 等. 钨丝捕获-电热蒸发原子荧光光谱法直接测定饮料中痕量镉. 食品科学, 2013, 34(24): 131-134] | |
| Wang ZJ, Chen ZR. Determination of cadmium content in drinks by atomic fluorescence spectrometry. Beverage Industry, 2012, 15(5): 43-45 [王志嘉, 陈正荣. 原子荧光光谱法测定饮料中镉含量不确定度的分析. 饮料工业, 2012, 15(5): 43-45] | |
| Wen XD, Wu P, Chen L, et al. Determination of cadmium in rice and water by tungsten coil electrothermal vaporization-atomic fluorescence spectrometry and tungsten coil electrothermal atomic absorption spectrometry after cloud point extraction. Analytica Chimica Acta, 2009, 650(1): 33-38 DOI:10.1016/j.aca.2009.01.053 | |
| Wu CF, Liu H, Qin LX. Shanghai residents eat seafood probabilistic assessment of cadmium exposure. Environmental and Occupational Medicine, 2013, 30(2): 93-97 [吴春峰, 刘弘, 秦璐昕. 上海市居民食用水产品的镉暴露水平概率评估. 环境与职业学, 2013, 30(2): 93-97] | |
| Ye PL, Hu YF, Zhu HB, et al. Detection of lead, cadmium and chromium in instant tea by mixed matrix modifier-graphite furnace atomic absorption spectrometry. Journal of Food Safety and Quality, 2015, 869(5): 1597-1602 DOI:10.1016/j.aca.2015.03.010 [叶佩琳, 胡银凤, 朱宏斌, 等. 混合基体改进剂-石墨炉原子吸收光谱法测定速溶茶中的铅镉铬. 食品安全质量检测学报, 2015, 869(5): 1597-1602] | |
| Yu JF, Wang K, Xue JZ, et al. Determination and analysis of elements in standard samples of scallop by X-ray fluorescence spectrometry. Environmental Pollution and Control, 2014, 36(5): 40-42, 48 [俞佳锋, 王凯, 薛俊增, 等. 扇贝标准样品中元素的全反射X射线荧光光谱法测定分析. 环境污染与防治, 2014, 36(5): 40-42, 48] | |
| Zhang XH, Feng L, Lu D, et al. Solid sampling atomic fluorescence spectrometry for measurement of cadmium in agricultural products. Modern Scientific Instruments, 2012(6): 157-161 | |
| Zhang Y, Mao XF, Liu JX, et al. Direct determination of cadmium in foods by solid sampling electrothermal vaporization inductively coupled plasma mass spectrometry using a tungsten coil trap. Spectrochimica Acta Part B: Atomic Spectroscopy, 2016(118): 119-126 | |
| Zhang Y, Mao YF, Wang M, et al. Direct determination of cadmium in grain by solid sampling electrothermal vaporization atomic fluorescence spectrometry with a tungsten coil trap. Analytical Letters, 2015, 48(18): 2908-2920 DOI:10.1080/00032719.2015.1052976 | |
| Zhang Y, Wang JY, Mao XF, et al. Applications of solid sampling plasma spectrometric technology for elemental analysis in food. Food Industry Technology, 2015, 36(12): 353-357, 363 [张英, 王金玉, 毛雪飞, 等. 固体进样等离子体光谱技术在食品元素分析中的应用. 食品工业科技, 2015, 36(12): 353-357, 363] |



