 仔稚幼鱼消化酶活性变化研究
仔稚幼鱼消化酶活性变化研究
2. 中国水产科学研究院黄海水产研究所 农业农村部海洋渔业可持续发展重点实验室 青岛海洋科学与技术试点国家实验室海洋渔业科学与 食物产出过程功能实验室 青岛 266071;
3. 大连富谷水产有限公司 大连 116400
 (Seriola aureovittata)早期发育阶段的消化生理特性,测定了黄条
(Seriola aureovittata)早期发育阶段的消化生理特性,测定了黄条 胚胎、仔稚幼鱼阶段脂肪酶、淀粉酶、胰蛋白酶和碱性磷酸酶活性变化。结果显示,在黄条
胚胎、仔稚幼鱼阶段脂肪酶、淀粉酶、胰蛋白酶和碱性磷酸酶活性变化。结果显示,在黄条 仔鱼出膜前胚胎阶段,即能检测到脂肪酶、淀粉酶和碱性磷酸酶活性;初孵仔鱼体内(1 d)初次检测出胰蛋白酶的活性。脂肪酶和碱性磷酸酶比活力在仔鱼孵化后迅速增强(P < 0.05),在4 d开口时,2种酶比活力达最高值;淀粉酶比活力在7 d时达最大值;胰蛋白酶比活力在仔鱼阶段缓慢上升,15 d时比活力最大。稚鱼阶段内脏团中脂肪酶、碱性磷酸酶和胰蛋白酶活性基本维持稳定,幼鱼阶段内脏团脂肪酶、碱性磷酸酶和胰蛋白酶活性都呈现上升趋势;稚鱼和幼鱼阶段内脏团中淀粉酶活性下降并基本稳定于较低水平。研究表明,黄条
仔鱼出膜前胚胎阶段,即能检测到脂肪酶、淀粉酶和碱性磷酸酶活性;初孵仔鱼体内(1 d)初次检测出胰蛋白酶的活性。脂肪酶和碱性磷酸酶比活力在仔鱼孵化后迅速增强(P < 0.05),在4 d开口时,2种酶比活力达最高值;淀粉酶比活力在7 d时达最大值;胰蛋白酶比活力在仔鱼阶段缓慢上升,15 d时比活力最大。稚鱼阶段内脏团中脂肪酶、碱性磷酸酶和胰蛋白酶活性基本维持稳定,幼鱼阶段内脏团脂肪酶、碱性磷酸酶和胰蛋白酶活性都呈现上升趋势;稚鱼和幼鱼阶段内脏团中淀粉酶活性下降并基本稳定于较低水平。研究表明,黄条 仔稚幼鱼发育过程中,各种消化酶活性变化明显,且与其发育阶段和食性密切相关。在尚未摄食饵料的早期仔鱼体内已存在消化酶,认为其是母源传递而来,不是由外源性饵料所致;幼鱼阶段内脏团脂肪酶、碱性磷酸酶和胰蛋白酶比活力明显提高,这反映出随苗种生长发育,其肠道结构和消化机能逐渐完善,并且对脂肪、蛋白质的需求逐渐增强。
仔稚幼鱼发育过程中,各种消化酶活性变化明显,且与其发育阶段和食性密切相关。在尚未摄食饵料的早期仔鱼体内已存在消化酶,认为其是母源传递而来,不是由外源性饵料所致;幼鱼阶段内脏团脂肪酶、碱性磷酸酶和胰蛋白酶比活力明显提高,这反映出随苗种生长发育,其肠道结构和消化机能逐渐完善,并且对脂肪、蛋白质的需求逐渐增强。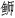 胚胎 仔稚幼鱼 消化酶 比活力
胚胎 仔稚幼鱼 消化酶 比活力 2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture and Rural Affairs, Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266071;
3. Dalian Fugu Fishery Co., Ltd., Dalian 116400
在鱼类规模化育苗生产中,鱼苗死亡率较高。Lauff等(1984)研究发现,消化酶是影响仔稚幼鱼死亡率的重要因素。消化酶活力高低直接影响鱼类的消化吸收,间接影响鱼类的生长发育。因此,对仔稚幼鱼消化酶活性的研究利于了解其消化生理特征,也有利于探索早期发育中鱼苗大量死亡的原因。目前,研究的鱼类消化酶主要包括脂肪酶、淀粉酶、胰蛋白酶和碱性磷酸酶等,脂肪酶主要由鱼类的肝胰脏分泌,起分解脂肪的作用;胰蛋白酶主要由胰脏分泌,鱼类早期发育蛋白消化主要靠胰蛋白酶完成;鱼类各消化器官都有淀粉酶存在,分解碳水化合物;碱性磷酸酶主要存在于鱼类肠道上皮细胞,与葡萄糖、Ca和P等的吸收有关,这些消化酶在鱼类早期发育过程的消化生理中起重要作用。目前,对仔稚幼鱼消化酶的研究日益增多,国内外学者对大菱鲆(Scophthalmus maximus)、半滑舌鳎(Cynoglossus semilaevis)、金头鲷(Sparus aurata)、古巴雀鳝(Atractosteus tristoechus)和日本黄姑鱼(Nibea iaponica)等仔稚幼鱼消化酶的分泌规律已有研究(陈慕雁等, 2005; 常青等, 2005; Moyano et al, 1996; Comabella et al, 2006; 孙敏等, 2012)。
黄条






实验所用的黄条


鱼苗培育期间,每天取样,观察仔稚幼鱼的生长发育情况。采集受精50 h的受精卵2份,每份1.5 g;孵化后第1、2、4、7、15和20天仔鱼,每个样品取2份(每份200~300尾);第25、30、40、50和60天稚幼鱼,每个样品取2份(每份20~ 100尾),随机取30尾仔稚幼鱼测量全长。为消除未消化饵料对消化酶的影响,每次在上午投喂前取样,取样后置于无饵料生物的海水中暂养3 h,保证样品鱼空腹。取样时,用双蒸水冲洗并用滤纸吸干表面水分,放入-80℃冰箱中冷冻保存。实验时,将样品于冰上解冻,20 d前的仔鱼较小,无法分离出内脏,故采取整体匀浆方法;20 d后的稚幼鱼解剖取其内脏团匀浆。每组3个平行,每个平行的取样量约为0.5 g,放入预冷的离心管中,按照w:v=1:9的比例加入预冷的生理盐水,进行组织匀浆,在4℃、5000 r/min的条件下离心30 min,取上清液作为粗酶提取液,4℃保存,并于24 h内测试完毕。黄条
使用碧云天的BCA蛋白浓度测定试剂盒,测量酶液中的蛋白浓度。脂肪酶用南京建成的脂肪酶试剂盒测定,活力单位定义:在37℃条件下,每毫升酶液在反应体系中与底物反应1 min,消耗1 mmol底物为1个酶活力单位。淀粉酶用南京建成淀粉酶试剂盒测定,其活力单位定义:组织中每毫克蛋白37℃、pH 7.0条件下与底物作用30 min,水解10 mg淀粉定义为1个淀粉酶活力单位。胰蛋白酶用南京建成胰蛋白酶试剂盒测定,其活力单位定义:在37℃、pH 8.0条件下,每毫克蛋白中含有的胰蛋白酶,每分钟使吸光度变化0.003,即为1个酶活力单位。碱性磷酸酶用南京建成碱性磷酸酶试剂盒测定,其活力单位定义:在37℃条件下,每克组织蛋白与基质作用15 min产生1 mg酚为1个酶活力单位。
1.3 数据处理采用SPSS 22.0软件对实验数据进行分析,运用单因子方差分析(One-way ANOVA)和Duncan氏检验法对各组数据进行显著性差异分析和多重比较,显著性水平为0.05,所有数值均采用平均值±标准差(Mean±SD)表示。
全长的平均日增长计算公式:
| $ {G_L} = ({L_2}--{L_1})/({t_2}--{t_1}) $ |
用指数函数分析全长和日龄的关系,公式:
| $ L = {\rm{a}}{{\rm{e}}^{{\rm{b}}t}} $ |
式中,L为全长(mm),t为日龄(d),a、b为常数。
2 结果 2.1 黄条
黄条

| $ L = 3.95{{\rm{e}}^{0.0358t}}, \;\;\;\;{R^2} = 0.9788 $ |
|
图 1 黄条 |
孵化后60 d内的仔稚幼鱼全长随着日龄的增加而加快增长,25 d后全长大幅增加。
2.2 黄条
黄条

|
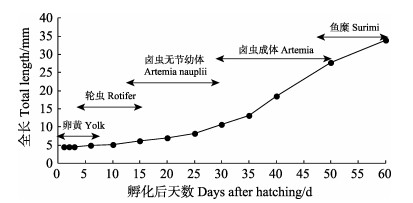
图 2 黄条 消化酶比活力存在显著差异(P < 0.05, n=3),下同 The embryonic stage is recorded as 0 d after hatching. Different lowercase letters mean significant differences in specific activities of digestive enzymes (P < 0.05, n=3), the same as below |
黄条

|
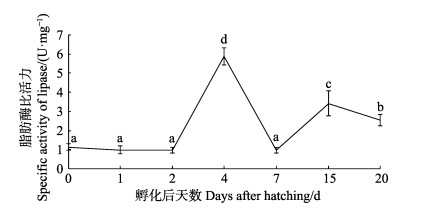
图 3 黄条 |
在黄条
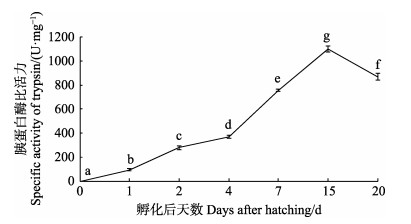
|
图 4 黄条 |
黄条
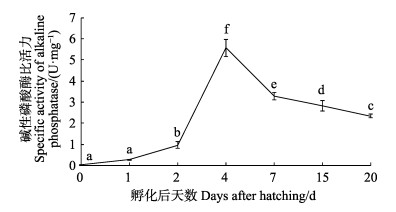
|
图 5 黄条 |

黄条

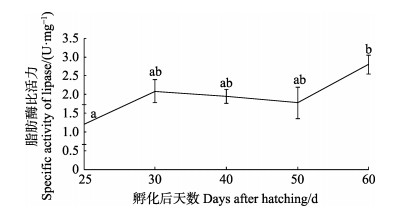
|
图 6 黄条 |
黄条

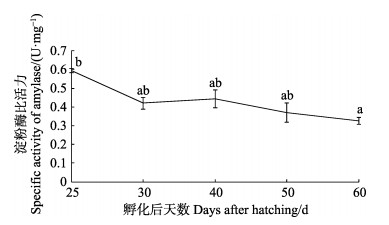
|
图 7 黄条 |
黄条

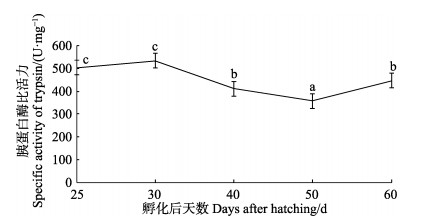
|
图 8 黄条 |
黄条


|
图 9 黄条 |
黄条

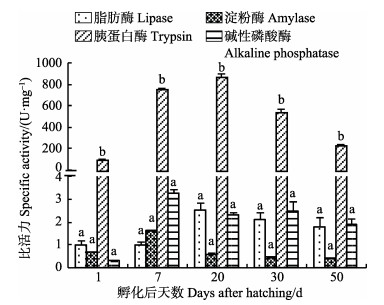
|
图 10 不同发育阶段黄条 |
鱼类早期发育过程中,由于饵料转换和消化器官的发育,消化酶在不同发育阶段的活性不同,消化酶的强弱是影响鱼类消化吸收能力强弱的重要因素,因此,消化酶是消化道中影响食物利用的重要因子,反映了仔稚幼鱼的消化能力和营养需求,与鱼类早期生长和存活也有很大关系(张云龙等, 2017)。本研究发现,黄条
本研究在黄条


脂肪酶主要是由鱼类的肝胰脏分泌的,在海水仔稚鱼的消化生理中占据很重要的地位。在不同鱼类中,脂肪酶变化趋势不同,Martínez等(1999)研究表明,塞内加尔舌鳎(Solea
senegalensis)孵化后第10天脂肪酶比活力最高。陈慕雁等(2005)研究表明,大菱鲆在15 d才检测出脂肪酶活性。Alvarez-González等(2008)研究表明,斑带副鲈中脂肪酶比活力随着生长发育而增加。本研究发现,黄条




本研究结果显示,黄条



本研究表明,仔鱼阶段胰蛋白酶比活力逐渐升高,稚幼鱼阶段也保持较高水平,说明整个发育阶段其对蛋白质的需求强烈。细点牙鲷胰蛋白酶比活力变化趋势和黄条


碱性磷酸酶是一种膜结合金属酶,主要存在于鱼类前肠上皮细胞的浅部和纹状缘,一般认为对脂类、葡萄糖、Ca和P的吸收有促进作用(Tengjaroenkul et al, 2000)。本研究结果显示,在仔鱼期黄条

黄条





Alvarez-González CA, Moyano-López FJ, Civera-Cerecedo R, et al. Development of digestive enzyme activity in larvae of spotted sand bass Paralabrax maculatofasciatus. 1. Biochemical analysis. Fish Physiology and Biochemistry, 2008, 34(4): 373-384 DOI:10.1007/s10695-007-9197-7 |
Babaei SS, Kenari AA, Nazari R, et al. Developmental changes of digestive enzymes in Persian sturgeon (Acipenser persicus) during larval ontogeny. Aquaculture, 2011, 318(1-2): 138-144 DOI:10.1016/j.aquaculture.2011.04.032 |
Cara JB, Moyano FJ, Cárdenas S, et al. Assessment of digestive enzyme activities during larval development of white bream. Journal of Fish Biology, 2003, 63(1): 48-58 DOI:10.1046/j.1095-8649.2003.00120.x |
Chang Q, Zhang XM, Chen SQ, et al. Variations in digestive enzymes activities in Tongue fish Cynoglossus semilaevis larvae and juveniles. Advances in Marine Science, 2005, 23(4): 472-476 [常青, 张秀梅, 陈四清, 等. 半滑舌鳎仔稚鱼消化酶活性的变化. 海洋科学进展, 2005, 23(4): 472-476 DOI:10.3969/j.issn.1671-6647.2005.04.012] |
Chen MY, Zhang XM, Lian JH. Development of some digestive enzymes and alkaline phosphatase activities in turbot Scophthalmus maximus larvae and juveniles. Periodical of Ocean University of China (Natural Sciences), 2005, 35(3): 483-486 [陈慕雁, 张秀梅, 连建华. 大菱鲆仔稚鱼期消化酶及碱性磷酸酶活性的变化. 中国海洋大学学报(自然科学版), 2005, 35(3): 483-486] |
Comabella Y, Mendoza R, Aguilera C, et al. Digestive enzyme activity during early larval development of the Cuban gar Atractosteus tristoechus. Fish Physiology and Biochemistry, 2006, 32(2): 147-157 |
Gisbert E, Giménez G, Fernández I, et al. Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture, 2009, 287(3-4): 381-387 DOI:10.1016/j.aquaculture.2008.10.039 |
Ji H, Sun HT, Tian JJ, et al. Digestive enzymes activities during early larval development of the paddlefish Polyodon spathula.. Acta Hydrobiologica Sinica, 2012, 36(3): 457-465 [吉红, 孙海涛, 田晶晶, 等. 匙吻鲟仔稚鱼消化酶发育的研究. 水生生物学报, 2012, 36(3): 457-465] |
Kim BG, Divakaran S, Brown CL, et al. Comparative digestive enzyme ontogeny in two marine larval fishes: Pacific threadfin (Polydactylus sexfilis) and bluefin trevally (Caranx melampygus). Fish Physiology and Biochemistry, 2001, 24(3): 225-241 DOI:10.1023/A:1014054431627 |
Lauff M, Hofer R. Proteolytic enzymes in fish development and the importance of dietary enzymes. Aquaculture, 1984, 37(4): 335-346 DOI:10.1016/0044-8486(84)90298-9 |
Li Q, Tang HY. Development of some digestive enzymes and alkaline phosphatase activities in Spinibarbus snensis larvae and juveniles. Journal of Hydroecology, 2010, 3(5): 82-85 [李芹, 唐洪玉. 中华倒刺鲃仔稚鱼期消化酶及碱性磷酸酶活性变化的研究. 水生态学杂志, 2010, 3(5): 82-85] |
Liu XZ, Xu YJ, Li R, et al. Analysis and evaluation of nutritional composition of the muscle of yellowtail kingfish (Seriola aureovittata). Progress in Fishery Sciences, 2017, 38(1): 128-135 [柳学周, 徐永江, 李荣, 等. 黄条  (Seriola aureovittata)肌肉营养组成分析与评价. 渔业科学进展, 2017, 38(1): 128-135] (Seriola aureovittata)肌肉营养组成分析与评价. 渔业科学进展, 2017, 38(1): 128-135] |
Liu YS, Shi YH, Zhang GY, et al. Growth, digestive enzyme and antioxidant enzyme activities of tawny puffer (Takifugu flavidus) larve. Journal of Zhejiang University(Agricultural and Life Science), 2014, 40(6): 688-696 [刘永士, 施永海, 张根玉, 等. 菊黄东方鲀仔稚鱼生长及其消化酶与抗氧化酶活性. 浙江大学学报(农业与生命科学版), 2014, 40(6): 688-696] |
Martínez I, Moyano FJ, Fernández-Díaz C, et al. Digestive enzyme activity during larval development of the Senegal sole (Solea senegalensis). Fish Physiology and Biochemistry, 1999, 21(4): 317-323 DOI:10.1023/A:1007802708459 |
Moran D, Gara B, Wells RMG. Energetics and metabolism of yellowtail kingfish (Seriola lalandi Valenciennes 1833) during embryogenesis. Aquaculture, 2007, 265(1-4): 359-369 DOI:10.1016/j.aquaculture.2007.02.003 |
Moyano FJ, Díaz M, Alarcón FJ, et al. Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiology and Biochemistry, 1996, 15(2): 121-130 DOI:10.1007/BF01875591 |
Oozeki Y, Bailey KM. Ontogenetic development of digestive enzyme activities in larval walleye pollock, Theragra chalcogramma. Marine Biology, 1995, 122(2): 177-186 |
Pan L, Fang H, Zhang SC, et al. The variation of digestive enzymes in larval and juvenile Hexagrammous otakii. Progress in Fishery Sciences, 2013, 34(3): 54-60 [潘雷, 房慧, 张少春, 等. 大泷六线鱼仔、稚、幼鱼期消化酶活力的变化. 渔业科学进展, 2013, 34(3): 54-60 DOI:10.3969/j.issn.1000-7075.2013.03.007] |
Poortenaar CW, Hooker SH, Sharp N. Assessment of yellowtail kingfish (Seriola lalandi) reproductive physiology, as a basis for aquaculture development. Aquaculture, 2001, 201(3-4): 271-286 DOI:10.1016/S0044-8486(01)00549-X |
Stuart KR, Drawbridge MA. Captive spawning and larval rearing of California yellowtail (Seriola lalandi). Aquaculture Research, 2013, 44(5): 728-737 |
Sun M, Chai XJ, Xu YJ, et al. Assessment of digestive enzymes activities during the early development of Nibea japonica. Journal of Shanghai Ocean University, 2012, 21(6): 965-970 [孙敏, 柴学军, 许源剑, 等. 日本黄姑鱼早期发育过程中消化酶活性变化研究. 上海海洋大学学报, 2012, 21(6): 965-970] |
Tengjaroenkul B, Smith BJ, Caceci T, et al. Distribution of intestinal enzyme activities along the intestinal tract of cultured Nile tilapia, Oreochromis niloticus L. Aquaculture, 2000, 182(3): 317-327 |
Tong SY, Ling CH, Wang XT. Appraisement and analysis of nutrient compositions for Moina mongolica Daday. Journal of Dalian Fisheries College, 1988(3、4): 29-33 [童圣英, 林成辉, 王雪涛. 蒙古裸腹溞营养成分分析与评价. 大连水产学院学报, 1988(3、4): 29-33] |
Yang SG, Hur SW, Ji SC, et al. Morphological development of embryo, larvae and juvenile in yellowtail kingfish, Seriola lalandi. Development and Reproduction, 2016a, 20(2): 131-140 DOI:10.12717/DR.2016.20.2.131 |
Yang SG, Ji SC, Lim SG, et al. Management of sexual maturation and natural spawning of captive-readed yellowtail kingfish, Seriola lalandi, in an indoor rearing tank. Development & Reproduction, 2016b, 20(2): 141-147 |
Zhang YL, Zhang HL, Wang LY, et al. Allometric growth and ontogenetic changes in nucleic acids and digestive enzymes during the early life stage in fish species: A review. Journal of Fishery Sciences of China, 2017, 24(3): 648-656 [张云龙, 张海龙, 王凌宇, 等. 鱼类早期发育阶段异速生长及核酸、消化酶变化的研究进展. 中国水产科学, 2017, 24(3): 648-656] |



