2. 全国水产技术推广总站 北京 100125
2. National Fisheries Technology Extension Center, Beijing 100125
近年来,黑皮质素(Melanocyte-stimulating hormone, MSH)系统备受关注,作为阿片黑素促皮质激素原(POMC)衍生物,在色素沉着、摄食、能量稳态、免疫调节以及繁殖活动中起着重要的作用(史学营等, 2015; Chen et al, 2017; Carter et al, 2018; Clément et al, 2018; Matsuda et al, 2018),特别是其对能量平衡的调节作用,使之成为研究热点。通过定位POMC神经元及其衍生物在下丘脑中的分布位置及配受体结合的药理学分析(Tao, 2010),发现黑皮质素系统可能通过调节下丘脑-垂体-性腺轴(HPG)从而对垂体中促性腺激素(Gth)的合成产生影响以调节生殖活动。然而,此类研究多集中于哺乳动物中,缺乏在低等脊椎动物中的相关研究。本文基于黑皮质素系统在物种间的保守性以及当前研究中对其在能量稳态和繁殖活动中的调控实验,重点论述了POMC、α-黑素细胞刺激素(α-MSH)以及黑皮质素受体-4(MC4R)在哺乳动物和鱼类中对能量稳态和繁殖活动的调节作用。
1 阿片黑素促皮质激素原POMC是黑皮质激素的前体物质,在机体中分布广泛,在脑、垂体和性腺中均有表达,特别是下丘脑弓状核中有大量表达(Kineman et al, 1989; 杜富宽等, 2017),经组织特异性转录调控加工成不同的衍生物。
1.1 POMC在能量稳态中的作用POMC神经元作为能量稳态的中央调节器(Cone, 1999),对摄食和能量平衡的调控作用一直备受关注。Cheung等(1997)在弓状核的POMC神经元中检测到瘦素表达; Caron等(2018)发现动物处于饥饿状态时,瘦素和POMC表达水平下降,摄食量增加。POMC与肥胖息息相关,研究发现,POMC突变患者出现早发性肥胖(Krude et al, 1998); 敲除POMC后,采用瘦素治疗也不能降低体重(Chhabra et al, 2016)。由此说明,POMC可能作为瘦素调控能量稳态的中间介质调节摄食和体重。
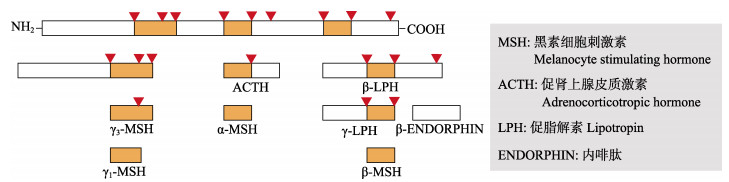
|
图 1 POMC翻译后加工产物 Fig.1 Post-translational processing of POMC (Butler et al, 2017) |
POMC除在能量调控中发挥重要作用外,在生殖活动中的作用也不可忽视。光镜和电镜下观察到GnRH的轴突末端与POMC细胞体并置(Sotonyi et al, 2010)。POMC神经元还可能通过Kisspeptin中间神经元影响GnRH的表达(Backholer et al, 2010)。Higo等(2016)在下丘脑弓状核中定位Kisspeptin发现,Kiss1r表达神经元中分布着大约63%的POMC神经元。因此,POMC与GnRH神经元在脑中的位置分布表明,POMC神经元可能对GnRH神经元具有重要的输入作用,它能够直接或间接地参与中枢神经系统中生殖功能的调控。目前,已通过脑内室注射(Intra- cerebroventricular injection, ICV)和基因敲除技术对POMC神经元在HPG轴中的作用进行探究。大鼠(Rattus norvegicus) ICV注射促性腺激素抑制激素(GnIH)降低了GnRH和POMC的表达水平(司丽娜等, 2017); POMC基因敲除小鼠(Mus musculus)的产仔率有显著下降(Faulkner et al, 2015),POMC可能通过与GnRH神经元相互联系,从而调控HPG轴中激素的合成而影响繁殖。
除神经肽外,POMC神经元释放的神经递质GABA和谷氨酸也显示出了对GnRH神经元的调节作用(Kuehl-Kovarik et al, 2002)。综上,POMC神经元及其释放的神经肽和神经递质均可能通过直接或间接的作用调节GnRH的表达,进而作用于繁殖功能,但此类研究目前多集中在哺乳动物中,且其作用机制还有待进一步研究。
2 黑素细胞刺激素(α-MSH)MSH包含α-、β-、γ-和δ-MSH,由POMC经组织特异性翻译加工而成,对色素沉着、摄食和能量稳态、免疫调节以及繁殖等生理活动具有重要作用(Cone, 2006; 朱学武等, 2017)。由于γ-MSH与受体的结合率较低(Tao, 2010),δ-MSH仅在软骨鱼中发现(Dores et al, 2003),且β-MSH发现时间较晚(Mayer et al, 2005),对此研究较少。目前,关于黑素细胞刺激素的研究多集中于α-MSH。因此,本文仅论述了α-MSH对能量稳态和繁殖活动的调控作用。研究表明,α-MSH在中枢神经系统表达量较高,主要包括背侧、外侧下丘脑以及弓状核(包新民等, 1990)。同时,在垂体、皮肤、胃肠道及性腺中也检测到α-MSH的分布,但表达量较低(Bardin et al, 1987; 何英等, 2013)。
2.1 α-MSH在能量稳态中的功能哺乳动物中,Leptin-Melanocortin系统是调控能量平衡的重要系统。脂肪组织分泌的瘦素穿过血脑屏障,与位于下丘脑(特别是弓状核)的瘦素受体结合(Lee, 2009)。此后,瘦素受体激活α-MSH神经元活性,刺激下丘脑弓状核分泌α-MSH。与此同时,分泌刺鼠相关蛋白(Agouti-related protein, AgRP)的神经元被抑制,AgRP的合成与分泌减少(Ramos-Molina et al, 2016)。α-MSH和AgRP具有相互拮抗的生理功能,二者的动态平衡是有机体保证能量平衡的重要保障之一(图 2)。
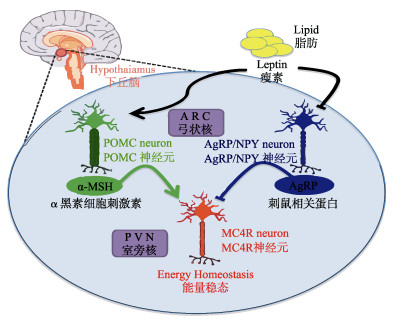
|
图 2 瘦素-黑皮质素调控能量代谢 Fig.2 Leptin-melanocortin pathway to regulate energy homeostasis (Ramos-Molina et al, 2016) |
鱼类中α-MSH相关研究结果与哺乳动物一致。α-MSH合成障碍的虹鳟出现食欲亢进、肝脏肿大和腹腔脂肪堆积现象(Yada et al, 2002)。给予金鱼(Carassius auratus)ICV注射NDP-α-MSH(α-MSH的高效类似物),观察到剂量依赖性的抑制食物摄入,且在中脑定位到了α-MSH的免疫性反应,发现α-MSH可能通过促肾上腺皮质激素释放激素(CRH)信号通路抑制食物摄入(Cerdá-Reverter et al, 2003a; Matsuda et al, 2008)。
2.2 α-MSH在繁殖活动中的功能α-MSH也参与繁殖活动的调控,早期研究多集中在哺乳动物中。用α-MSH多肽刺激小鼠卵巢,卵巢内孕酮(P)含量显著增加(Durando et al, 1998); ICV注射α-MSH能够影响小鼠的性行为(Thody et al, 1979),并且能够降低卵巢摘除小鼠中下丘脑和垂体组织中的GnRH、LH和FSH的表达水平(杨松鹤等, 2010)。随后发现,α-MSH紧邻GnRH和Kisspeptin神经元(Cardoso et al, 2015),α-MSH处理可以激活70%的GnRH神经元(Roa et al, 2012)。上述实验证实,α-MSH除可以在中枢神经系统和外周组织中直接调控HPG轴,也可通过Kisspeptin间接调控生殖活动。
硬骨鱼类中的研究证实,α-MSH在鱼类中同样能够调控HPG轴,表明其调控繁殖的生理功能具有物种间的保守性。在欧洲狼鲈(Dicentrarchus labrax)和黑鲷(Sparus aurata)的卵泡颗粒细胞及卵原细胞的胞浆中发现α-MSH,且在繁殖期表达水平最高(Mosconi et al, 1994),推测α-MSH在鱼类卵巢组织中可能直接调控性类固醇激素的合成,从而影响卵泡成熟和排卵。
3 黑皮质素受体-4(MC4R)黑皮质素受体-4(Melanocortin-4 receptor, MC4R)主要在下丘脑的室旁核(Paraventricular nucleus, PVN)表达。作为α-MSH的重要受体之一,能够调控摄食与能量稳态、糖类与脂质代谢、心血管功能和繁殖等多种生理活动(Chen et al, 2017; Huszar et al, 1997; Nogueiras et al, 2007; da Silva et al, 2008)。人类的MC4R由332个氨基酸编码而成,是典型的G蛋白偶联受体(GPCR),具有7个跨膜螺旋结构域(Gantz et al, 1993),其N端和C端分别位于细胞外和细胞内,内环和外环较短,使之成为GPCR超家族中最短的成员之一(Tao, 2010)。目前,已在人、狗、猫、猪等哺乳类动物和鱼、鸡、鸽子等非哺乳动物中克隆得到MC4R序列(Tao, 2010),其在物种间高度保守。除MC4R外,在高等哺乳动物中,还观察到MC3R也是α-MSH的受体。MC3R与MC4R在下丘脑中高水平表达,共同参与能量调节,二者的功能并不重合。MC3R主要调节摄食行为和摄食效率,而MC4R主要调节摄食量和能量消耗(Tao, 2010)。在硬骨鱼类中,早期观点认为,硬骨鱼类的MC3R在进化过程中丢失,仅保留MC4R调控鱼类的能量代谢(Cortés et al, 2014)。近年来,部分硬骨鱼类的基因组数据鉴定出MC3R(Logan et al, 2003),但其生理学功能与药理学特征尚需进一步研究。
3.1 MC4R信号通路MC4R的经典信号通路是与异源G蛋白三聚体偶联(Mountjoy et al, 2001),MC4R被激动剂,如α-MSH、β-MSH、γ-MSH、ACTH激活后,引起环腺苷酸环化酶(Adenylate cyclase, AC)活化,使细胞内环磷酸腺苷酸(Cyclic adenosine monophosphate, cAMP)增加,进而激活下游的蛋白激酶A(PKA),PKA催化蛋白质的磷酸化,进而调控蛋白质功能。与此同时,被PKA激活的某些蛋白可以作为转录因子,进入细胞核激活或抑制相关基因的转录,从而调节细胞功能。除GPCRs经典的Gs-cAMP-PKA通路外,MC4R还能够激活丝裂原活化蛋白激酶-细胞外信号调节激酶(MEK-ERK1/2)信号通路。早期实验观察到,MTⅡ诱导的抑制食物摄入依赖于MEK-ERK1/2信号通路(Sutton et al, 2005)。在稳定表达的人MC4R的中国仓鼠卵巢(Chinese hamster ovary, CHO)细胞中,MEK- ERK1/2信号通路可以被NDP-α-MSH激活,并表现出时间和剂量依赖性(Vongs et al, 2004),从而调节下游蛋白功能或相关基因的转录。
3.2 MC4R组织分布MC4R研究较为广泛,早期在小鼠的丘脑、下丘脑及海马体中观察到了MC4R的分布(Mountjoy et al, 1994)。后续研究发现,MC4R不仅存在于中枢神经系统中,也在外周组织中表达。哺乳动物中,MC4R在脑中的表达量显著高于其他组织,外周组织如在小鼠、山羊和猪的性腺、肌肉、肾和心脏中也有少量表达(Mountjoy et al, 2003; 张子军等, 2012; 何夏萍等, 2013)。禽类中,MC4R在脑中与外周组织中的表达量差异不显著,在鸡、鸭、鹅的脑、脂肪、肾、胃和肠中均有较高表达(Takeuchi et al, 1998; 王婕等, 2011; 原昊等, 2011)。MC4R在低等脊椎动物中的组织表达模式区别较大。例如,角鲨(Squalus acanthias)的MC4R只在脑中表达(Ringholm et al, 2003),而在其他多种鱼类中,除在脑中表达外,外周组织中也检测到了MC4R的分布,如金鱼(Cerdá-Reverter et al, 2003a)、条斑星鲽(Verasper moseri)(Kobayashi et al, 2008)、花鲈(Lateolabrax maculatus)(Zhang et al, 2019)及金钱鱼(Scatophagus argus)(Li et al, 2016)的性腺、肝脏和垂体中均有MC4R的表达。然而,MC4R在斑马鱼(Danio rerio)的眼中也有分布(Ringholm et al, 2002),而上述鱼类的眼中未检测到MC4R的表达。MC4R在脑、胃、肠中的分布,表明其能够参与摄食和能量稳态的调控作用。在哺乳动物和鱼类中性腺的分布,推测其可能对生殖活动产生影响。但MC4R的表达具有一定的组织特异性及物种特异性,其在外周组织中的功能还有待进一步研究。
3.3 MC4R在能量稳态中的功能MC4R作为瘦素介导食欲调节的最末端基因,在摄食和能量稳态中的功能备受关注。Huszar等(1997)发现,敲除MC4R基因的小鼠食欲旺盛,体重增加。Williams等(2002)将MC4R的激动剂MTⅡ对小鼠进行ICV注射,发现其能抑制摄食,而随后注射的抑制剂SHU9119缓解了这一现象。在瘦素基因敲除的小鼠中注射MC4R的激动剂,观察到小鼠食欲减退,体重下降(Clément et al, 2018)。上述研究表明,MC4R不但在瘦素调控能量稳态的过程中起到中间介质的作用,还能够直接调控摄食、减少体重。
在硬骨鱼中关于MC4R对摄食的调控也有研究。给予金鱼和虹鳟ICV注射MTⅡ,观察到食物摄入被抑制; 而注射MC4R特异性拮抗剂HS024会增加食物摄入(Cerdá-Reverter et al, 2003b; Schjolden et al, 2009)。齐口裂腹鱼(Schizothorax prenanti)经过短期禁食后,脑中MC4R的表达量显著增加(Wei et al, 2013)。在处于食物短缺环境中的墨西哥洞穴鱼(Astyanax mexicanus)中发现,其MC4R基因的保守残基出现了突变,该突变增加了墨西哥洞穴鱼抗饥饿的能力以及对黑暗环境的适应能力(Aspiras et al, 2015)。此外,在MC4R的药理学研究中发现,MC4R的反向激动剂(Inverse agonist)可以降低鱼类MC4R的组成性活性,刺激鱼类摄食,为养殖带来更高的效益。在黄鳝(Monopterus albus)中发现1种小分子物质ML00253764,可以作为MC4R的反向激动剂(Yi et al, 2018)。小分子配体可以直接与饵料混合,避免肌肉注射对鱼类的胁迫。然而,目前ML00253764的生产成本较高,制约了其在养殖中的应用(Yi et al, 2018)。如若今后可以鉴定出廉价、高效的鱼类MC4R小分子反向激动剂,则有进一步应用前景。
3.4 MC4R在繁殖活动中的功能MC4R在繁殖调控中的生理功能研究较少,且主要集中在哺乳动物中。实验发现,AgRP过表达导致成年小鼠不育(Granholm et al, 1986); 通过基因敲降技术得到的MC4R缺陷的雄性小鼠除表现出肥胖外,其勃起功能受损,性行为受到影响(van der Ploeg et al, 2002); MC4R缺陷雌性小鼠发情周期不规律(Chen et al, 2017),且出现排卵率和受精率下降及早衰现象(Sandrock et al, 2009)。为进一步明确MC4R是否参与繁殖过程,对妊娠小鼠下丘脑弓状核中MC4R的含量进行了测定,结果显示,在妊娠早期,MC4R的表达量较高,随着妊娠进行,表达水平下降(Asadi-Yousefabad et al, 2015),表明MC4R除影响性功能及性行为外,也参与到妊娠的维持过程。但Stanley等(1999)研究发现,给予小鼠ICV注射MC4R的抑制剂刺鼠相关蛋白(AgRP)后,GnRH、LH和FSH的表达水平上升,且在小鼠子宫内,MC4R和雌激素(E2)呈负相关(Cheung et al, 2001)。这与之前所述结果不同,可能与MC4R激动剂或抑制剂的处理浓度相关,然而具体原因还未得到实验验证,有待进一步探究。
在硬骨鱼中(如条斑星鲽、金鱼、金钱鱼),大量实验观察到MC4R在性腺中表达量较高(Cerdá-Reverter et al, 2003a; Li et al, 2016; Kobayashi et al, 2008),推测其参与繁殖调控。同时,近期实验观察到黄鳝MC4R的表达具有性别特异性。雌性黄鳝脑和性腺的MC4R基因表达显著高于雄性,推测MC4R参与大脑雌雄二象性的调控和性腺的分化与发育(Yi et al, 2018)。此外,在金钱鱼中,使用MC4R的激动剂THIQ刺激体外培养的脑和垂体,发现其能够促进GnRH、LHβ和FSHβ的表达,而拮抗剂SHU9119作用相反,表明MC4R除能够作用于GnRH调控生殖活动外,也可以直接作用于垂体调节Gth的分泌(Jiang et al, 2017)。
3.5 MC4R的其他生理功能哺乳动物中,对MC4R的研究较为广泛,除上述在能量稳态和繁殖活动中的调控作用外,其在心血管系统及糖类和脂质代谢中的功能也受到关注,如注射MC4R拮抗剂的小鼠和猕猴(Macaca arctoides)心率和动脉压下降(Tallam et al, 2004; Kievit et al, 2013),MC4R敲除小鼠脂质的吸收和甘油三酸酯的合成增加,白色脂肪组织中脂肪积累,而注射MTⅡ的小鼠脂肪利用率增加,脂肪形成减少(Nogueiras et al, 2007)。由于鱼类在生理结构上与哺乳动物有较大差别,其组织和器官的分化不显著,目前,在鱼类中未有关于MC4R在心血管系统及糖类和脂质代谢中功能的研究。
4 总结与展望黑皮质素系统在色素沉着、摄食和能量稳态、免疫调节以及繁殖等生理活动中具有重要作用。目前,关于黑皮质素系统中α-MSH和MC4R对能量稳态和繁殖活动调控作用的研究和应用还不充分,仍存在较多未知。能否开发出廉价的新型小分子配体作为MC4R的反向激动剂,促进养殖动物增加体重?MC4R被配体激活后对HPG轴是否存在负反馈调控?MC4R在种间的组织表达模式存在较大差异,其对繁殖活动的调控能力是否存在物种特异性?随着基因敲除技术和药理学的不断发展,今后可以更加深入地探索其在能量稳态和繁殖活动中的调节通路和作用机理,并将研究结果充分应用于实际生产,以期其在动物的生长发育和遗传育种中具有更加广阔的应用前景。
Asadi-Yousefabad SL, Sarvestani FS, Tamadon A, et al. Agouti- related peptide and melanocortin-4 receptor mRNAs expressions in arcuate nucleus during the pregnancy and lactation of rats. Veterinarski Arhiv, 2015, 85(6): 689-700 |
Aspiras AC, Rohner N, Martineau B, et al. Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient- poor conditions. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(31): 9668-9673 DOI:10.1073/pnas.1510802112 |
Backholer K, Smith JT, Rao A, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology, 2010(5): 2233-2243 |
Bao XM, Shu SY. Comparison and distributions of the perikaryal and fibers of the pro-opiomelanocortin derived peptidergic neurons (ACTH, β-END, α-MSH) in the rat hypothalamus. Chinese Journal of Neuroanatomy, 1990, 6(1): 43-48 [包新民, 舒斯云. 大鼠下丘脑中前阿黑皮素来源的肽能神经元(ACTH, β-END, α-MSH)胞体和纤维的分布和比较. 神经解剖学杂志, 1990, 6(1): 43-48] |
Bardin CW, Chen CL, Morris PL, et al. Proopiomelanocortin- derived peptides in testis, ovary, and tissues of reproduction. Recent Progress in Hormone Research, 1987, 43: 1-28 |
Butler AA, Girardet C, Mavrikaki M, et al. A life without hunger: The ups (and downs) to modulating melanocortin-3 receptor signaling. Frontiers in Neuroscience, 2017, 11: 128 |
Cardoso RC, Alves BRC, Sharpton SM, et al. Nutritional programming of accelerated puberty in heifers: Involvement of pro-opiomelanocortin neurones in the arcuate nucleus. Journal of Neuroendocrinology, 2015, 27(8): 647-657 DOI:10.1111/jne.12291 |
Caron A, Dungan Lemko HM, Castorena CM, et al. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. Elife, 2018, 7: e33710 DOI:10.7554/eLife.33710 |
Carter DW, Sood RF, Seaton ME, et al. MC1R gene polymorphisms are associated with dysfunctional immune responses and wound infection after burn injury. Journal of Surgical Research, 2018, 231: 448-452 DOI:10.1016/j.jss.2018.07.018 |
Cerdá-Reverter JM, Ringholm A, Schiöth HB, et al. Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: Involvement in the control of food intake. Endocrinology, 2003a, 144(6): 2336-2349 DOI:10.1210/en.2002-0213 |
Cerdá-Reverter JM, Schiöth HB, Peter RE. The central melanocortin system regulates food intake in goldfish. Regulatory Peptides, 2003b, 115(2): 101-113 |
Chen X, Huang L, Tan HY, et al. Deficient melanocortin-4 receptor causes abnormal reproductive neuroendocrine profile in female mice. Reproduction, 2017, 153(3): 267-276 DOI:10.1530/REP-16-0341 |
Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology, 1997, 138(10): 4489-4492 DOI:10.1210/endo.138.10.5570 |
Cheung CC, Thornton JE, Nurani SD, et al. A reassessment of leptin's role in triggering the onset of puberty in the rat and mouse. Neuroendocrinology, 2001, 74(1): 12-21 |
Chhabra KH, Adams JM, Jones G, et al. Hypothalamic pomc- deficiency impairs the function of leptin to decrease food intake and bodyweight. FASEB Journal, 2016, 30(1): 1293 |
Clément K, Biebermann H, Farooqi IS, et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nature Medicine, 2018, 24(5): 551-555 DOI:10.1038/s41591-018-0015-9 |
Cone RD. Studies on the physiological functions of the melanocortin system. Endocrine Reviews, 2006, 27(7): 736-749 DOI:10.1210/er.2006-0034 |
Cone RD. The central melanocortin system and energy homeostasis. Trends in Endocrinology and Metabolism, 1999, 10(6): 211-216 DOI:10.1016/S1043-2760(99)00153-8 |
Cortés R, Navarro S, Agulleiro MJ, et al. Evolution of the melanocortin system. General and Comparative Endocrinology, 2014, 209: 3-10 DOI:10.1016/j.ygcen.2014.04.005 |
da Silva AA, Carmo JMD, Kanyicska B, et al. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension, 2008, 51(4): 884-890 |
Dores RM, Cameron E, Lecaude S, et al. Presence of the delta- MSH sequence in a proopiomelanocortin cDNA cloned from the pituitary of the galeoid shark, Heterodontus portusjacksoni. General and Comparative Endocrinology, 2003, 133(1): 71-79 |
Du FK, Xu GC, Li Y, et al. Cloning of POMC cDNA and the stress response in Coilia nasus. Journal of Fishery Sciences of China, 2017, 24(2): 231-238 [杜富宽, 徐钢春, 黎燕, 等. 刀鲚POMC基因的cDNA克隆及其应激应答. 中国水产科学, 2017, 24(2): 231-238] |
Durando PE, Celis ME. In vitro effect of α-MSH administration on steroidogenesis of prepubertal ovaries. Peptides, 1998, 19(4): 667-675 DOI:10.1016/S0196-9781(97)00458-0 |
Faulkner LD, Dowling AR, Stuart RC, et al. Reduced melanocortin production causes sexual dysfunction in male mice with POMC neuronal insulin and leptin insensitivity. Endocrinology, 2015, 156(4): 1372-1385 DOI:10.1210/en.2014-1788 |
Gantz I, Miwa H, Konda Y, et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. Journal of Biological Chemistry, 1993, 268(20): 15174-15179 |
Granholm NH, Jeppesen KW, Japs RA. Progressive infertility in female lethal yellow mice (Ay/a; strain C57BL/6J). Journal of Reproduction and Fertility, 1986, 76(1): 279-287 DOI:10.1530/jrf.0.0760279 |
He XP, Zhou YN, Yan ZX, et al. Molecular cloning and analysis and tissue distribution of melanocortin receptor 3 and 4 genes in pigs (Sus scrofa). Journal of Sichuan University (Natural Science), 2013, 50(4): 899-907 [何夏萍, 周彦妮, 阎振鑫, 等. 猪黑皮质素受体3和4基因克隆及分析. 四川大学学报(自然科学版), 2013, 50(4): 899-907 DOI:10.3969/j.issn.0490-6756.2013.04.042] |
He Y, Liao Y. Research progress on the anti-inflammatory effects of α-msh in the early stage of severe burns. Medical Information, 2013, 11061106(21): 663 [何英, 廖毅. α-msh在严重烧伤早期的抗炎作用研究进展. 医学信息, 2013, 11061106(21): 663] |
Higo S, Iijima N, Ozawa H. Characterisation of Kiss1r (Gpr54)- expressing neurones in the arcuate nucleus of the female rat hypothalamus. Journal of Neuroendocrinology, 2016, 29(2) |
Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell, 1997, 88(1): 131-141 DOI:10.1016/S0092-8674(00)81865-6 |
Jiang DN, Li JT, Tao YX, et al. Effects of melanocortin-4 receptor agonists and antagonists on expression of genes related to reproduction in spotted scat, Scatophagus argus. Journal of Comparative Physiology B, 2017, 187(4): 603-612 DOI:10.1007/s00360-017-1062-0 |
Kievit P, Halem H, Marks DL, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes, 2013, 62(2): 490-497 |
Kineman DR, Kraeling RR, Crim JW, et al. Localization of proopiomelanocortin (POMC) immunoreactive neurons in the forebrain of the pig. Biology of Reproduction, 1989, 40(5): 1119-1126 DOI:10.1095/biolreprod40.5.1119 |
Kobayashi Y, Tsuchiya K, Yamanome T, et al. Food deprivation increases the expression of melanocortin-4 receptor in the liver of barfin flounder, Verasper moseri. General and Comparative Endocrinology, 2008, 155(2): 280-287 |
Krude H, Biebermann H, Luck W, et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nature Genetics, 1998, 19(2): 155-157 DOI:10.1038/509 |
Kuehl-Kovarik MC, Pouliot WA, Halterman GL, et al. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. Journal of Neuroscience, 2002, 22(6): 2313-2322 DOI:10.1523/JNEUROSCI.22-06-02313.2002 |
Lee YS. The role of leptin-melanocortin system and human weight regulation: Lessons from experiments of nature. Annals of Academy of Medicine, Singapore, 2009, 38(1): 34-111 |
Li JT, Yang Z, Chen HP, et al. Molecular cloning, tissue distribution, and pharmacological characterization of melanocortin-4 receptor in spotted scat, Scatophagus argus. General and Comparative Endocrinology, 2016, 230-231: 143-152 DOI:10.1016/j.ygcen.2016.04.010 |
Logan DW, Bryson-Richarson RJ, Taylor MS, et al. Sequence characterization of teleost fish melanocortin receptors. Annals of the New York Academy of Sciences, 2003, 994(1): 319-330 DOI:10.1111/j.1749-6632.2003.tb03196.x |
Matsuda K, Kojima K, Shimakura S, et al. Corticotropin-releasing hormone mediates α-melanocyte-stimulating hormone- induced anorexigenic action in goldfish. Peptides, 2008, 29(11): 1930-1936 DOI:10.1016/j.peptides.2008.06.028 |
Matsuda N, Kasagi S, Nakamaru T, et al. Left-right pigmentation pattern of Japanese flounder corresponds to expression levels of melanocortin receptors (MC1R and MC5R), but not to agouti signaling protein 1 (ASIP1) expression. General and Comparative Endocrinology, 2018, 262: 90-98 DOI:10.1016/j.ygcen.2018.03.019 |
Mayer JP, Hsiung HM, Flora DB, et al. Discovery of a β-MSH- derived MC-4R selective agonist. Journal of Medicinal Chemistry, 2005, 48(9): 3095-3098 DOI:10.1021/jm0501432 |
Mosconi G, Carnevali O, Facchinetti F, et al. Ovarian melanotropic peptides and adaptation in two teleostean species: Sparus aurata L. and Dicentrarchus labrax L. Peptides, 1994, 15(5): 927-931 |
Mountjoy KG, Jenny Wu CS, Dumont LM, et al. Melanocortin-4 receptor messenger ribonucleic acid expression in rat cardiorespiratory, musculoskeletal, and integumentary systems. Endocrinology, 2003, 144(12): 5488-5496 DOI:10.1210/en.2003-0570 |
Mountjoy KG, Kong PL, Taylor JA, et al. Melanocortin receptor- mediated mobilization of intracellular free calcium in HEK293 cells. Physiological Genomics, 2001, 5(1): 11-19 DOI:10.1152/physiolgenomics.2001.5.1.11 |
Mountjoy KG, Mortrud MT, Low MJ, et al. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Molecular Endocrinology, 1994, 8(10): 1298-1308 |
Nogueiras R, Wiedmer P, Pereztilve D, et al. The central melanocortin system directly controls peripheral lipid metabolism. Journal of Clinical Investigation, 2007, 117(11): 3475-3488 DOI:10.1172/JCI31743 |
Ramos-Molina B, Martin MG, Lindberg I. PCSK1 variants and human obesity. Progress in Molecular Biology and Translational Science, 2016, 140: 47-74 DOI:10.1016/bs.pmbts.2015.12.001 |
Ringholm A, Fredriksson R, Poliakova N, et al. One melanocortin 4 and two melanocortin 5 receptors from zebrafish show remarkable conservation in structure and pharmacology. Journal of Neurochemistry, 2002, 82(1): 6-18 DOI:10.1046/j.1471-4159.2002.00934.x |
Ringholm A, Klovins J, Fredriksson R, et al. Presence of melanocortin (MC4) receptor in spiny dogfish suggests an ancient vertebrate origin of central melanocortin system. European Journal of Biochemistry, 2003, 270(2): 213-221 DOI:10.1046/j.1432-1033.2003.03371.x |
Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology, 2012, 153(11): 5587-5599 DOI:10.1210/en.2012-1470 |
Sandrock M, Schulz A, Merkwitz C, et al. Reduction in corpora lutea number in obese melanocortin-4-receptor-deficient mice. Reproductive Biology and Endocrinology, 2009, 7: 24 DOI:10.1186/1477-7827-7-24 |
Schjolden J, Schiöth HB, Larhammar D, et al. Melanocortin peptides affect the motivation to feed in rainbow trout (Oncorhynchus mykiss). General and Comparative Endocrinology, 2009, 160(2): 134-138 |
Shi XY, Xu YJ, Wu NN, et al. Preliminary studies on blind-side hypermelanosis of Cynoglossus semilaevis: Chromatophores observation and expression of proopiomelanocortin. Progress in Fishery Sciences, 2015, 36(2): 45-54 [史学营, 徐永江, 武宁宁, 等. 半滑舌鳎(Cynoglossus semilaevis)体表色素细胞观察及POMC表达特性分析. 渔业科学进展, 2015, 36(2): 45-54] |
Si LN, Wu D, Su W, et al. Observation of plasma GnRH, FSH levels and expression of POMC protein in hypothalamus in rats with lateral ventricle injection of GnIH. Shandong Medical Journal, 2017, 57(27): 34-36 [司丽娜, 吴迪, 苏玮, 等. 侧脑室注射微量GnIH大鼠的血浆GnRH、FSH水平及下丘脑组织POMC蛋白表达观察. 山东医药, 2017, 57(27): 34-36 DOI:10.3969/j.issn.1002-266X.2017.27.009] |
Sotonyi P, Mezei G, Racz B, et al. Gonadotropin-releasing hormone fibers contact POMC neurons in the hypothalamic arcuate nucleus. Reproductive Sciences, 2010, 17(11): 1024-1028 DOI:10.1177/1933719110378346 |
Stanley SA, Small CJ, Kim MS, et al. Agouti related peptide (Agrp) stimulates the hypothalamo pituitary gonadal axis in vivo and in vitro in male rats. Endocrinology, 1999, 140(11): 5459-5462 DOI:10.1210/endo.140.11.7248 |
Sutton GM, Duos B, Patterson LM, et al. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology, 2005, 146(9): 3739-3747 DOI:10.1210/en.2005-0562 |
Takeuchi S, Takahashi S. Melanocortin receptor genes in the chicken—Tissue distributions. General and Comparative Endocrinology, 1998, 112(2): 220-231 |
Tallam LS, Kuo JJ, da Silva AA, et al. Cardiovascular, renal, and metabolic responses to chronic central administration of agouti-related peptide. Hypertension, 2004, 44(6): 853-858 DOI:10.1161/01.HYP.0000148993.47498.b2 |
Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocrine Reviews, 2010, 31(4): 506-543 DOI:10.1210/er.2009-0037 |
Thody AJ, Wilson CA, Everard D. Facilitation and inhibition of sexual receptivity in the female rat by α-MSH. Physiology and Behavior, 1979, 22(3): 447-450 |
van der Ploeg LH, Martin WJ, Howard AD, et al. A role for the melanocortin 4 receptor in sexual function. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(17): 11381-11386 DOI:10.1073/pnas.172378699 |
Vongs A, Lynn NM, Rosenblum CI. Activation of MAP kinase by MC4-R through PI3 kinase. Regulatory Peptides, 2004, 120(1-3): 113-118 DOI:10.1016/j.regpep.2004.02.018 |
Wang J, Liu XL, Hou SS, et al. Cloning and expression pattern of MC4R gene in Beijing duck. Acta Agriculturae Boreali- Occidentalis Sinica, 2011, 20(1): 29-34 [王婕, 刘小林, 侯水生, 等. 北京鸭MC4R基因的克隆及其组织表达的差异. 西北农业学报, 2011, 20(1): 29-34 DOI:10.3969/j.issn.1004-1389.2011.01.006] |
Wei R, Yuan D, Zhou C, et al. Cloning, distribution and effects of fasting status of melanocortin 4 receptor (MC4R) in Schizothorax prenanti. Gene, 2013, 532(1): 100-107 |
Williams DL, Grill HJ, Weiss SM, et al. Behavioral processes underlying the intake suppressive effects of melanocortin 3/4 receptor activation in the rat. Psychopharmacology, 2002, 161(1): 47-53 |
Yada T, Moriyama S, Suzuki Y, et al. Relationships between obesity and metabolic hormones in the "cobalt" variant of rainbow trout. General and Comparative Endocrinology, 2002, 128(1): 36-43 |
Yang SH, Zhao LZ, Wang XQ, et al. Effect of Leptin on the neuroendocrine reproductive axis by regulating proopiomelanocortin neurons. Chinese Journal of Family Planning, 2010, 18(3): 143-146 [杨松鹤, 赵连志, 王小强, 等. 瘦素经POMC神经元对神经内分泌-生殖轴的影响. 中国计划生育学杂志, 2010, 18(3): 143-146 DOI:10.3969/j.issn.1004-8189.2010.03.004] |
Yi TL, Yang LK, Ruan GL, et al. Melanocortin-4 receptor in swamp eel (Monopterus albus): Cloning, tissue distribution, and pharmacology. Gene, 2018, 678: 79-89 DOI:10.1016/j.gene.2018.07.056 |
Yuan H, Zhang HY, Tian Y, et al. The melanocortin-4 receptor gene (MC4R) expression detected by SYBR green I real- time quantitative PCR in landes geese before and after overfeeding. Journal of Agricultural Biotechnology, 2011, 19(4): 692-697 [原昊, 张红艳, 田勇, 等. SYBR Green I荧光定量PCR法检测朗德鹅填饲前后黑素皮质素受体-4基因(MC4R)的表达. 农业生物技术学报, 2011, 19(4): 692-697 DOI:10.3969/j.issn.1674-7968.2011.04.014] |
Zhang KQ, Hou ZS, Wen HS, et al. Melanocortin-4 receptor in spotted sea bass, Lateolabrax maculatus: Cloning, tissue distribution, physiology, and pharmacology. Frontiers in Endocrinology (Lausanne), 2019, 10: 700-705 DOI:10.3389/fendo.2019.00700 |
Zhang ZJ, Cheng X, Liu HY, et al. cDNA cloning, sequence analysis and tissue expression of goat MC4R gene. Journal of Northwest A & F University(Natural Science), 2012, 40(12): 20-26 [张子军, 程箫, 刘洪瑜, 等. 山羊MC4R基因cDNA的克隆和序列分析及组织表达研究. 西北农林科技大学学报(自然科学版), 2012, 40(12): 20-26] |
Zhu XW, Xu YJ, Liu XZ, et al. Physiological mechanisms for degeneration of blind-side hypermelanosis in pond-cultured Japanese flounder (Paralichthys olivaceus). Progress in Fishery Sciences, 2017, 38(1): 103-110 [朱学武, 徐永江, 柳学周, 等. 池塘养殖牙鲆(Paralichthys olivaceus)无眼侧体色黑化消褪机理. 渔业科学进展, 2017, 38(1): 103-110] |



