2. 上海海洋大学水产与生命学院 上海 201306;
3. 青岛海洋科学与技术国家实验室海洋渔业科学与食物产出过程功能实验室 青岛 266071
2. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306;
3. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071
凡纳滨对虾(Litopenaeus vannamei)作为优良的养殖品种,20世纪80年代引入我国,现已成为我国主要的养殖虾类,在我国渔业经济中占有重要地位。2015年该品种养殖产量达162.5万t,占我国对虾总产量的80%以上(农业部渔业渔政管理局, 2016)。但同其他经济水生养殖动物一样,各类疫病频发的现状已严重威胁到包括凡纳滨对虾在内的对虾养殖业的可持续发展(Flegel, 2012; Thitamadee et al, 2016)。
2010年以来,早期死亡综合症(Early mortality syndrome, EMS)/急性肝胰腺坏死病(Acute hepatopancreatic necrosis disease, AHPND)在全球虾类主要养殖地区肆虐,给越南、中国、马来西亚、泰国、墨西哥和菲律宾等国对虾产业带来巨大损失(Tran et al, 2013; Joshi et al, 2014; Nunan et al, 2014; Dabu et al, 2017; de la Peña et al, 2015; Kongrueng et al, 2015; Soto-Rodriguez et al, 2015)。该疫病因在早期(投苗30 d左右)引起虾苗不寻常的高死亡率而被称之为EMS。2012年8月,亚太水产养殖中心网络(NACA,2012)针对感染该疫病的对虾肝胰腺盲管从中段到末端进行性变性,B、F和R细胞功能紊乱,部分细胞核膨大,肝胰腺盲管上皮细胞坏死或脱落,在后期肝胰腺盲管间或盲管内血细胞浸润,肝胰腺被细菌二次感染等组织病理学特征,正式命名该疫病为AHPND,以区别也可引起对虾早期死亡的其他疾病,但在该会上并未确定该疫病的致病原因。世界动物卫生组织(World Organisation for Animal Health, OIE)当前给出的AHPND定义也是参照上述的研究结果。目前认为对虾AHPND是由部分致病性弧菌所引起的,这些弧菌均携带着编码毒力蛋白PirVP的质粒。已报道的可引起对虾AHPND的弧菌种类包括副溶血弧菌(Vibrio parahaemolyticus)、哈维氏弧菌(Vibrio harveyi)、欧文斯氏弧菌(Vibrio owensii)和坎贝氏弧菌(Vibrio campbellii) (Lee et al, 2015; Kondo et al, 2015; Liu et al, 2015; Dong et al, 2017)。
2016年3月山东省潍坊市某凡纳滨对虾养殖场下塘38 d的虾苗(2~4 cm)出现大规模急性死亡,发病虾表现为空肠空胃,肝胰腺颜色变浅或发白,伴有肌肉轻微浑浊等症状,与AHPND临床特征相似(Tran et al, 2013)。以肝胰腺DNA为模板的病原检测中,AHPND致病相关的pirAVP和pirBVP基因的检测结果也呈阳性。以此为基础,本研究进行疑似AHPND感染对虾的细菌病原的分离鉴定及致病性分析,以期掌握该病原的基本生物学特征,为虾类AHPND病原的流行病学及其药物防控研究提供基础资料。
1 材料与方法 1.1 实验虾患病凡纳滨对虾于2016年3月采自山东省潍坊市某养殖场,体长约为2~4 cm。健康凡纳滨对虾购自山东省潍坊市另一养殖场,平均体长为(3.1±0.2) cm。健康对虾采用室内水箱(60 cm×30 cm×40 cm)养殖,实验期间,水温为(28±1)℃,正常投饵管理,24 h充气,每天吸污、换水1/2。实验前暂养1周。
1.2 细菌分离用无菌接种环蘸取患病虾肝胰腺样品,划线接种于添加了2% NaCl的TSA+固体平板(北京陆桥技术股份有限公司)上,28℃恒温培养,并对形态一致的优势菌进行纯化,菌株编号为20160303005-1。
1.3 菌株的API-20NE鉴定按照API-20NE鉴定试剂盒(梅里埃公司)说明书对菌株20160303005-1进行鉴定。
1.4 菌株的血清型鉴定按照血清检测试剂盒(天津生物芯片技术有限责任公司)说明书,对纯化的菌株20160303005-1进行O和K抗原血清型鉴定,该试剂盒含有O抗原(13种单价,1种多价)和K抗原(65种单价,9种多价)。
1.5 菌株特定基因的PCR扩增及测序以煮沸菌液的上清为模板,按Premix Ex Taq Version2.0(TaKaRa,大连)说明书,进行菌株各基因的PCR扩增,包括16S rRNA、分子伴侣蛋白groEL基因、与AHPND致病相关的pirAVP和pirBVP基因、耐热直接溶血毒素基因tdh和相对耐热直接溶血毒素基因trh。所用引物见表 1。25 μl的PCR反应体系包含Premix Ex Taq 12.5 μl,正、反向引物(10 μm/L)各0.5 μl,模板DNA 1 μl,ddH2O 10.5 μl。扩增条件为94℃预变性7 min;94℃变性40 s,适当温度退火30 s,72℃延伸一定时间,30个循环;72℃温育10 min。其中,16S rRNA基因退火温度为54℃,72℃延伸90 s;groEL退火温度为58℃,72℃延伸1 min;pirAVP和pirBVP基因退火温度为60℃,72℃延伸30 s。PCR产物用2%琼脂糖凝胶电泳分离,用胶回收试剂盒(OMEGA,美国)纯化,再用pEASY-T5克隆载体试剂盒(北京全式金生物技术有限公司)进行连接转化。阳性克隆送生工生物工程(上海)股份有限公司测序。采用NCBI中的BLAST工具进行核酸序列的比对分析,MEGA7软件用于基于邻接法(Neighbor-Joining, NJ法)的groEL基因的系统进化树的构建,并进行1000次的Bootstraps重复检验。
|
|
表 1 本实验所用的引物 Table 1 Primers used in this study |
健康凡纳滨对虾随机分成3个组(感染组、对照组和空白组),15尾/组,每组设3个平行。急性感染参照Tran等(2013)的方法。
感染组:将400 ml浓度为2.01×109 CFU/ml的菌液(含2% NaCl的TSB+液体培养基)加入到3.6 L海水中,充气;将实验对虾放入其中浸浴15 min后,再移入40 L添加了40 ml浓度为2.01×109 CFU/ml菌液的海水中,充气观察。
对照组:参照感染组,但养殖海水中仅添加无菌TSB+;空白组:对虾放入40 L海水中养殖。
取感染组的濒死虾及对照组和空白组虾的肝胰腺、胃、肠及鳃组织,置于Davidson’s AFA固定液中,固定24 h后更换为75%乙醇保存。按常规病理组织学方法制备石蜡切片,HE染色,镜检观察。
1.7 菌株的致病力分析健康凡纳滨对虾随机分成7个组(6个感染组和1个对照组),12尾/组,每组设3个平行。
感染组:将1.64×109 CFU/ml浓度的菌液离心并重悬于海水中,再10倍梯度稀释为1.64×102~ 1.64×107 CFU/ml的6个梯度。
对照组:对虾放入不含有菌液的海水中养殖。实验期间,对虾正常饲养管理,每天换水量为总水体的1/2,并相应补充菌液以维持海水中原菌液浓度。连续观察7 d,记录对虾死亡情况。根据不同细菌浓度浸泡下的实验虾的累积死亡数进行细菌致病力评估,采用Reed等(1938)方法计算LD50。
实验期间,随机取18尾濒死虾的肝胰腺,接种于TSA+平板,用其分离菌株为模板,进行pirAVP和pirBVP基因的PCR检测分析。
1.8 菌株的药敏性分析按照ATB G-5肠细菌药敏试剂盒(梅里埃公司)说明书,进行菌株的药物敏感性分析,该试剂盒含有阿莫西林等21种药物。
2 结果 2.1 菌株20160303005-1的API-20NE鉴定及理化特征API-20NE鉴定系统对菌株的理化指标检测结果如表 2所示,该鉴定结果显示,菌株20160303005-1为副溶血弧菌,其鉴定百分率值为99.1%。
|
|
表 2 菌株20160303005-1的理化特征 Table 2 Phenotypic characteristics of isolated 20160303005-1 |
基于16S rRNA基因序列分析结果显示,20160303005-1株属于弧菌属,与副溶血弧菌、溶藻弧菌(Vibrio alginolyticus)等弧菌的相似度超过99%,但其菌种无法确定。进一步对groEL基因进行序列分析,所构建系统进化树显示,该菌株与副溶血弧菌聚为一支(图 1)。上述结果再结合菌株的理化特征,将该分离菌株鉴定为副溶血弧菌。
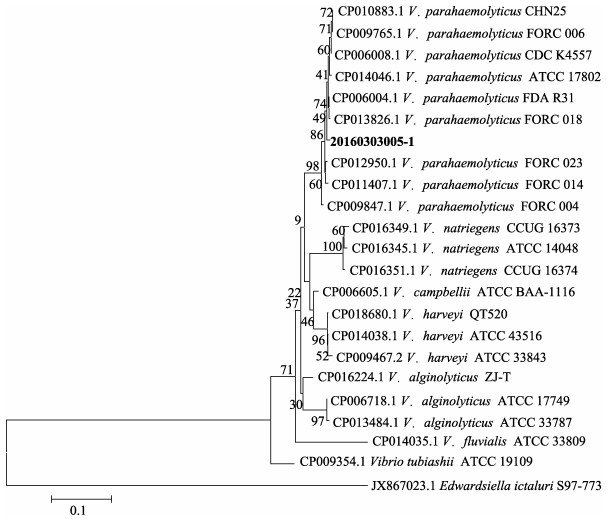
|
图 1 基于Neighbor-Joining方法的groEL基因系统进化树 Figure 1 The phylogenetic tree based on groEL gene sequences using the neighbour-joining method 1000次的Bootstraps重复检验,比例尺代表遗传距离 1000 bootstrap replicates, and scale bars represented distance values |
血清试剂盒检测结果显示,菌株20160303005-1的O抗原为O1型,但其K抗原不属于检测试剂盒的65种K抗原中的任何一种,即为不可分型(Untypeable)类,也即该菌株血清型为O1:KUT(K untypeable)。
2.4 致病基因的PCR扩增PCR检测结果显示,菌株20160303005-1的AHPND相关毒力蛋白pirAVP和pirBVP基因的PCR扩增结果均为阳性(图 2),其PCR扩增产物的核酸测序结果也与Han等(2015)研究中的相应序列完全一致(GenBank No. BAVF00000000.1);副溶血弧菌的临床致病基因tdh和trh基因的PCR扩增结果均为阴性。
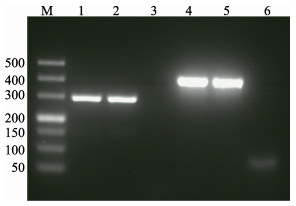
|
图 2 菌株20160303005-1的pirAVP和pirBVP基因的PCR扩增结果 Figure 2 PCR screening results of pirAVP and pirBVP genes from the isolated strain 20160303005-1 M:Marker DL500;泳道1~3和4~6为分别用pirAVP和pirBVP基因的引物的扩增产物。泳道1,2,4和5以菌液为模板,泳道3和6为阴性对照 M: Marker DL500. Lanes 1~3 and 4~6: PCR amplification used the primers of pirAVP and pirBVP gene, respectively. Lanes 1, 2, 4 and 5: Boiled cells were used as templates for PCR amplification. Lanes 3 and 6: Negative control |
急性感染的凡纳滨对虾,感染后3 h没有明显症状,但个别对虾开始死亡;6 h对虾出现空肠空胃,肝胰腺颜色变浅,死亡虾增多;9 h对虾肝胰腺呈浅白色并萎缩变小(图 3A),死亡数过半;12 h对虾整个身体几近透明,部分对虾甲壳变软;甲壳变软症状在18 h更为明显,24 h对虾死亡率达到100%。而对照组对虾(图 3B)摄食正常,活力良好,无死亡。
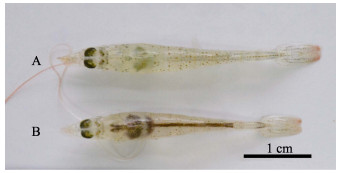
|
图 3 感染20160303005-1组与对照组凡纳滨对虾 Figure 3 The shrimp infected by the isolated strain 20160303005-1 and normal L. vannamei A:感染组虾(感染9 h);B:对照组虾 A: 20160303005-1 infected shrimp (9 h post-challenged); B: Normal shrimp in the control group |
对感染后12 h的濒死虾进行组织病理切片观察,与对照组(图 4A)相比,感染组凡纳滨对虾的肝胰腺小管上皮细胞严重萎缩,大量坏死、脱落,导致肝胰腺小管变薄、崩塌(图 4B),呈典型的AHPND病理特征。
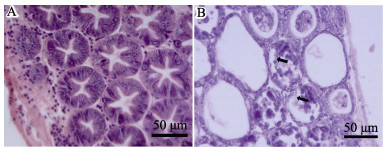
|
图 4 凡纳滨对虾的肝胰腺HE染色组织切片观察 Figure 4 HE-stained histological sections of the hepatopancreas of L. vannamei A:对照组;B:AHPND特征感染组(浸泡12 h后),箭头表示脱落的肝胰腺小管上皮细胞 A: Normal shrimp from the control group; B: Infected shrimp with the lesions characteristic of AHPND from the immersion treatments at 12 h post-challenged. Necrotic sloughing of hepatopancreas (HP) tubule epithelial cells (arrows) are shown in the HP tubule lumens |
用菌株20160303005-1浸泡感染凡纳滨对虾,各组的7天累积死亡情况如图 5所示,经计算,其LD50值为7.96×103 CFU/ml。以随机所取18尾濒死虾肝胰腺中分离的细菌为模板,pirAVP和pirBVP基因的PCR扩增结果均为阳性,该结果提示对虾死于该病原感染。实验期间,对照组对虾正常无死亡。
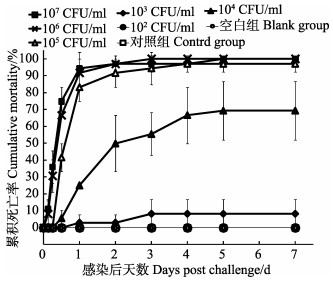
|
图 5 实验各组的累积死亡率(平均值±标准差) Figure 5 The cumulative mortality of L. vannamei in different treatments (Mean±SD) |
菌株20160303005-1的药敏结果见表 3。从表 3可以看出,菌株20160303005-1对庆大霉素、环丙沙星等16种药物敏感,但对阿莫西林、替卡西林、头孢噻吩、头孢呋辛、复方新诺明这5种药物表现为耐药。
|
|
表 3 菌株20160303005-1的药物敏感性 Table 3 Antimicrobial susceptibility testing of isolated 20160303005-1 |
AHPND是近年来导致全球对虾养殖大规模减产的重要病害之一,对其疫病病原的准确鉴定是进行后续病原防控的基础。本研究从疑似患AHPND临床症状的凡纳滨对虾中分离得到一株细菌,传统及分子鉴定的方法确定了该菌株为副溶血弧菌。鉴定中所选择的groEL基因的聚类分析结果也证实了该基因具有普遍性、保守性及种间变异性的优点,可作为弧菌属细菌分子鉴定的靶基因(Hossain et al, 2012; Hossain et al, 2013; Ahmed et al, 2016)。
病原菌的血清学特征可为流行病学调查及临床用药提供参考。目前,国际上副溶血弧菌多采用基于13种O抗原(菌体抗原)和71种K抗原(荚膜抗原)的血清型分型系统(Ishibashi et al, 2000)。我国临床副溶血弧菌的血清型主要为O3:K6、O4:K8、O4:K68和O1:K36等,而水产品中分离的副溶血弧菌主要为O2、O1和O11型等,基于K抗原自身的多样性,以及抗原检测方法多是依据临床菌株的原因,目前水产品中副溶血弧菌的K抗原很多都无法定型(K untypeable, KUT)(Chen et al, 2016; Xu et al, 2016)。本研究分离到的菌株20160303005-1的血清型为O1:KUT,这与Kongrueng等(2015)报道的可引起AHPND的副溶血弧菌泰国株的血清型一致。目前,我们正对近4年全国虾类流行病学调查中收集到的100多株副溶血弧菌的血清型进行分类鉴定工作,以期找到我国虾类副溶血弧菌致病株的血清型特征。
作为一种食源性致病菌,国内外均有副溶血弧菌引起食物中毒的报道。tdh和trh基因作为该病原的重要毒力分子标志基因,常用于人源副溶血弧菌致病菌的分子检测中(Honda et al, 1988)。目前包括本研究在内,国内水产品源副溶血弧菌的tdh和thr基因检测结果均为阴性。但国外已有虾源副溶血弧菌的tdh和thr基因检测结果为阳性的报道(Sujeewa et al, 2009; Rahimi et al, 2010),应当引起我国水产品质量监测部门的关注。
本研究分离到的副溶血弧菌菌株20160303005-1对凡纳滨对虾具有较强的致病力,浸泡感染实验中的LD50为7.96×103 CFU/ml。当菌液浓度为1.64×103 CFU/ml时,对虾开始出现死亡,且死亡率随着菌液浓度的提高而显著增加,当菌液浓度达到1.64×106 CFU/ml时,实验虾在感染第5天即达到100%的死亡率,这与Joshi(2014)和Soto-Rodriguez等(2015)的研究结果相似,即副溶血弧菌细菌剂量与对虾死亡率具有相关性,只有当致病副溶血弧菌浓度达到一定值时,才会导致对虾发病死亡。AHPND感染虾表现有明显的空肠空胃,肝胰腺发白等特征,病理学观察结果证实,该菌株可引起凡纳滨对虾的AHPND典型症状,诸如肝胰腺小管崩塌,上皮细胞严重脱落等(Tran et al, 2013; Joshi et al, 2014; Soto-Rodriguez et al, 2015)。Joshi等(2014)报道的该菌株AP2引物(http://www.enaca.org/modules/library/publication.php?Publication_id=1128)的检测结果为阳性,但该病原感染凡纳滨对虾后,不引起AHPND的肝胰腺小管上皮细胞严重坏死病变,却可引起小管上皮细胞的崩解,并在E细胞中形成特有的大量空泡等特征。提示OIE目前关于AHPND病理特征的界定尚待进一步明确。
目前,对虾AHPND的产生机制是本领域的研究热点。Lee等(2015)研究发现,VP-AHPND中均含有一个约69 kb的质粒,该质粒中携带有产生AHPND的2个相关毒力蛋白的pirAVP和pirBVP基因,蛋白结构及信息学分析发现,尽管2个蛋白与昆虫致病菌苏云金芽孢杆菌(Bacillus thuringiensis)分泌的1种Cry毒素蛋白(Cry insecticidal toxin-like protein)的基因序列同源性很低(< 10%),但却在结构上十分相似,提示两菌株间可能存在一定的关联性。Lee (2015)还发现,用重组表达的PirAVP+PirBVP及PirBVP蛋白均可引起AHPND的症状,据此推测pirBVP可能是引起AHPND的主要致病基因。Lai等(2015)采用免疫印渍法(Western blotting)和酶联免疫吸附实验(ELISA)的方法进行了VP-AHPND致病蛋白在对虾各组织的定位分析及病理学研究,其结果也提示了该病原的单独的PirBVP蛋白也可引起感染对虾AHPND的病理症状。不同于上述的Pir蛋白与AHPND直接相关的结果,Joshi等(2014)研究发现,携带该质粒却产生不同于AHPND典型病理特征的弧菌。因此,该2种Pir蛋白在AHPND的产生机制及致病中作用还有待深入研究。携带PirVP基因的质粒也存在水平转移的可能,继引起AHPND的副溶血弧菌报道之后,哈维氏弧菌、欧文斯氏弧菌和坎贝氏弧菌均带有该质粒的事实也提示了这种水平转移存在的可能性(Lee et al, 2015; Kondo et al, 2015; Liu et al, 2015; Dong et al, 2017),阐释该质粒/Pir致病基因的致病及其水平转移的机制,可为该病原的防控提供理论依据。
本研究分离菌株对阿莫西林等5种药物表现为耐药特征,较国内外副溶血弧菌不同株耐药谱不尽一致的结果,应主要与菌株分离来源及抗生素药物使用等因素有关,其中抗生素使用所造成的选择压力也客观上促进了耐药菌株的产生(Lai et al, 2015; Elmahdi et al, 2016; Chen et al, 2016; Bennett, 2008; Freel et al, 2013)。本研究分离的耐药菌株的结果也再次提醒当前水产菌株耐药及食品安全等问题的严重性。近年来,通过生物安保来实现动物疫病的预防与控制的目的已为健康养殖业所接受,联合国粮食及农业组织(FAO)和世界动物卫生组织(OIE)对生物安保的定义是为了降低病原对水产养殖系统的传入、定植和扩散的风险,在管理、技术和设施上执行的一整套措施(FAO渔业委员会水产养殖分委员会, 2010; OIE, 2015)。黄倢研究团队在OIE和国内生物安保概念的发展和应用推广中做出了引领性的工作,该理念所提倡的包括疫病防控在内的与水产养殖健康相关的各种措施的集成来保障水生生物的健康养殖(黄倢等, 2016),有望克服当前水产养殖业中,因针对各类病害被动防控的药物使用,所带来的诸如食品安全、环境恶化及耐药菌株等系列问题。相信水产生物安保的推广实施,可有效保障我国水产健康养殖业的可持续发展,也应成为今后水产养殖业健康发展的理论基础。
| Ahmed R, Rafiquzaman SM, Hossain MT, et al. Species-specific detection of Vibrio alginolyticus in shellfish and shrimp by real-time PCR using the groEL gene. Aquaculture International, 2016, 24(1): 157-170 DOI:10.1007/s10499-015-9916-5 | |
| Bennett PM. Plasmid encoded antibiotic resistance:Acquisition and transfer of antibiotic resistance genes in bacteria. British Journal of Pharmacology, 2008, 153(S1): S347-S357 | |
| Bureau of Fisheries and Fisheries Law Enforcement, Ministry of Agriculture. China fishery statistical yearbook. Beijing: China Agriculture Press, 2016: 28, 30, 71. [农业部渔业渔政管理局. 中国渔业统计年鉴. 北京: 中国农业出版社, 2016: 28, 30, 71.] | |
| Chen Y, Chen X, Yu F, et al. Serology, virulence, antimicrobial susceptibility and molecular characteristics of clinical Vibrio parahaemolyticus strains circulating in southeastern China from 2009 to 2013. Clinical Microbiology and Infection, 2016, 22(3): 258(e9-e16) | |
| COFI Sub-committee on Aquaculture. Aquatic biosecurity: A key for sustainable aquaculture development. Phuket: CoFI Sub-Committee on Aquaculture, 2010 [FAO渔业委员会水产养殖分委员会. 水生生物安保: 可持续水产养殖发展的一个关键. 普吉: FAO渔业委员会水产养殖分委员会, 2010] | |
| Dabu IM, Lim JJ, Arabit PMT, et al. The first record of acute hepatopancreatic necrosis disease in the Philippines. Aquaculture Research, 2017, 48(3): 792-7998 DOI:10.1111/are.2017.48.issue-3 | |
| de la Peña LD, Cabillon NA, Catedral DD, et al. Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeus vannamei and P. monodon cultured in the Philippines. Diseases of Aquatic Organisms, 2015, 116(3): 251-254 DOI:10.3354/dao02919 | |
| Dong X, Wang HL, Xie GS, et al. An isolate Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerging Microbes and Infections, 2017, 6(1): e2 DOI:10.1038/emi.2016.131 | |
| Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries:A review. Food Microbiology, 2016, 57: 128-134 DOI:10.1016/j.fm.2016.02.008 | |
| Flegel TW. Historic emergence, impact and current status of shrimp pathogens in Asia. Journal of Invertebrate Pathology, 2012, 110(2): 166-173 DOI:10.1016/j.jip.2012.03.004 | |
| Freel KC, Millán-Aguiñaga N, Jensen PR. Multilocus sequence typing reveals evidence of homologous recombination linked to antibiotic resistance in the genus Salinispora. Applied and Environmental Microbiology, 2013, 79(19): 5997-6005 DOI:10.1128/AEM.00880-13 | |
| Han JE, Tang KF, Tran LH, et al. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Diseases of Aquatic Organisms, 2015, 113(1): 33-40 DOI:10.3354/dao02830 | |
| Honda T, Yuxin NI, Miwatani T. Purification and characteriza-tion of a hemolysin produced by a clinical isolate of Kanagawa phenomenon-negative Vibrio parahaemolyticus and related to the thermostable direct hemolysin. Infection and Immunity, 1988, 56(4): 961-965 | |
| Hossain MT, Kim EY, Kim YR, et al. Application of groEL gene for the species-specific detection of Vibrio parahaemolyticus by PCR. Letters in Applied Microbiology, 2012, 54(1): 67-72 DOI:10.1111/lam.2011.54.issue-1 | |
| Hossain MT, Kim EY, Kim YR, et al. Development of a groEL gene-based species-specific multiplex polymerase chain reaction assay for simultaneous detection of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus. Journal of Applied Microbiology, 2013, 114(2): 448-456 | |
| Huang J, Zeng LB, Dong X, et al. Trend analysis and policy recommendation on aquatic biosecurity in China. Engineering Sciences, 2016, 18(3): 15-21 [黄倢, 曾令兵, 董宣, 等. 水产生物安保发展趋势与政策建议. 中国工程科学, 2016, 18(3): 15-21] | |
| Ishibashi M, Ohta K, Shimada T, et al. Current status of OK serotype combinations of Vibrio parahaemolyticus. Nippon Saikingaku Zasshi, 2000, 55: 539-541 DOI:10.3412/jsb.55.539 | |
| Joshi J, Srisala J, Truong VH, et al. Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND). Aquaculture, 2014, 428-429: 297-302 DOI:10.1016/j.aquaculture.2014.03.030 | |
| Kondo H, Van PT, Dang LT, et al. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announcements, 2015, 3(5): e00978-15 | |
| Kongrueng J, Yingkajorn M, Bunpa S, et al. Characterization of Vibrio parahaemolyticus causing acute hepatopancreatic necrosis disease in southern Thailand. Journal of Fish Diseases, 2015, 38(11): 957-966 DOI:10.1111/jfd.12308 | |
| Lai HC, Ng TH, Ando M, et al. Pathogenesis of acute hepatopancreatic necrosis disease (AHPND) in shrimp. Fish and Shellfish Immunology, 2015, 47(2): 1006-1014 | |
| Lee CT, Chen IT, Yang YT, et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(34): 10798-10803 DOI:10.1073/pnas.1503129112 | |
| Liu LY, Xiao JZ, Xia XM, et al. Draft genome sequence of Vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announcements, 2015, 3(6): e01395-15 | |
| NACA. Report of the Asia Pacific emergency regional consultation on the emerging shrimp disease: Early mortality syndrome (EMS)/acute hepatopancreatic necrosis syndrome (AHPNS). 2012 | |
| Nunan L, Lightner D, Pantoja C, et al. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Diseases of Aquatic Organisms, 2014, 111(1): 81-86 | |
| Rahimi E, Ameri M, Doosti A, et al. Occurrence of toxigenic Vibrio parahaemolyticus strains in shrimp in Iran. Foodborne Pathogens and Disease, 2010, 7(9): 1107-1111 | |
| Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. American Journal of Hygiene, 1938, 27(3): 493-497 | |
| Soto-Rodriguez SA, Gomez-Gil B, Lozano-Olvera R, et al. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico. Applied and Environmental Microbiology, 2015, 81(5): 1689-1699 | |
| Sujeewa AKW, Norrakiah AS, Laina M. Prevalence of toxic genes of Vibrio parahaemolyticus in shrimps (Penaeus monodon) and culture environment. International Food Research Journal, 2009, 16: 89-95 | |
| Tey YH, Jong KJ, Fen SY, et al. Genetic variation in Vibrio parahaemolyticus isolated from the aquacultural environ-ments. Letters in Applied Microbiology, 2015, 60(4): 321-327 | |
| Thitamadee S, Prachumwat A, Srisala J, et al. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture, 2016, 452: 69-87 | |
| Tran L, Nunan L, Redman RM, et al. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Diseases of Aquatic Organisms, 2013, 105(1): 45-55 | |
| World Organization for Animal Health (OIE). Aquatic animal health code. Paris: OIE, 2015 | |
| Xu X, Cheng J, Wu Q, et al. Prevalence, characterization, and antibiotic susceptibility of Vibrio parahaemolyticus isolated from retail aquatic products in North China. BMC Microbiology, 2016, 16: 32-41 |



