2. 内江师范学院生命科学院 内江 641100;
3. 重庆市梁平区水产站 重庆 405200
2. Department of Aquaculture, College of Life Sciences, Neijiang Normal University. Neijiang641100;
3. Fisheries Adminstration of Liangping, Chongqing405200
摄食是包括进食及觅食的复杂行为(Simpson et al, 2016),能为动物提供所必需的营养物质与能量,维持动物生长、繁殖等活动。与哺乳动物一样,鱼类摄食调控是一个复杂过程,由大脑神经中枢调控,通过接收和处理来自大脑和外周组织内分泌的因子进行调控(Volkoff, 2016)。迄今为止,在鱼类上克隆和鉴定了大量与摄食调控相关的因子,如Ghrelin (Zhou et al, 2014、2016)、Leptin(穆方申等, 2017)、CRH (Corticotropin-releasing hormone)(Wang et al, 2014),CCK (Cholecystokinin) (Zhang et al, 2017),NPY (Neuropeptide Y) (Han et al, 2014)等,然而,由于种间特异性,同一个基因在不同鱼类的摄食调控作用不尽相同(Liu et al, 2014; Jonsson et al, 2010; 卢荣华等, 2015; Zhang et al, 2013; Michel et al, 2016; Fuentes et al, 2012)。CRH是脊椎动物中一种含41个氨基酸的脑神经递质调节肽(Vale et al, 1981),具有调节神经内分泌、自主反馈应激等作用(Chrousos et al, 2017)。CRH可以影响哺乳动物的各种生理行为,如CRH对应激后小鼠行为具有一定调节作用(Fuzesi et al, 2016),CRH通过刺激POMC(Proopiomelanocortin)和ACTH (Adreno-cortico-tropic-hormone)的分泌,可以影响绵羊繁殖(周梅等, 2017),还具有维持能量平衡(Rabasa et al, 2016)及调控食欲(Rayatpour et al, 2017)等功能。鱼类CRH可以影响生长发育、调节能量(王宇等, 2014)、调控摄食(Matsuda, 2013)等。就摄食调控而言,目前已有实验探究了齐口裂腹鱼(Schizothorax prenanti) (Wang et al, 2014)、金鱼(Carassius auratus) (Matsuda et al, 2006) crh基因对食欲的调节作用,在其他鱼类上的研究还较为欠缺。
银鲫(Carassius auratus gibelio)是中国淡水养殖的重要品种之一,营养价值高、生长速度快(方旭等, 2017),在中国养殖范围广,市场需求旺盛,价格稳定,养殖效益明显。到目前为止,银鲫的摄食调控研究主要集中在饲料添加剂、环境调控等方面(张亚卓等, 2016; 尹子煜等, 2017),从分子层面上对银鲫摄食影响因子与摄食调控、能量代谢之间关系的研究较少。
该研究克隆了银鲫crh基因的cDNA全长序列,并进行了序列分析,同时利用实时荧光定量PCR技术,对crh基因在银鲫各组织及餐前、餐后和禁食状态下的相对表达量进行了研究,以期通过对crh基因结构与功能的探索,为建立合理的银鲫投饲策略和促银鲫快速生长提供参考资料。
1 材料与方法 1.1 实验动物及实验设计银鲫购于重庆荣昌双河鱼技中心,买回后放入西南大学荣昌校区实验基地10个鱼缸(110 cm×44 cm× 44 cm),暂养2周,水温为(23.0±2.0)℃,光照为自然光(12 h L:12 h D),每天09:00和17:00使用通威2.0饲料进行投喂,投喂量为鱼体重的2%,所有实验鱼在摄食和行为上无明显差异。
用于基因克隆的银鲫平均体重为(293.5±17.5) g,用于组织表达实验的银鲫平均体重为(316.33±11.7) g,雌雄比例均为1:1。
餐前、餐后实验。银鲫平均体重为(308.7±17.5) g,以17:00为采样时间节点0 h,分别在餐前3 h(-3 h)、餐前1 h(-1 h)、餐点(0 h)、餐后1 h(+1 h)、餐后3 h(+3 h)采取银鲫下丘脑组织,餐后1 h(+1 h),餐后3 h(+3 h)分别有1组禁食组,每组采样6个重复。
长时间禁食实验。银鲫平均体重为(458.33± 20.10) g,随机选5组鱼作为长时间禁食组,5组为正常投喂组,分别在禁食第1、第3、第5、第7天的17:30对禁食组和正常投喂组进行采样。第9天禁食组重新投食,并在第9、第11、第14天的17:30对复投喂组及正常投喂组进行采样(每组采样6个重复)。
1.2 主要试剂Primer Script® RT reagent kit、RNAiso Plus试剂、pMD19-T克隆载体、DNA Marker和Loading Buffer均购于大连宝生物工程公司。SMARTerTM RACE cDNA Amplification Kit购自Clontech公司。去离子水、DNA胶回收试剂盒、2×Taq PCR Master Mix及DH5α感受态细胞购于天根生化科技公司。X-gal、氨苄青霉素和IPTG购于生工生物工程(上海)股份有限公司。Gold View购自北京索莱宝科技公司。琼脂糖购自AITIresco公司。
1.3 组织采样及RNA提取crh基因cDNA克隆使用脑组织,组织表达实验采集了端脑、下丘脑、中脑、小脑、延脑、垂体、眼、心脏、肝胰脏、鳃、脾脏、前肠、中肠、后肠、头肾、中肾、红肌、白肌、皮肤、精巢及卵巢共21种组织样品。餐前、餐后和长时间禁食实验所取组织为下丘脑。采样时,使用MS-222将实验鱼麻醉,迅速取出所需组织,用0.6%生理盐水冲洗干净,立即用滤纸吸干生理盐水,再转移至液氮中冷冻,最后转入-80℃冰箱保存备用。取出-80℃冻存的样品,采用大连宝生物工程公司的RNAiso Plus试剂提取总RNA,测定其光密度(OD),将OD260 nm/OD280 nm在1.8~2.0的RNA置于-80℃备用。
1.4 银鲫crh基因的克隆从GenBank中下载硬骨鱼类crh基因序列进行比对,获得保守区一致性的序列,采用Premier 3.0设计PCR引物(表 1)。25 μl反应体系:12.5 μl的2×dNTP Mix (Invitrogen),上下游引物各1.25 μl,2.5 μl的cDNA及7.5 μl ddH2O(Invitrogen)。PCR反应参数:预变性94℃ 5 min,94℃变性30 min,58℃退火30 s,72℃延伸30 s,共35个循环,最后延伸72℃ 8 min。扩增后的目的条带经琼脂糖割胶回收,连接至pMD®19-T载体(TaKaRa, 大连),热激转化DH5α感受态细胞后均匀涂布在含氨苄西林(Amp+)的LB固体培养基上,置于37℃培养箱中过夜,将转化后形成的白色单菌落接种进2 ml含Amp+的LB培养基中,所有操作均按照快速抽提质粒的试剂盒说明书进行。酶切使用SalⅠ和BamHⅠ,取大约500 ml的经1%琼脂糖凝胶电泳鉴定后显示为阳性的克隆菌液送到大连宝生物工程公司测序。
|
|
表 1 实验所用引物 Tab.1 The primers used in this study |
根据已获得crh核心序列设计RACE的巢式引物:3' Race特异性引物和5' Race特异性引物(表 1)。参照Clontech公司的SMARTer RACE cDNA试剂盒说明书制备5' -RACE-Ready cDNA,采用Touchdown PCR反应进行3' 和5' 末端扩增,扩增产物经胶回收、连接、转化后,筛选阳性的克隆菌液送到大连宝生物工程公司测序。
1.5 基因序列分析运用BLAST(http://blast.ncbi.nlm.nih.gov/Blast.cgi)对cDNA测序结果进行同源性比较。使用SignalP Ver. 4.0(http://www.cbs.dtu.dk/services/SignalP/)确认信号肽分位点和成熟肽。运用ClustalW (http://www.ddbj.nig.ac.jp/search/clustalw-e.html)多重序列比对分析氨基酸序列。通过Mega 5.1,采用邻接法(Neighbour- joining,N-J)构建系统进化树,自展(Bootstrap)重复1000次。
1.6 实时荧光定量PCR通过实时荧光定量检测crh基因的组织表达谱、餐前餐后及禁食表达量。所有操作过程在CFX96 Real-Time PCR检测系统(Bio-Rad)上完成。荧光定量反应试剂采用SYBR Green (TaKaRa, 大连),20 μl反应体系:SYBR 10 μl;上下游引物各0.8 μl;无菌水6 μl;cDNA模板2 μl; RoxⅡ0.4 μl。反应参数:95℃预变性30 s,95℃变性5 s,60℃退火30 s,72℃延伸30 s,共45个循环。每个样品设置3个重复,内参基因为18S rRNA和β-actin,相对表达量通过公式E-ΔΔCt(E为扩增效率)计算。
1.7 数据统计及分析用平均值±标准误(Mean±SE)表示实验数据,并使用SPSS 21.0处理结果。用单因素方差分析法(One-way ANOVA)分析方差同质性检验后的数据,P < 0.05表示差异显著,P < 0.01表示差异极显著。
2 结果 2.1 银鲫crh基因的克隆及序列分析银鲫crh基因cDNA(登录号KY861383)全长920 bp,其中,5' -UTR为53 bp,3' -UTR为378 bp,开放阅读框(ORF)为489 bp。推导出银鲫CRH蛋白由162个氨基酸组成,其中包含24个氨基酸组成的信号肽、41个氨基酸构成的成熟肽和11个氨基酸构成的保守区(图 1)。通过氨基酸序列的多重比较分析发现,银鲫CRH的氨基酸序列与鲤科鱼类具有较高同源性,其中,与金鱼的同源性为99%,与鲤的同源性为96%,而与其他硬骨鱼及哺乳动物的相似性较低(图 2)。根据这些物种的氨基酸序列,使用MEGA 5.1软件,运用邻接法(N-J法)绘制系统进化树(图 3),结果显示,银鲫与金鱼亲缘关系很近,与鲤科鱼同源性最高,单独聚为一类,与其他硬骨鱼类的联系紧密,但与哺乳动物则相对较远。
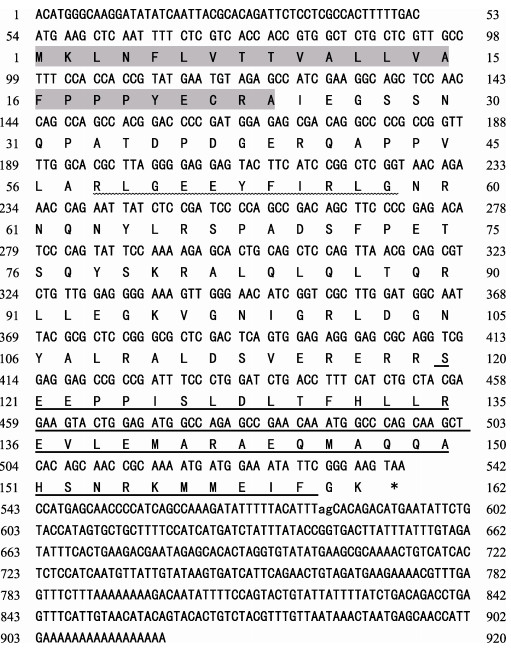
|
图 1 银鲫crh基因cDNA序列及推导的氨基酸序列 Fig.1 Nucleotide and predicted amino acid sequences ofC. auratus gibelio crh gene 阴影部分表示信号肽区域。成熟肽氨基酸编码区用下划线,保守区用双下划线表示,*表示终止密码子 The signal peptide region is in in shade background. Mature peptide amino acid coding region is underlined. The conserved region in the cryptic peptide is shown in double underlined. The asterisk indicates the stop codon |
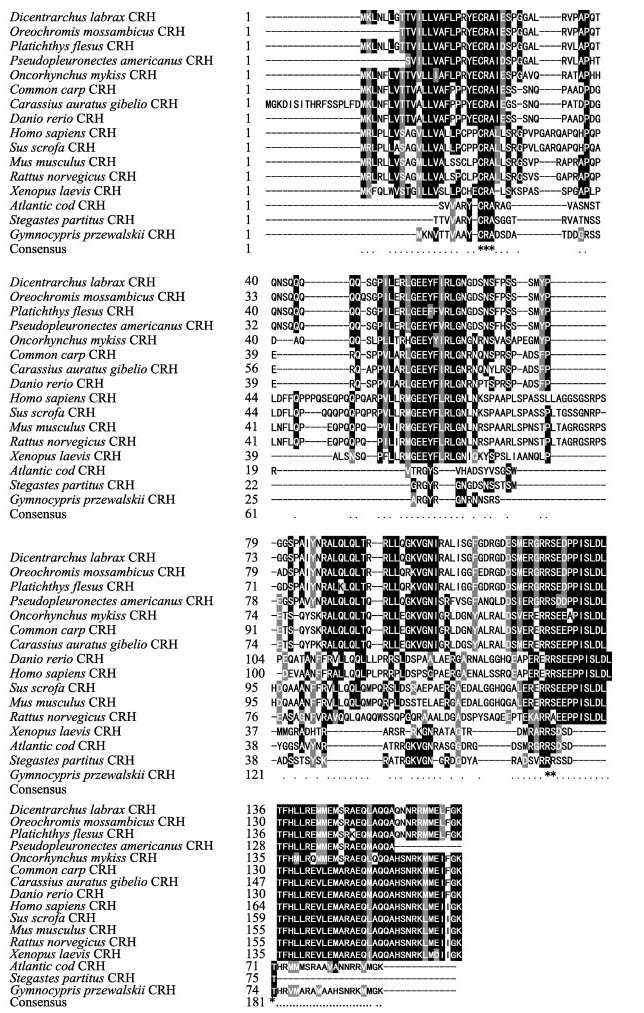
|
图 2 不同物种crh基因的氨基酸序列多重比较 Fig.2 Amino acid sequences alignment of crh gene 阴影表示列表中超过一半的多肽残基序列为保守序列。“*”表示完全一致的氨基酸序列 More than half of the peptide residue sequences in the shading list are conserved sequences.The asterisk indicates that the amino acid sequence is exactly the same |
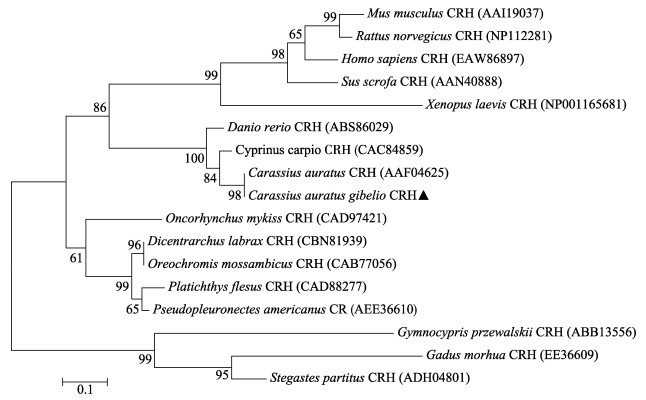
|
图 3 CRH的氨基酸序列系统进化分析 Fig.3 The phylogenetic analysis of the amino acid sequences of crh gene from C. auratus gibelio 每个残基替代率由比例尺表示,步长值由节点上的数字表示 Scale bar indicates residue replacement rate. The bootstrap value is represented by the numbers at the node |
如图 4所示,在银鲫的端脑、下丘脑、中脑、小脑、延脑、垂体、心脏、脾脏、肝胰脏、皮肤、眼、鳃、前肠、中肠、后肠、头肾、中肾、红肌、白肌、精巢、卵巢中均检测到了crh基因的表达,其中,下丘脑里的表达量最高,端脑和心脏次之,其余组织相对表达量较低。
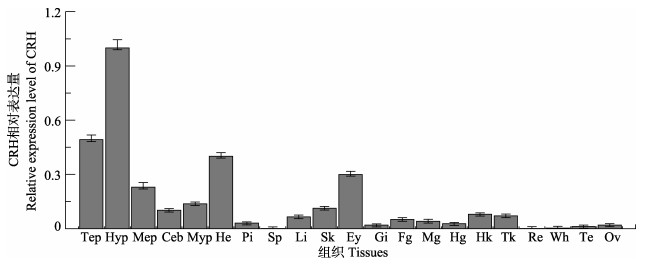
|
图 4 银鲫crh基因的组织分布 Fig.4 Tissue distribution of crh gene in C. auratus gibelio Tep:端脑; Hyp:下丘脑; Mep:中脑; Ceb:小脑; Myp:延脑; Pi:垂体; He:心脏; Sp:脾脏; Li:肝胰脏; Sk:皮肤; Ey:眼; Gi:鳃; Fg:前肠; Mg:中肠; Hg:后肠; Hk:头肾; Tk:中肾; Re:红肌; Wh:白肌; Te:精巢; Ov:卵巢 Tep: Telencephalon; Hyp: Hypothalamus; Mep: Mesencephalon; Ceb: Cerebellum; Myp: Myelencephalon; Pi: Pituitary; He: Heart; Sp: Spleen; Li: Liver; Sk: Skin; Ey: Eye; Gi: Gill; Fg: Foregut; Mg: Midgut; Hg: Hindgut; Hk: Head kidney; Tk: Trunk kidney; Re: Red muscle; Wh: White muscle; Te: Testis; Ov: Ovary |
结果显示,从餐前3 h至餐点0 h,银鲫下丘脑中crh基因的表达量逐渐降低,但差异不显著(P > 0.05)。与禁食组相比,餐后1 h投喂组下丘脑中crh基因表达量明显升高,但未达到统计学差异显著水平(P > 0.05);在餐后3 h投喂组下丘脑中,crh基因表达量又降低到与禁食组基本一致(P > 0.05)(图 5)。
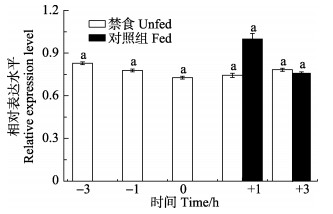
|
图 5 餐前餐后银鲫下丘脑中crh基因的表达情况 Fig.5 Postprandial changes of the crh gene expression in the hypothalamus of C. auratus gibelio 图柱字母表示禁食组和投喂组(+1 h、+3 h)之间差异显著性 The fed and unfed groups that differ significantly are indicated by different letters above bars |
从禁食第1~7天,禁食组下丘脑的crh基因表达量呈总体下降趋势,第5、第7天禁食组下丘脑crh基因表达量极显著低于正常投喂组(P < 0.01)。在第9、第11天复投喂后,实验组下丘脑crh基因表达量大幅上升,极显著高于正常投喂组(P < 0.01)(图 6)。
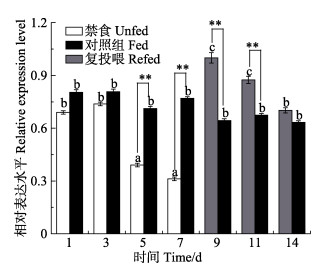
|
图 6 禁食和复投喂对银鲫下丘脑crh基因表达的影响 Fig.6 Effects of fasting and refeeding on crh gene expression in C. auratus gibelio hypothalamus 图柱上的字母表示禁食组、正常投喂组及复投喂组之间的差异显著性;“*”表示同一时间实验组和正常投喂组间的差异显著性。*: P < 0.05,**: P < 0.01 Different letters on the bar represent significant differences between experimental groups. "*" represents significant differences between the experimental groups at the sametime point. *: P < 0.05, **: P < 0.01 |
该研究首次克隆了银鲫crh基因cDNA的全长序列,鉴定出crh基因的1种亚型。同样,在齐口裂腹鱼(Wang et al, 2014)、刀鲚(Coilia nasus)(王宇等, 2014)和黄斑篮子鱼(Siganus canaliculatus)(游翠红等, 2014)中也只鉴定出crh基因的1种亚型,但有趣的是,在草鱼(殷新平, 2015)、虹鳟(Oncorhynchus mykiss) (Doyon et al, 2006)和鲤(Huising et al, 2004)中鉴定出crh基因的2种亚型,可能与鱼类基因组复制相关(Meyer et al, 2005; Sundstrom et al, 2008)。这些结果表明,硬骨鱼祖先额外的第3次全基因组复制导致的crh基因加倍后,部分硬骨鱼可能丢失了其中1个拷贝,而有些硬骨鱼在进化过程中可能保留或从其他途径获得2个拷贝,可能是导致目前观察到硬骨鱼基因组crh基因亚型个数多样性的重要原因。crh基因进化可能在不同硬骨鱼进化中发挥非常大的作用,但其加倍后在硬骨鱼基因组产生多个亚型的具体机制以及各自生物学功能还有待深入研究。通过氨基酸序列结构的比较可知,银鲫crh基因的信号肽和成熟肽均较保守。通过对系统进化树分析发现,银鲫和金鱼亲缘关系很近,与其他硬骨鱼类的联系紧密,但与哺乳动物则相对较远。
3.2 银鲫crh基因组织表达的分析本实验使用RT-qPCR检测了银鲫crh基因在端脑、下丘脑、中脑等21个组织中的表达情况,结果显示,在银鲫下丘脑、心脏、肝脏等21个组织中均检测到crh基因表达,其中,crh基因在下丘脑里表达量最高。此外,crh基因的表达产物也广泛分布在褐牙鲆(Paralichthys olivaceus)(沈娇娇等, 2017)、鲤(Mazon et al, 2006; Huising et al, 2007)、草鱼(Ctenopharynodon idellus)(殷新平, 2015)的各个组织中,脑部表达量最高。此外,在团头鲂(Megalobrama amblycephala)(陈凯等, 2016),斑马鱼(Chandrasekar et al, 2010)、金鱼(Bernier et al, 1999)、比目鱼(Pleuronichthys cornutus)(Lu et al, 2004)的各组织中,crh基因在脑部的表达量最高。在脊椎动物上已证实,下丘脑神经元回路对摄食行为和能量的调节起着重要作用(王国文等, 2016),并且Wang等(2014)对齐口裂腹鱼摄食调控的研究发现,crh基因也在脑部的表达量最高。上述结果推测crh基因可能通过下丘脑对银鲫的摄食行为进行调控。此外,在外周组织如肝胰脏和精巢、卵巢中检测到crh基因的表达,说明crh基因可能参与到免疫应答(Vamvakopoulos et al, 2013; Tort, 2011)及繁殖过程(Jeong et al, 1999; Chabbi et al, 2014),具体机制还有待进一步研究。
3.3 银鲫crh基因对摄食调控的影响摄食调控是一个复杂的生理过程,涉及中枢神经系统与外周神经系统相互作用,以及内分泌系统与消化之间的相互作用(Matsuda et al, 2007)。调控鱼类摄食行为因子的表达受摄食状态的影响,如饱食、饥饿及限制摄食等(Hoskins et al, 2012)。在哺乳动物与非哺乳动物中,crh基因已被证明具有调节摄食行为的作用(Rayatpour et al, 2017; Volkoff, 2016)。本研究中,餐后1 h与禁食组和餐前相比,进食组下丘脑crh基因的表达量升高,但差异不显著(P > 0.05)。同样,对齐口裂腹鱼的短期禁食发现,齐口裂腹鱼下丘脑中crh基因的表达量不受短期禁食影响(Wang et al, 2014),推测在银鲫中crh基因的表达量不受短期禁食的影响。
在长时间禁食实验中,第1、第3天禁食组的表达量低于正常投喂组,但差异不显著(P > 0.05),第5、第7天禁食组crh基因表达量与正常投喂组相比,出现极显著下降(P < 0.01)。第9天,复投喂组crh基因表达量与正常投喂组相比,出现极显著升高(P < 0.01)。在齐口裂腹鱼的禁食实验中也得到相似结果(Wang et al, 2014),在第1、第3、第5天禁食组的crh基因表达量与对照组没有差异,第7天禁食组crh基因的表达量显著低于进食组。在金鱼中,长时间禁食会引起crh基因表达量的降低,复投喂后又显著增加(Matsuda et al, 2006)。根据以上结果推测,crh基因可能作为一个饱感因子,参与银鲫的摄食调控,并且受不同禁食时间的影响,crh基因表达量受长时间禁食影响,而不受短期禁食调控。此外,Maruyama等(2006)发现,给金鱼脑室注射CRH后,金鱼摄食能力降低,活动能力有所提升;此外,给金鱼注射哺乳动物的CRH也会抑制其食欲(Matsuda et al, 2013),说明鱼类和哺乳动物crh基因在摄食调控中具有相似的生物学功能,还有实验证明鱼类食欲可以被环境(秦希获等, 2017)、生理应激(穆小生, 2013)等抑制。Graf等(2012)证明,由应激引起的食欲抑制效应与crh基因及其相关肽表达量升高,和下丘脑-垂体-肾上腺(HPI)应激轴激活紧密相关,由于crh基因在下丘脑-垂体-肾上腺的应激过程中扮演着重要调节作用(殷新平等, 2014),因此,银鲫的crh基因可能通过激活下丘脑-垂体-肾上腺应激轴参与银鲫的摄食调控。
综上所述,该研究确定了银鲫crh基因的cDNA序列,并对其进行了分析。crh基因广泛表达在银鲫各组织中,下丘脑中表达量最高,且下丘脑中crh基因的表达量受长期禁食的影响,这些结果提示crh基因可能是银鲫的饱感因子,对银鲫摄食调控起到一定的作用,但具体机制有待进一步研究。
Bernier NJ, Lin X, Peter RE. Differential expression of corticotropin-releasing factor (CRF) and urotensin I precursor genes, and evidence of CRF gene expression regulated by cortisol in goldfish brain. General and Comparative Endocrinology, 1999, 116(3): 461-477 DOI:10.1006/gcen.1999.7386 |
Chabbi A, Ganesh CB. Glucocorticoid synthesis inhibitor metyrapone blocks stress-induced suppression along luteinizing hormone secreting cells-ovary axis in the fish Oreochromis mossambicus. Journal of Experimental Zoology Part A Ecological and Integrative Physiology, 2014, 321(3): 125-134 |
Chandrasekar G, Lauterg, Hauptmann G. Distribution of corticotropin-releasing hormone in the developing zebrafish brain. Journal of Comparative Neurology, 2010, 505(4): 337-351 |
Chen K, Xi BW, Teng T, et al. Molecular cloning, characterization, and regulation of Megalobrama amblycephala corticotropin-releasing hormone receptor expression. Journal of Fishery Sciences of China, 2016, 23(4): 833-845 [ 陈凯, 习丙文, 滕涛, 等. 团头鲂促肾上腺皮质激素释放激素受体基因序列特征及表达分析. 中国水产科学, 2016, 23(4): 833-845] |
Chrousos GP, Zoumakis E. Milestones in CRH Research. Current Molecular Pharmacology, 2017, 10(4): 259 |
Doyon C, Leclair J, Trudeau VL, et al. Corticotropin-releasing factor and neuropeptide Y mRNA levels are modified by glucocorticoids in rainbow trout, Oncorhynchus mykiss. General and Comparative Endocrinology, 2006, 146(2): 126-135 |
Fang X, Teng SQ, Yang JL, et al. Pond high-yield, efficient and healthy aquaculture technology of Carassius auratus gibelio. China Fisheries, 2017(3): 91-92 [ 方旭, 滕淑芹, 杨建利, 等. " 中科3号" 异育银鲫池塘高产高效健康养殖技术. 中国水产, 2017(3): 91-92] |
Fuentes EN, Kling P, Einarsdotti IE, et al. Plasma leptin and growth hormone levels in the fine flounder (Paralichthys adspersus) increase gradually during fasting and decline rapidly after refeeding. General and Comparative Endocrinology, 2012, 177(1): 120 |
Fuzesi T, Daviu N, Wamsteeker CJI, et al. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nature Communications, 2016, 7: 11937 DOI:10.1038/ncomms11937 |
Graf C, Kuehne C, Panhuysen M, et al. Corticotropin-releasing hormone regulates common target genes with divergent functions in corticotrope and neuronal cells. Molecular and Cellular Endocrinology, 2012, 362(1-2): 29-38 DOI:10.1016/j.mce.2012.05.007 |
Han J, Liu B, Jiang Z, et al. cDNA cloning of neuropeptide Y gene of turbot and its expression analysis for the strategy of starvation and feeding in industrial culture. Engineering Sciences, 2014, 09 |
Hoskins LJ, Volkoff H. The comparative endocrinology of feeding in fish: Insights and challenges. General and Comparative Endocrinology, 2012, 176(3): 327-335 |
Huising MO, Lm VDA, Metz JR, et al. Corticotropin-releasing factor (CRF) and CRF-binding protein expression in and release from the head kidney of common carp: evolutionary conservation of the adrenal CRF system. Journal of Endocrinology, 2007, 193(3): 349-357 |
Huising MO, Metz JR, Van SC, et al. Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response. Journal of Molecular Endocrinology, 2004, 32(3): 627-648 |
Jeong KH, Jacobson L, Widmaier EP, et al. Normal suppression of the reproductive axis following stress in corticotropin- releasing hormone-deficient mice. Endocrinology, 1999, 140(4): 1702-1708 |
Jonsson E, Kaiya H, Bjornsson BT. Ghrelin decreases food intake in juvenile rainbow trout (Oncorhynchus mykiss) through the central anorexigenic corticotropin-releasing factor system. General and Comparative Endocrinology, 2010, 166(1): 39 |
Liu L, Liang XF, Li J, et al. Feed intake, feed utilization and feeding-related gene expression response to dietary phytic acid for juvenile grass carp (Ctenopharyngodon idellus). Aquaculture, 2014, 424-425(2): 201-206 |
Lu RH, Sun JJ, Liang XF, et al. Biological characteristics and functions of Leptin in fish. Acta Hydrobiologica Sinica, 2015, 39(3): 583-589 [ 卢荣华, 孙君君, 梁旭方, 等. 鱼类leptin的生物学特性及功能. 水生生物学报, 2015, 39(3): 583-589] |
Lu W, Dow L, Gumusgoz S, et al. Coexpression of corticotropin-releasing hormone and urotensin i precursor genes in the caudal neurosecretory system of the euryhaline flounder (Platichthys flesus): A possible shared role in peripheral regulation. Endocrinology, 2004, 145(12): 5786 DOI:10.1210/en.2004-0144 |
Matsuda K, Hagiwara Y, Shibata H, et al. Ovine corticotropin- releasing hormone (oCRH) exerts an anxiogenic-like action in the goldfish, Carassius auratus. General and Comparative Endocrinology, 2013, 188(1): 118-122 |
Matsuda K, Maruyama K. Regulation of feeding behavior by pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal polypeptide (VIP) in vertebrates. Peptides, 2007, 28(9): 1761-1766 DOI:10.1016/j.peptides.2007.03.007 |
Matsuda K, Miura T, Uchiyama M, et al. Relationship between anorexigenic action of pituitary adenylate cyclase-activating polypeptide (PACAP) and that of corticotropin-releasing hormone (CRH) in the goldfish (Carassius auratus). Peptides, 2006, 27(7): 1820-1826 DOI:10.1016/j.peptides.2006.01.013 |
Matsuda K. Regulation of feeding behavior and psychomotor activity by corticotropin-releasing hormone (CRH) in fish. Frontiers in Neuroscience, 2013, 7(7): 91 |
Mazon AF, Verburg-van Kemenade BM, Flik G, et al. Corticotropin-releasing hormone-receptor 1 (CRH-R1) and CRH-binding protein (CRH-BP) are expressed in the gills and skin of common carp Cyprinus carpio L. and respond to acute stress and infection. Journal of Experimental Biology, 2006, 209(3): 510-517 |
Meyer A, Vav DPY. From 2R to 3R: Evidence for a fish-specific genome duplication (FSGD). Bioessays, 2005, 27(9): 937-945 DOI:10.1002/(ISSN)1521-1878 |
Michel M, Page-Mccaw PS, Chen W, et al. Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(11): 3084 DOI:10.1073/pnas.1513212113 |
Mu FS, Miao L, Li MY, et al. Cloning and expression of Leptin gene in Acrossocheilus fasciatus fasciatus during fasting and refeeding. Oceanologia et Limnologia Sinica, 2017, 48(4): 822-829 [ 穆方申, 苗亮, 李明云, 等. 光唇鱼(Acrossocheilus fasciatus) Leptin基因克隆及禁食-再投喂对其表达的影响. 海洋与湖沼, 2017, 48(4): 822-829] |
Mu XS. Gene cloning, specific expression in different tissues and recombinant protein preparation of 4 biomarkers in Scophthalmus maximus. Masterxs Thesis of Ocean University of China, 2013, 91-92 [穆小生.大菱鲆4种生物标志物基因的克隆、组织特异性表达研究及重组蛋白制备.中国海洋大学硕士研究生学位论文, 2013, 91-92] http://cdmd.cnki.com.cn/Article/CDMD-10423-1013369173.htm
|
Qin XH, Wu YJ, Shi XT, et al. Effects of light intensity on the hatching rate of fertilized Schizothorax chongi eggs and on the growth and feeding of larvae. Journal of Hydroecology, 2017, 38(5): 97-102 [ 秦希获, 邬玉娇, 石小涛, 等. 光照对细鳞裂腹鱼受精卵孵化率及仔鱼生长、摄食的影响. 水生态学杂志, 2017, 38(5): 97-102] |
Rabasa C, Dickson S L. Impact of stress on metabolism and energy balance. Current Opinion in Behavioral Sciences, 2016, 9(9): 71-77 |
Rayatpour A, Ghasemi M, Radahmadi M, et al. Effect of intraparaventricular administration of corticotropin releasing hormone on food intake in food-deprived rats. Journal of Isfahan Medical School, 2017, 35(436): 70-775 |
Shen JJ, Lü WQ. Effects of thyroid hormone on CRH gene expression in Paralichthys olivaceus at early development stage. Journal of Southern Agriculture, 2017, 48(2): 323-327 [ 沈娇娇, 吕为群. 褐牙鲆早期发育阶段CRH基因表达及受甲状腺激素的影响. 南方农业学报, 2017, 48(2): 323-327] |
Simpson EH, Balsam PD. Behavioral neuroscience of motivation. Springer International Publishing., 2016, 1-12 |
Sundstrom G, Larsson TA, Brenner S, et al. Evolution of the neuropeptide Y family: New genes by chromosome duplications in early vertebrates and in teleost fishes. General and Comparative Endocrinology, 2008, 155(3): 705 DOI:10.1016/j.ygcen.2007.08.016 |
Tort L. Stress and immune modulation in fish. Developmental and Comparative Immunology, 2011, 35(12): 1366 DOI:10.1016/j.dci.2011.07.002 |
Vale W, Spiess J, Rivier C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science, 1981, 213(4514): 1394 DOI:10.1126/science.6267699 |
Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Journal of Clinical Investigation, 2013, 92(4): 1896 |
Volkoff H. The neuroendocrine regulation of food intake in fish: A review of current knowledge. Frontiers in Neuroscience, 2016, 10: 540 |
Wang GW, Shao BP, Ding YP. The orexigenic role and mechanism of neuropeptide Y. Chinese Journal of Animal Nutrition, 2016(2): 385-391 [ 王国文, 邵宝平, 丁艳平. 神经肽Y的促摄食作用及其调控机制. 动物营养学报, 2016(2): 385-391 DOI:10.3969/j.issn.1006-267x.2016.02.011] |
Wang T, Zhou C, Yuan D, et al. Schizothorax prenanti, corticotropin-releasing hormone (CRH): Molecular cloning, tissue expression, and the function of feeding regulation. Fish Physiology and Biochemistry, 2014, 40(5): 1407-1415 DOI:10.1007/s10695-014-9935-6 |
Wang Y, Lu DQ, Li WP, et al. The effect of acute handling stress on the stress-related neuroendocrine factor in Coilia nasus. Journal of Fisheries of China, 2014, 38(6): 803-812 [ 王宇, 卢丹琪, 李伟萍, 等. 急性操作胁迫对刀鲚应激反应相关神经内分泌因子的影响. 水产学报, 2014, 38(6): 803-812] |
Yin XP, Su JM, Pu L, et al. Research on the effect of corticotropin-releasing hormone in stress reaction in the fishes. Chinese Journal of Fisheries, 2014(3): 57-61 [ 殷新平, 苏建明, 蒲龄, 等. 促肾上腺皮质激素释放激素在鱼类应激反应中的作用. 水产学杂志, 2014(3): 57-61 DOI:10.3969/j.issn.1005-3832.2014.03.013] |
Yin XP. Cloning and expression of CRH, CRH-R1 from Ctenopharyngodon idella. Masterxs Thesis of Hunan Agricultural University, 2015, 42-43 [殷新平.草鱼CRH、CRH-R1基因的全长克隆及表达分析.湖南农业大学硕士研究生学位论文, 2015, 42-43] http://cdmd.cnki.com.cn/Article/CDMD-10537-1017033736.htm
|
Yin ZY, Xue M, Zheng YH, et al. Effect of yeast extract as feeding stimulants on diet preference of Gibel carp. Feed Industry, 2017, 38(8): 4-8 [ 尹子煜, 薛敏, 郑银桦, 等. 酵母提取物在异育银鲫饲料中促摄食作用的研究. 饲料工业, 2017, 38(8): 4-8] |
You CH, Lu YB, Wang SQ, et al. Cloning of gene encoding corticotrophin-releasing hormone (CRH) and its mRNA expression after shallow water stress in Siganus canaliculatus. Journal of Shantou University (Natural Science), 2014, 29(2): 43-51 [ 游翠红, 卢玉标, 王树启, 等. 黄斑蓝子鱼CRH基因的克隆及其在浅水应激后的mRNA表达. 汕头大学学报(自然科学版), 2014, 29(2): 43-51] |
Zhang H, Chen H, Zhang Y, et al. Molecular cloning, characterization and expression profiles of multiple leptin genes and a leptin receptor gene in orange-spotted grouper (Epinephelus coioides). General and Comparative Endocrinology, 2013, 181(1): 295-305 |
Zhang X, Tang N, Qi J, et al. CCK reduces the food intake mainly through CCK1R in Siberian sturgeon (Acipenser baerii Brandt). Scientific Reports, 2017, 7(1): 12413 DOI:10.1038/s41598-017-12646-3 |
Zhang YZ, Zheng ZM, Hua JQ, et al. Bio-floc nutrition and nitrogen uptake by shrimp and silver prussian carp in polyculture brackish water. Journal of Ningbo University, 2016, 29(3): 29-33 [ 张亚卓, 郑忠明, 华建权, 等. 低盐度条件下生物絮团的营养组分及凡纳滨对虾和银鲫对其摄食效率的研究. 宁波大学学报(理工版), 2016, 29(3): 29-33] |
Zhou C, Zhang X, Liu T, et al. Schizothorax davidi ghrelin: cDNA cloning, tissue distribution and indication for its stimulatory character in food intake. Gene, 2014, 534(1): 72 DOI:10.1016/j.gene.2013.10.012 |
Zhou C, Zheng J, Luo L, et al. Evidence that ghrelin, may be associated with the food intake of gibel carp (Carassius auratus gibelio). Fish Physiology and Biochemistry, 2016, 42(6): 1-10 |
Zhou M, Di R, Hu WP. Different expression of CRH and POMC genes in reproductive related tissues in small-tail han sheep and sunite sheep. China Herbivore Science, 2017, 37(4): 1-4 [ 周梅, 狄冉, 胡文萍, 等. 小尾寒羊和苏尼特羊CRH和POMC基因在繁殖相关组织中的差异性表达. 中国草食动物科学, 2017, 37(4): 1-4 DOI:10.3969/j.issn.2095-3887.2017.04.001] |



