鲑鳟鱼类即鲑科鱼类的统称。鲑鱼属深海冷水性鱼类,也是一种溯河洄游鱼类,它于生殖季节在淡水江河上游产卵,产卵后再回到海洋肥育。鲑鱼主要分布于太平洋、大西洋北部及北冰洋海区和沿岸诸水系,包括大西洋鲑(Salmo salar)、太平洋鲑属(Oncorhynchus spp.)、北鲑(Stenodus leucichthys nelma)等;而鳟鱼通常分布于淡水中,栖息在流速较缓的水域或湖泊,包括虹鳟(Oncorhynchus mykiss)、金鳟(Oncorhynchus aguabonita)、河鳟(Salmo fario Linnaeus)等。三文鱼通常指鲑科鱼类中的鲑鱼,主要为大西洋鲑。鲑鳟鱼在欧美国家养殖极为普遍,随着渔业生产技术的不断发展,我国鲑鳟鱼类养殖范围也在不断扩大,养殖品种也由单一的虹鳟扩大到金鳟、银鲑、大西洋鲑等(邰如玉等, 2019)。在养殖产业化和集约化程度不断提高的同时,寄生虫病也随之出现(陈国生, 2011)。寄生虫的感染可导致鱼体生长缓慢,抵抗力下降,组织损伤和死亡。鲑鳟鱼感染的寄生虫多数具有简单生活史和直接发育型(不需要中间宿主),这无疑加大了寄生虫病的治疗难度,大多数寄生虫病的防治至今仍是一个难题。深入了解鲑鳟鱼寄生虫的生活习性和危害,能在生产上预判疾病的发展规律,提前做好相关措施,减少损失。为此,本文就目前鲑鳟鱼类危害较为严重的寄生虫病(表 1)的病原学、生活史、危害性及防治方法的研究进行介绍,以期为国内养殖业者提供参考与借鉴。
|
|
表 1 鲑鳟鱼类常见寄生虫病 Tab.1 Common parasitic diseases of salmon and trout |
三代虫病(Gyrodactylus salaris)的病原是三代虫(Gyrodactylus),属单殖吸虫纲(Monogenoidea)、三代虫目(Gyrodactylidea)、三代虫科(Gyrodactylidae)、三代虫属(Gyrodactylus)。三代虫是一种专性寄生虫,胎生,生活史类型是直接型,无中间宿主。在一个生命周期中,三代虫个体一般产出2~4代子代,子代出生后24 h内就可产出子二代(Harris et al, 1994)。
三代虫的寄生是通过固着器的1对中央大钩和8对边缘小钩固着在鱼的体表和鳃上(图 1)。位于虫体前端的附着盘与后端的固着器相互配合,使三代虫在鱼体上做“尺蠖式”运动(李冉冉等, 2014)。少量寄生时无明显症状,大量寄生时,可导致鱼鳃丝黏液增多、鳃部浮肿、鳃组织损伤、体表发白,进而导致鱼窒息死亡。此外,三代虫的附着和摄食破坏了宿主鳃和表皮的完整性(图 2),导致其抵抗细菌及病毒的能力下降,进而有可能引发潜在的继发性感染(Busch et al, 2003)。对此病的诊断可用显微镜检查鳃的临时压片,若发现有大量三代虫寄生(每片鳃上有50只以上,或在低倍显微镜下,每个视野中有3~4只)时,可确诊为三代虫病。

|
图 1 三代虫固着器扫描电子显微照片 Fig.1 Scanning electron micrograph of Gyrodactylus fixator (Jørgensen et al, 2007) a:中央大钩 Hamulus;b:边缘小钩 Marginal hook |

|
图 2 感染三代虫病的虹鳟尾部严重溃烂 Fig.2 Caudal region of O. mykiss showing extensive caudal erosion associated with severe infections of G. salaris (You et al, 2006) |
温度是影响三代虫病暴发的最主要环境因子。在一定范围内,三代虫种群增长速率与水温呈正相关,其具体表现在随水温升高,虫体产出子代所需时间变短。因此,夏秋等高温季节有利于三代虫的流行与传播(Sereno-Uribe et al, 2012)。三代虫可寄生于宿主的所有发育阶段,但更偏好寄生于幼龄宿主(Cable et al, 2000)。该病是淡水养殖鲑鳟鱼类较为常见的寄生虫性疾病,大量寄生能引发虹鳟体表损伤甚至死亡。
养殖者广泛采取池水中泼洒福尔马林(37%甲醛)来治疗鲑鳟鱼类三代虫病(Stoltze et al, 2001)。在丹麦,Buchmann等(2003)用甲醛对虹鳟进行浸浴处理,暴露在浓度为20 mg/L的甲醛中18 h,可杀灭寄生虫;而5和10 mg/L的处理可显著减少寄生虫。一些抗虫药物(如H2O2)也可用于三代虫病的治疗。在美国,Rach等(2000)以严重患有三代虫病的虹鳟稚鱼为对象,按高浓度(560 mg/L) H2O2药浴30 min/d,连续处理3 d,结果显示,H2O2药浴可将病鱼体表寄生虫清除。Bowker等(2012a)以50 mg/L H2O2隔天药浴30 min自然感染的成年虹鳟,三代虫平均丰度减少99%。
2 鱼虱病鱼虱病(Fish lice disease)是由鱼虱寄生于鱼体表引起的疾病。鱼虱属于节肢动物门(Arthropoda)、甲壳纲(Crustaceans)、桡足亚纲(Copepoda)、鱼虱目(Caligoida)、鱼虱科(Argulidae)、鱼虱属(Argulus)。20世纪70年代,在大西洋鲑海水网箱养殖于挪威兴起之际,鱼虱病迅速出现。目前,该病已发展成为挪威、英格兰、爱尔兰、加拿大等国家海水养殖大西洋鲑最重要的寄生虫性疾病(Denholm et al, 2002),主要危害品种有大西洋鲑、大马哈鱼(Oncorhynchus kisutch)及虹鳟,其中,大西洋鲑和虹鳟是鱼虱最易感染的2个物种(Bravo et al, 2014)。
鲑鱼虱有11个形态不同的阶段(图 3)。雌虱具有2个卵囊,其中充满正在发育的胚胎。随着胚体孵化,I期无节幼体在水中产出,以旋转的运动方式离开。在10℃左右水温条件下,约4 d后,无节幼体变态成为感染性桡足幼体。此后,桡足幼体开始以宿主的黏液和表皮组织为食,经过4个附着幼体期和2个前体成虫期,最终发育至生殖成虫期(Gonzalez et al, 2003)。
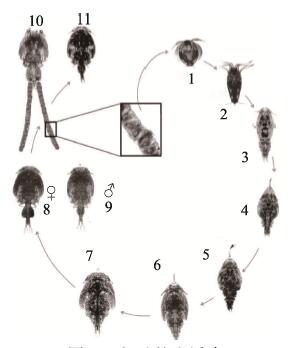
|
图 3 鱼虱的生活史 Fig.3 Life cycle of C. rogercresseyi (Gonzalez et al, 2003) 1:第1期无节幼体(0.43 mm);2:第2期无节幼体(0.46 mm);3:桡足幼体(0.66 mm);4:第1期幼体(0.83 mm);5:第2期幼体(1.27 mm);6:第3期幼体(2.15 mm);7:第4期幼体(3.15 mm);8:前体成虫期(雌, 4.10 mm);9:前体成虫期(雄, 4.10 mm);10:成虫(雌, 4.83 mm);11:成虫(雄, 4.79 mm) 1: First nauplius (0.43 mm); 2: Second nauplius (0.46 mm); 3: Copepodid (0.66 mm); 4: First chalimus (0.83 mm); 5: Second chalimus (1.27 mm); 6: Third chalimus (2.15 mm); 7: Fourth chalimus (3.15 mm); 8: Pre-adult stage (female, 4.10 mm); 9: Pre-adult stage (male, 4.10 mm); 10: Adult (female, 4.83 mm); 11: Adult (male, 4.79 mm) |
鱼虱流行季节为5~10月,7、8月最严重,25℃~30℃为其适宜流行水温(Bjørn et al, 2001)。鱼虱以寄主的的黏液、表皮组织和血液为食,造成体表机械损伤。早期寄生期的皮肤损伤通常相对较小,呈轻微感染(图 4A)。而较大和可移动的鱼虱成虫寄生可导致大面积的皮肤侵蚀,并伴随宿主的渗透调节问题和潜在的宿主死亡(图 4B)。对此病采取镜检的诊断方法,镜下观察有大量鱼虱寄生,即可确诊。
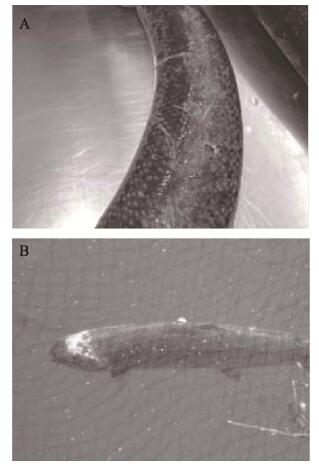
|
图 4 大西洋鲑感染鱼虱后的皮肤损伤 Fig.4 Skin damage of Atlantic salmon infected with fish lice (Burka et al, 2012) A:轻微感染,造成轻微磨损及失水B:严重感染,鱼虱已经穿过皮肤和肌肉进入头骨 A: Mild infection causing minor abrasion and fluid loss B: Severe infection where the lice have eaten through skin and flesh thereby exposing the skull |
对于鲑鳟鱼类鱼虱病的治疗对策,挪威养殖者采取降低放养密度、定期空箱休养、世代分群放养等措施对鱼虱病进行预防。20世纪90年代早期,有养殖者使用敌百虫等有机杀虫剂治疗鲑鳟鱼类鱼虱病(Costello, 1993),但敌百虫会对环境产生不利影响,且疗效不稳定。之后,在美国、加拿大、苏格兰、爱尔兰、挪威和智利等国,H2O2被广泛用于海水养殖大西洋鲑鱼虱病防治。Thomassen等(1993)研究表明,当被虱子感染的鲑鱼暴露在1500 mg/L的H2O2浓度下20 min,85%~100%的虱子能被有效清除。不过,依据Kiemer等(1997)的研究,H2O2有效处理浓度范围较窄(1200~1500 mg/L),过高H2O2浓度可能引起鳃组织损伤,并导致死亡。近年来,在挪威、芬兰等国开始使用合成除虫菊酯(氯氰菊酯、溴氰菊酯)药浴治疗鱼虱病(Aaen et al, 2015)。除药浴治疗外,饲料中预混杀虫药物也是世界上主要鲑鱼生产国综合防控鱼虱战略的重要组成部分(Ritchie et al, 2002; Bowker et al, 2012b)。表 2为控制鱼虱的常用方法。
|
|
表 2 控制鱼虱的常用方法 Tab.2 Common methods for controlling fish lice |
变形虫性鳃病(Amoebic gill disease, AGD)是由新阿米巴原虫引起的体外寄生虫病(Zilberg et al, 2001)。该病病原新阿米巴原虫隶属肉足鞭毛门(Sarcomastiugophora)、肉足纲(Lobosasida)、阿米巴目(Amoebida)。阿米巴原虫生活史分为滋养体(Trophont)和包囊期(Tomont) 2个阶段。其滋养体内外质分明,内质丰富,有大量的细小颗粒性内容物;外质透明,运动时外质伸出,形成伪足,能做定向运动(Young et al, 2014)。滋养体是摄食、活动和增殖的生活时期。包囊期是具有保护性外壁的生活阶段,无致病性(黄道超等, 2006)。包囊多呈球形,直径为10~16 μm,未染色时为一个折光性圆形小体,碘染色后呈黄色。AGD最早于1986年在塔斯马尼亚岛养殖的大西洋鲑中被发现,从那时起,世界上许多国家在不同的海洋有鳍鱼类中报道了AGD (Green et al, 2005)。近年来,AGD已成为欧洲大西洋鲑养殖业最严重的寄生虫病之一,给养殖业造成了严重的经济损失(Rodger, 2013)。
寄生虫与宿主的相互作用导致鳃上出现肉眼可见的多灶白色黏液斑块、黏液分泌过多,呼吸窘迫。严重患病的鱼鳃上部分白斑连成一片,局灶性鳃丝增生,板层囊肿(图 5A)。AGD的组织学证据是鳃组织出现原发性和继发性上皮增生、片层融合(图 5B)。
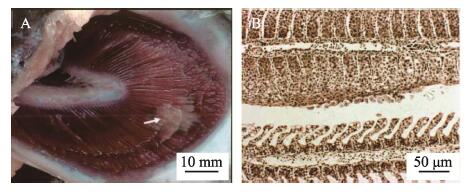
|
图 5 感染变形虫性鳃病的大西洋鲑临床症状 Fig.5 Clinical symptoms of Atlantic salmon infected with AGD (Johnson-MacKinnon et al, 2016) A:大西洋鲑鳃上出现白色的黏液斑块;B:受变形虫性鳃病影响的大西洋鲑鳃病理表现为上皮增生、片层融合 A: Pale mucoid patches (arrowhead) appearing upon the gills of Atlantic salmon; B: The pathological manifestations of the Atlantic salmon gills affected by AGD include epithelial hyperplasia and lamellar fusion |
大西洋鲑AGD发病主要受环境温度的影响(Lundebye et al, 1999)。AGD最初仅局限于夏季暴发,现在呈现出全年性问题。大西洋鲑AGD发病率呈双峰分布,第1个峰值较大,出现于夏季,第2个峰值较小,出现于秋季(Bridle et al, 2010)。此外,Clark等(1999)研究表明,盐度对AGD也起着重要作用。阿米巴原虫是一种海洋变形虫,对淡水的耐受性较差。因此,大西洋鲑在溯河洄游产卵期几乎不受感染。Kent等(1988)初步研究发现,阿米巴原虫在盐分短暂降低后就能从患病鱼鳃上清除。
Munday等(1993)研究发现,大多数外用治疗药物并不能根除该病,而通过淡水浴治疗可以迅速将变形虫从患病鱼鳃中根除。病原体的生长和生存需要海水,在盐度低于10的环境下生长缓慢,迄今为止这仍然是首选且有效的治疗方法(Powell et al, 2015)。在夏季AGD的高发期,每隔4~6周重复实施淡水浴,具有较好的杀虫效果(Powell et al, 2015)。在澳大利亚,淡水浴作为大西洋鲑AGD控制技术于20世纪80年代末最初引进时,实施2~3次即足以缓解AGD症状。但目前,在相同生产时期内,可能需要实施多达10次才会成功避免AGD蔓延(Adams et al, 2004)。另外,也有研究表明,在200~400 mg/L的剂量下,持续18~22 min H2O2浴对控制AGD感染也具有较好的效果(Rodger, 2013)。目前,研究者对AGD抗性的选择性育种进行了研究。在塔斯马尼亚进行的实验表明,褐鳟(Salmo trutta)和大西洋鲑杂交品种对AGD的抗性有了显著提高。具体来说,在遭受自然AGD感染的患病鱼中,大西洋鲑需要4次淡水浴治疗,杂交鲑鱼只需浸浴1次,同时,该杂交品种保持与大西洋鲑相当的存活率和生长速度(Maynard et al, 2016)。
4 鲑鱼旋转病鲑鱼旋转病(Salmonid whirling disease)的病原为脑碘泡虫,隶属黏体动物门(Myxozoa)、粘孢子虫纲(Myxosporea)、碘泡科(Myxobolidae)、碘泡虫属(Myxobolus)。脑碘泡虫有2个专性寄主,感染在鲑科鱼类宿主(如虹鳟)和淡水寡毛纲宿主颤蚓(Tubifex tubifex)之间交替发生(Nehring et al, 2016)。当寄生虫的黏孢子从受感染鱼的软骨中释放出来时(图 6A),就开始了其生命周期(图 6)。释放的黏孢子壳面呈卵圆形或圆形,缝面观呈瓜子形,2个等大的极囊位于孢子前端(图 6B)。要使生命周期继续下去,粘孢子需寄生在颤蚓的肠道内进行无性繁殖(图 6C)。生活史的最后阶段,虫体形成三叉放射孢子,正面呈锚状,孢子体与尾突之间有长约150 μm的柄,3个尾突等长,约200 μm (图 6D)。虫子释放到水体,在水体中感染下一个鱼宿主。
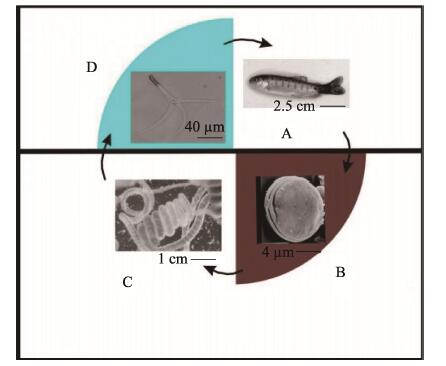
|
图 6 脑碘泡虫生活史 Fig.6 The life cycle of M. cerebralis (Andree et al, 1997) |
由脑碘泡虫引起的鲑鱼旋转病是存在于鲑鱼养殖场和野生种群的一个重大健康问题。当虫体迁移至鱼体软骨组织发育并开始吞噬软骨细胞后,鱼的听觉前庭器官严重受损并导致其保持身体直立方向的能力受损,引起病鱼的螺旋游泳运动。同时,寄生虫以鱼体软骨为食,引起鱼体脊髓和下脑干区域的收缩,可导致病鱼脊椎骨畸形。此外,脊柱后部的炎症也对负责控制色素沉积的尾神经造成损伤,从而导致黑尾(Rose et al, 2000; Chiaramonte et al, 2018)。在普通光学显微镜下观察,在鱼体脑组织中见到呈卵圆形或圆形、缝面呈瓜子形的孢子,可确诊为鲑鱼旋转病。
该病最早于100年前在欧洲的虹鳟身上发现,并不引起严重疾病,随着易感宿主虹鳟通过渔业贸易进入北美地区后,病原得以大量增殖(Bartholomew et al, 2002; Hogge et al, 2004)。随后,又随着渔业跨境贸易传播至非洲、大洋洲等地。目前,该病流行于北美、欧洲及日本等鲑鱼养殖区,对养殖幼苗期的鲑鱼危害严重,常常导致大量苗种死亡,死亡率可达90%以上。这种寄生虫能够感染包括大西洋鲑和淡水虹鳟在内的多个鲑科鱼类,如大西洋鲑、红大麻哈鱼(Oncorhynchus nerka)、虹鳟、金鳟、溪红点鲑(Salvelinus fontinalis)(Hedrick et al, 1999)。
脑碘泡虫的放射孢子期(Triactinomyxon, TAM),在水生寡毛纲宿主的消化道上皮细胞之间发育,是最易被破坏而失活的阶段。因此,针对TAM阶段的控制策略可能卓有成效(Wagner et al, 2003)。在水产养殖和渔业管理中,次氯酸钠(5.25%溶液,即漂白剂)、聚维酮碘(10%溶液)和H2O2被广泛用作消毒剂治疗外部寄生虫和病原体(Dubey et al, 2007)。除化学消毒外,病原控制还可通过冷冻、加热和干燥等物理手段来实现。Wagner等(2003)研究发现,冷冻、干燥1 h、温度高于75℃持续5 min均可有效杀灭TAM。此外,设备消毒能防止病原向未感染部位转移,也是控制该寄生虫病的重要措施。
5 增生性肾病增生性肾病(Proliferative kidney disease, PKD)的病原为苔藓鲑四囊虫,该病原隶属黏体动物门(Myxozoa)、软孢子虫纲(Malacosporea)。这种寄生虫的生命周期在淡水苔藓虫和鲑鳟鱼类之间交替进行(Morris et al, 2007)。处于单细胞阶段的寄生虫表现为隐性感染(Covert infection),此后,在苔藓虫体腔内增殖发育成多细胞孢囊(直径可达350 μm),寄生虫发展为显性感染(Overt infection)(Canning et al, 2000)。孢子(直径约为20 μm)在成熟孢囊内产生,每个孢子内有4个极囊。从苔藓虫体腔内释放到水中的孢子,通过极囊内的极丝附着在鱼身上,从而造成鱼的感染(图 7)。增生性肾病(PKD)被认为是鲑鳟鱼类最严重的寄生虫病之一,是造成养殖场和野生鱼类种群高死亡率的原因(Canning et al, 2003)。

|
图 7 苔藓鲑四囊虫的生活史 Fig.7 The life cycle of T. bryosalmonae (Okamura et al, 2015) |
从淡水苔藓虫中释放出来的寄生孢子主要通过鳃和皮肤侵入鱼体。随后,寄生虫经血液循环系统迁移至肾脏组织,并在其中进一步发育,引起炎症反应,损害肾组织(Longshaw et al, 2002)。PKD的外部临床症状包括鱼体发白、鳃贫血和腹部肿胀(Chilmonczyk et al, 2002);组织病理学特征包括肾间质增生、肾小管萎缩、白细胞浸润和肉芽肿性肾炎(图 8)。

|
图 8 无增生性肾病(左)和有增生性肾病(右)的褐鳟 Fig.8 Brown trout without proliferative kidney disease (PKD) (left) and with PKD(right)(Okamura et al, 2011) |
PKD是一种严重的季节性疾病。该病主要发生于5~11月,8、9月达到高峰(Wahli et al, 2002)。PKD的发生与温度有关,最适温度在12℃~18℃之间。温度升高会促进其他疾病的发生,进而加剧PKD的感染(Bettge et al, 2009)。该病主要感染幼鱼,死亡率高达95%(Okamura et al, 2011)。虹鳟、褐鳟、大西洋鲑、奇努克三文鱼(Oncorhynchus tshawytscha)和银大马哈鱼(O. kisutch)等多种鲑鳟鱼类均为易感品种(Sterud et al, 2007)。大西洋鲑在海水养殖季节与产卵季节均可受其感染,但因其产卵期在秋、冬季,感染率较低。
孔雀石绿和烟曲霉素等化学制剂对PKD的治疗有效,但由于其对人体的毒性,这些化学药物无法获得许可。因此,在缺乏有效控制措施的情况下,免疫治疗可能是一种有效的方法。存活下来的鱼对PKD再次感染具有免疫力。在生产上,养殖者在夏末或秋季气温开始下降时,将鱼苗暴露于苔藓虫感染,暴露在这种环境下的鱼,在接下来的一年里,当温度升高时,不会严重感染PKD,或者表现出PKD临床症状的减少(Kallert et al, 2011)。
6 库道虫病库道虫病(Kudoasis)的病原为库道虫属,该病原隶属于粘体动物门(Myxozoa)、粘孢子虫纲(Myxosporea)、多壳目(Multivalvulida)、库道虫科(Kudoidae)(Moran et al, 1999)。目前,世界上发现的库道虫有100多种,均寄生于海水鱼类中,淡水养殖的虹鳟不易感染(Kristmundsson et al, 2014)。关于库道虫的生命周期及其与寄主的关系尚不清楚,但与其他粘孢子虫一样,它被认为存在寄生于鱼类宿主和另一种无脊椎宿主的2个发育阶段(Jones et al, 2016)。
在世界范围内,库道虫已对开放式海水网箱中的大西洋鲑和银大马哈鱼造成严重感染,引起肌肉液化症(Soft-flesh syndrome)(Chase et al, 2001; Henning et al, 2012)。感染时,寄生虫寄生在宿主的骨骼肌细胞内,被结缔组织包围形成白色极性孢囊(图 9)。感染是隐性的,没有临床症状的发展,不会导致寄主死亡。但由寄生虫产生的蛋白酶会在鱼体死后降解鱼肉,影响鱼片纹理和肌肉的价值,严重影响鲑鱼的销售价值(Funk et al, 2008)。
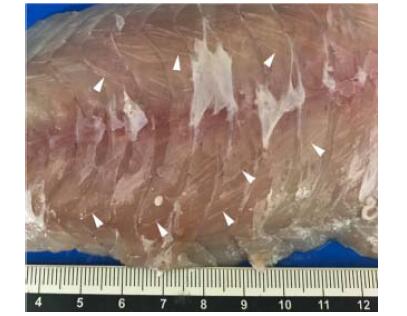
|
图 9 生长在肌纤维中的库道虫孢子(箭头所示白色条纹) Fig.9 Spores of K. thyrsites growing in the myofiber, and some of them (white stripes) are indicated by arrowheads (Sakai et al, 2019) |
库道虫感染在夏、秋季最易发生,在冬季和早春月份不易发生。感染的进程与环境温度密切相关,随水温的升高,库道虫感染的速度加快(Moran et al, 1999)。
受库道虫特异性疫苗缺乏和获批治疗方法的限制,目前,鲑鱼养殖者对库道虫的控制以预防为主,如饲料中预混化学药物和早期检测。几种口服化合物已被证明对治疗鲑鱼黏体寄生虫的感染有效。如烟曲霉素可用于治疗大西洋鲑宿主中的几种黏液寄生虫(Athanassopoulou et al, 2004);在养殖过程中,饲喂含有尼卡巴嗪(Nicarbazin)的鱼饲料对控制库道虫也有一定效果(Jones et al, 2012)。另外,预防养殖鲑鱼库道虫的感染也可通过早期检测的手段,可通过对新鲜组织的显微镜检查直接实现,也可通过血清学或分子方法间接实现(Taylor et al, 2005)。
7 其他寄生虫病除上述几种危害较为严重的鲑鳟鱼类寄生虫病外,还有一些由微孢子虫、多子小瓜虫、萨氏角形虫等病原引起的鲑鳟鱼类寄生虫病,对鲑鳟鱼类养殖业也造成了不同程度的影响。
7.1 多子小瓜虫多子小瓜虫属于纤毛门(Ciliophora)、寡膜纲(Oligohymenophorea)、膜口目(Hymenostomalida)、凹口科(Ophryoglenidae)、小瓜虫属(Ichthyophthirius)。虫体呈卵圆形或球形,全身密布短而均匀的纤毛。由该病原引起的小瓜虫病仅对淡水养殖的虹鳟和金鳟的稚鱼危害较大。小瓜虫主要寄生在鱼类的皮肤、鳍、鳃、头、口腔和眼等部位,形成肉眼可见的白色点状胞囊。发病的鱼游动缓慢,反应迟钝,肉眼可见其背部、头部和尾部等部位有许多针尖大小的白点,俗称“白点病”(姚嘉赟等, 2019)。小瓜虫生长的适宜水温为15℃~25℃,在水温降至10℃以下或升至28℃以上时小瓜虫停止发育。因此,温度升高或者降低都可控制病情(张其中等, 2010)。
7.2 微孢子虫微孢子虫属于微孢子虫纲(Microsporididae)、微孢子目(Microsporida)、微孢子虫属(Microsporidia)。该病原主要寄生于鲑鳟鱼的鳃部,引起微孢子虫鳃病(Microsporidial gill disease, MGD)(Ramsay et al, 2003)。在鱼鳃的内皮细胞和支柱细胞内,产生充满孢子的异种瘤,异种瘤在孢子成熟时破裂,引发严重的鳃部发炎和上皮增生性反应,导致呼吸窘迫,直至死亡(Rodríguez-Tovar et al, 2003)。该病发生在春季水温上升的时候,每年夏末、秋初季节流行趋势较强(Kent et al, 1995)。微孢子虫主要感染太平洋鲑鱼属的虹鳟和奇努克三文鱼(Shaw et al, 2000)。用灭活的孢子腹腔注射接种于虹鳟,能使虹鳟对微孢子虫的再次感染产生抗性,保护养殖三文鱼免受MGD的感染具有可行性(Rodríguez-Tovar et al, 2006)。
7.3 萨氏角形虫萨氏角形虫是一种黏体动物寄生虫,主要存在于太平洋西北部和加利福尼亚的一些水域,主要感染虹鳟等鲑科鱼类。萨氏角形虫是一种组织内寄生虫,以侵入宿主肠道的方式感染鲑鳟鱼类,引起肠道角化病。该病以肠出血和坏死为特征,寄生虫侵入肠道组织,引发急性炎症反应,从而导致鱼类宿主死亡(Bjork et al, 2009)。感染主要发生在4~12月。感染的严重程度取决于多种因素,如水温、鱼宿主的固有抗性和寄生虫剂量(Zatti et al, 2017)。目前,还没有发现彻底治愈肠道角化病的药物,但药物和化学治疗在降低萨氏角形虫感染的严重程度方面被证明是有效的。如烟曲霉素的产物富马西林可在一定程度上减少虹鳟萨氏角形虫的自然感染(Whipple et al, 2002)
8 总结与展望随着鲑鳟鱼类养殖规模的增加,鲑鳟鱼类寄生虫病的研究已经成为水产疾病研究的热点。目前,人们对大部分鲑鳟鱼类寄生虫的生活史、流行情况、危害已基本明确,并结合生产实践总结和探索出一些行之有效的防控措施。但是,由于鲑鳟鱼类寄生虫的种类繁多,涉及到原生动物和甲壳动物的许多种和属,且在养殖环境逐渐变差的情况下,可能会出现新的寄生虫病。因此,在寄生虫病的治疗方面至今还没有效果显著的药物。中草药提取液中含活性杀菌成分,开发中草药药物杀灭寄生虫是研究的热点。鱼体表黏液中提取的蛋白也具有一定的抗虫效果,是今后寄生虫病防治的研究热点。此外,在一些海水鱼的寄生虫病防治上,抗寄生虫疫苗也相继开发出来,可采取类似的手段,针对鲑鳟鱼寄生虫病开发相应的疫苗。
相信在不久的将来,随着科学技术的发展,研究方法和手段的不断更新,鲑鳟鱼类寄生虫病的研究能不断取得突破性成果,为寄生虫病的防治开辟新天地。
Aaen SM, Helgesen KO, Bakke MJ, et al. Drug resistance in sea lice: A threat to salmonid aquaculture. Trends in Parasitology, 2015, 31(2): 72-81 DOI:10.1016/j.pt.2014.12.006 |
Adams MB, Nowak BF. Sequential pathology after initial freshwater bath treatment for amoebic gill disease in cultured Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 2004, 27(3): 163-173 DOI:10.1111/j.1365-2761.2004.00531.x |
Andree KB, Gresoviac SJ, Hedrick RP. Small subunit ribosomal RNA sequences unite alternate actinosporean and myxosporean stages of Myxobolus cerebralis the causative agent of whirling disease in salmonid fish. Journal of Eukaryotic Microbiology, 1997, 44(3): 208-215 DOI:10.1111/j.1550-7408.1997.tb05702.x |
Athanassopoulou F, Karagouni E, Dotsika E, et al. Efficacy and toxicity of orally administrated anti-coccidial drugs for innovative treatments of Myxobolussp. infection in Puntazzo puntazzo. Diseases of Aquatic Organisms, 2004, 62(3): 217-226 |
Bartholomew JL, Reno PW. The history and dissemination of whirling disease. American Fisheries Society Symposium, 2002(29): 3-24 |
Bettge K, Segner H, Burki R, et al. Proliferative kidney disease (PKD) of rainbow trout: Temperature- and time- related changes of Tetracapsuloides bryosalmonae DNA in the kidney. Parasitology, 2009, 136(6): 615-625 DOI:10.1017/S0031182009005800 |
Bjork SJ, Bartholomew JL. Effects of Ceratomyxa shasta dose on a susceptible strain of rainbow trout and comparatively resistant chinook and coho salmon. Diseases of Aquatic Organisms, 2009, 86(1): 29-37 |
Bjørn PA, Finstad B, Kristoffersen R. Salmon lice infection of wild sea trout and Arctic char in marine and freshwaters: The effects of salmon farms. Aquaculture Research, 2001, 32(12): 947-962 DOI:10.1046/j.1365-2109.2001.00627.x |
Bowker JD, Carty D, Dotson MM. Efficacy of 35% PEROX- AID (hydrogen peroxide) in reducing an infestation of Gyrodactylus salmonis in freshwater-reared rainbow trout. North American Journal of Aquaculture, 2012a, 74(2): 154-159 DOI:10.1080/15222055.2012.675992 |
Bowker JD, Carty DG, Wandelear N, et al. Efficacy of SLICE premix (0.2% Emamectin Benzoate) for reducing infestations of Salmincola spp. on freshwater-reared rainbow trout.. North American Journal of Aquaculture, 2012b, 74(3): 428-437 DOI:10.1080/15222055.2012.676019 |
Bravo S, Silva MT, Treasurer J. Factors affecting the abundance of Caligus rogercresseyi (Boxshall and Bravo) on farmed salmonids in Chile in the period 2006–2007. Aquaculture, 2014, 434: 456-461 DOI:10.1016/j.aquaculture.2014.09.009 |
Bridle AR, Crosbie PBB, Cadoret K, et al. Rapid detection and quantification of Neoparamoebaperurans in the marine environment. Aquaculture, 2010, 309(1–4): 56-61 |
Buchmann K, Kristensson RT. Efficacy of sodium percarbonate and formaldehyde bath treatments against Gyrodactylus derjavini infestations of rainbow trout. North American Journal of Aquaculture, 2003, 65(1): 25-27 DOI:10.1577/1548-8454(2003)065<0025:EOSPAF>2.0.CO;2 |
Burka JF, Fast MD, Revie CW. Lepeophtheirus salmonis and Caligus rogercresseyi. In: Woo PTK, Buchmann K. Fish parasites: Pathobiology and protection. Guelph, Ontario, Canada: University of Guelph, 2012, 350
|
Busch S, Dalsgaard I, Buchmann K. Concomitant exposure of rainbow trout fry to Gyrodactylus derjavini and Flavobacterium psychrophilum: Effects on infection and mortality of host. Veterinary Parasitology, 2003, 117(1–2): 117-112 |
Cable J, Harris PD, Bakke TA. Population growth of Gyrodactylus salaris (Monogenea) on Norwegian and Baltic Atlantic salmon (Salmo salar) stocks. Parasitology, 2000, 121(6): 621-629 |
Canning EU, Curry A, Feist SW, et al. A new class and order of myxozoans to accommodate parasites of bryozoans with ultrastructural observations on Tetracapsula bryosalmonae (PKX organism). Journal of Eukaryotic Microbiology, 2000, 47(5): 456-468 DOI:10.1111/j.1550-7408.2000.tb00075.x |
Canning EU, Okamura B. Biodiversity and evolution of the Myxozoa. Advances in Parasitology, 2003, 56: 43-131 DOI:10.1016/S0065-308X(03)56002-X |
Chase JC, Dawson-Coates JA, Haddow JD, et al. Analysis of Kudoa thyrsites (Myxozoa: Myxosporea) spore antigens using monoclonal antibodies. Diseases of Aquatic Organisms, 2001, 45(2): 121-129 |
Chen GS. Epidemiological investigation and analysis of parasitic diseases of cage-cultured fish in Luoyuan Bay. Fujian Journal of Animal Husbandry and Veterinary, 2011, 33(3): 11-14 |
陈国生. 罗源湾网箱养殖鱼类寄生虫病流行病学调查与分析. 福建畜牧兽医, 2011, 33(3): 11-14 |
Chiaramonte LV, Burbank D, Scott R, et al. Comparison of sampling and detection methods for chinook salmon and steelhead naturally infected with Myxobolus cerebralis. Journal of Aquatic Animal Health, 2018, 30(1): 57-64 DOI:10.1002/aah.10008 |
Chilmonczyk S, Monge D, de Kinkelin P. Proliferative kidney disease: Cellular aspects of the rainbow trout, Oncorhynchus mykiss (Walbaum), response to parasitic infection. Journal of Fish Diseases, 2002, 25(4): 217-226 DOI:10.1046/j.1365-2761.2002.00362.x |
Clark A, Nowak BF. Field investigations of amoebic gill disease in Atlantic salmon, Salmo salar L, in Tasmania. Journal of Fish Diseases, 1999, 22(6): 433-443 DOI:10.1046/j.1365-2761.1999.00175.x |
Costello MJ. Review of methods to control sea-lice (Caligidae: Crustacea) infestations on salmon farms. In: Boxshall GA, Defaye D (Ed.). Pathogens of wild and farmed fish: Sea lice. Chichester: Ellis Horwood Limited Publisher, 1993
|
Denholm I, Devine GJ, Horsberg TE, et al. Analysis and management of resistance to chemotherapeutants in salmon lice, Lepeophtheirus salmonis (Copepoda: Caligidae). Pest Management Science, 2002, 58(6): 528-536 DOI:10.1002/ps.482 |
Dubey RJ, Caldwell CA, Gould WR. Relative susceptibility and effects on performance of Rio Grande Cutthroat trout and rainbow trout challenged with Myxobolus cerebralis. Transactions of the American Fisheries Society, 2007, 136(5): 1406-1414 DOI:10.1577/T06-251.1 |
Funk VA, Olafson RW, Raap M, et al. Identification, characterization and deduced amino acid sequence of the dominant protease from Kudoa paniformis and K. thyrsites: A unique cytoplasmic cysteine protease. Comparative Biochemistry and Physiology Part B Biochemistry and Molecular Biology, 2008, 149(3): 477-489 DOI:10.1016/j.cbpb.2007.11.011 |
Gonzalez L, Carvajal J. Life cycle of Caligus rogercresseyi, (Copepoda: Caligidae) parasite of Chilean reared salmonids. Aquaculture, 2003, 220(1): 101-117 |
Green TJ, Powell MD, Harris JO, et al. Effects of dissolved organic carbon and hardness in freshwater used to treat amoebic gill disease. Aquaculture Research, 2005, 36(4): 398-404 DOI:10.1111/j.1365-2109.2005.01221.x |
Harris PD, Jansen PA, Bakke TA. The population age structure and reproductive biology of Gyrodactylus salaris Malmberg (Monogenea). Parasitology, 1994, 108(2): 167-173 DOI:10.1017/S0031182000068268 |
Hedrick RP, McDowell TS, Gay M, et al. Comparative susceptibility of rainbow trout Oncorhynchus mykiss and brown trout Salmo trutta to Myxobolus cerebralis, the cause of salmonid whirling disease. Diseases of Aquatic Organisms, 1999, 37(3): 173-183 |
Henning SS, Hoffman LC, Manley M. A review of Kudoa-induced myoliquefaction of marine fishes in South Africa and other countries. South African Journal of Science, 2012, 109(11–12): 1-15 |
Hogge C, Campbell M, Johnson K. Discriminating between a neurotropic Myxobolusspand M. cerebralis, the causative agent of salmonid whirling disease. Journal of Aquatic Animal Health, 2004, 16(3): 137-144 DOI:10.1577/H04-007.1 |
Huang DC, Yang GY, Wang Q, et al. Progress on amoebiasis of human and animals. Progress in Veterinary Medicine, 2006, 27(5): 51-55 |
黄道超, 杨光友, 王强, 等. 人和动物阿米巴原虫病研究进展. 动物医学进展, 2006, 27(5): 51-55 |
Johnson-MacKinnon J, Oldham T, Nowak B. Amoebic gill disease: A growing threat. Microbiology Australia, 2016, 37(3): 140-142 DOI:10.1071/MA16048 |
Jones SRM, Cho S, Nguyen J, et al. Acquired resistance to Kudoa thyrsites in Atlantic salmon Salmo salar following recovery from a primary infection with the parasite. Aquaculture, 2016, 451: 457-462 DOI:10.1016/j.aquaculture.2015.10.002 |
Jones SRM, Forster I, Liao XJ, et al. Dietary nicarbazin reduces prevalence and severity of Kudoa thyrsites (Myxosporea: Multivalvulida) in Atlantic salmon Salmo salar post-smolts. Aquaculture, 2012, 342–343: 1-6 |
Jørgensen TR, Larsen TB, Jørgensen LG, et al. Characterisation of a low pathogenic form of Gyrodactylus salaris from rainbow trout. Diseases of Aquatic Organisms, 2007, 73(3): 235-244 |
Kallert DM, Bauer W, Haas W, et al. No shot in the dark: Myxozoans chemically detect fresh fish. International Journal for Parasitology, 2011, 41(3–4): 271-276 |
Kent ML, Dawe SC, Speare DJ. Transmission of Loma salmonae (Microsporea) to chinook salmon in sea water. Canadian Veterinary Journal, 1995, 36(2): 98-101 |
Kent ML, Sawyer TK, Hedrick RP. Paramoeba pemaquidensis (Sarcomastigophora: Paramoebidae) infestation of the gills of coho salmon Oncorhynchus kisutch reared in sea water. Diseases of Aquatic Organisms, 1988, 5(3): 163-169 |
Kiemer MCB, Black KD. The effects of hydrogen peroxide on the gill tissues of Atlantic salmon, Salmo solar L.. Aquaculture, 1997, 153(3–4): 181-189 |
Li RR, Li WX, Wu XD, et al. Identification of Gyrodactylus species in goldfish (Carassius auratus) through morphological study and the analysis of the rDNA ITS sequence. Acta Hydrobiologica Sinica, 2014, 38(5): 903-909 |
李冉冉, 李文祥, 吴旭东, 等. 金鱼寄生三代虫的形态学及基于rDNA ITS序列的分子鉴定. 水生生物学报, 2014, 38(5): 903-909 |
Longshaw M, Le Deuff RM, Harris AF, et al. Development of proliferative kidney disease in rainbow trout, Oncorhynchus mykiss (Walbaum), following short-term exposure to Tetracapsula bryosalmonae infected bryozoans. Journal of Fish Diseases, 2002, 25(8): 443-449 DOI:10.1046/j.1365-2761.2002.00353.x |
Lundebye AK, Berntssen MHG, Bonga SEW, et al. Biochemical and physiological responses in Atlantic salmon (Salmo salar) following dietary exposure to copper and cadmium. Marine Pollution Bulletin, 1999, 39(1–12): 137-144 |
Maynard BT, Taylor RS, Kube PD, et al. Salmonid heterosis for resistance to amoebic gill disease (AGD). Aquaculture, 2016, 451: 106-112 DOI:10.1016/j.aquaculture.2015.09.004 |
Moran JDW, Kent ML, Whitaker DJ. Kudoa thyrsites (Myxozoa: Myxosporea) infections in pen-reared Atlantic salmon in the Northeast Pacific Ocean with a survey of potential nonsalmonid reservoir hosts. Journal of Aquatic Animal Health, 1999, 11(2): 101-109 DOI:10.1577/1548-8667(1999)011<0101:KTMMII>2.0.CO;2 |
Morris DJ, Adams A. Sacculogenesis and sporogony of Tetracapsuloides bryosalmonae (Myxozoa: Malacosporea) within the bryozoan host Fredericella sultana (Bryozoa: Phylactolaemata). Parasitology Research, 2007, 100(5): 983-992 DOI:10.1007/s00436-006-0371-0 |
Munday BL, Lange K, Foster C, et al. Amoebic gill disease of sea-caged salmonids in Tasmanian waters. Tasmanian Fish Research, 1993, 28: 14-19 |
Nehring RB, Schisler GJ, Chiaramonte L, et al. Accelerated deactivation of Myxobolus cerebralis myxospores by susceptible and non-susceptible Tubifex tubifex. Diseases of Aquatic Organisms, 2016, 121(1): 37-47 DOI:10.3354/dao03025 |
Okamura B, Gruhl A, Reft AJ. Cnidarian origins of the Myxozoa. Myxozoan Evolution Ecology and Development, 2015, 45-68 |
Okamura B, Hartikainen H, Schmidt-Posthaus H, et al. Life cycle complexity, environmental change and the emerging status of salmonid proliferative kidney disease. Freshwater Biology, 2011, 56(4): 735-753 DOI:10.1111/j.1365-2427.2010.02465.x |
Parsons H, Nowak B, Fisk D, et al. Effectiveness of commercial freshwater bathing as a treatment against amoebic gill disease in Atlantic salmon. Aquaculture, 2001, 195(3–4): 205-210 |
Powell MD, Reynolds P, Kristensen T. Freshwater treatment of amoebic gill disease and sea-lice in seawater salmon production: Considerations of water chemistry and fish welfare in Norway. Aquaculture, 2015, 448: 18-28 DOI:10.1016/j.aquaculture.2015.05.027 |
Rach JJ, Gaikowski MP, Ramsay RT. Efficacy of hydrogen peroxide to control parasitic infestations on hatchery-reared fish. Journal of Aquatic Animal Health, 2000, 12(4): 267-273 DOI:10.1577/1548-8667(2000)012<0267:EOHPTC>2.0.CO;2 |
Ramsay JM, Speare DJ, Becker JA, et al. Loma salmonae- associated xenoma onset and clearance in rainbow trout, Oncorhynchus mykiss (Walbaum): Comparison of per os and cohabitations exposure using survival analysis. Aquaculture Research, 2003, 34(14): 1329-1335 DOI:10.1046/j.1365-2109.2003.00952.x |
Ritchie G, Rønsberg SS, Hoff KA, et al. Clinical efficacy of teflubenzuron (Calicide) for the treatment of Lepeophtheirus salmonis infestations of farmed Atlantic salmon Salmo salar at low water temperatures. Diseases of Aquatic Organisms, 2002, 51(2): 101-106 |
Rodger HD. Amoebic gill disease (AGD) in farmed salmon (Salmo salar) in Europe. Fish Veterinary Journal, 2013, 14: 16-27 |
Rodríguez-Tovar LE, Becker JA, Markham RJF, et al. Induction time for resistance to microsporidial gill disease caused by Loma salmonae following vaccination of rainbow trout (Oncorhynchus mykiss) with a spore-based vaccine. Fish and Shellfish Immunology, 2006, 21(2): 170-175 DOI:10.1016/j.fsi.2005.11.009 |
Rodríguez-Tovar LE, Wright GM, Wadowska DW, et al. Ultrastructural study of the late stages of Loma salmonae development in the gills of experimentally infected rainbow trout. Journal of Parasitology, 2003, 89(3): 464-474 DOI:10.1645/0022-3395(2003)089[0464:USOTLS]2.0.CO;2 |
Rose JD, Marrs GS, Lewis C, et al. Whirling disease behavior and its relation to pathology of brain stem and spinal cord in rainbow trout. Journal of Aquatic Animal Health, 2000, 12(2): 107-118 DOI:10.1577/1548-8667(200006)012<0107:WDBAIR>2.0.CO;2 |
Sakai H, Kawai T, Zhang J, et al. New host records of three Kudoa spp. (K. yasunagai, K. thalassomi, and K. igami) with notable variation in the number of shell valves and polar capsules in spores. Parasitology Research, 2019, 118(1): 143-157 DOI:10.1007/s00436-018-6144-8 |
Kristmundsson Á, Freeman MA. Negative effects of Kudoa islandica n. sp. (Myxosporea: Kudoidae) on aquaculture and wild fisheries in Iceland. International Journal for Parasitology Parasites and Wildlife, 2014, 3(2): 135-146 DOI:10.1016/j.ijppaw.2014.06.001 |
Sereno-Uribe AL, Zambrano L, García-Varela M. Reproduction and survival under different water temperatures of Gyrodactylus mexicanus (Platyhelminthes: Monogenea), a parasite of Girardinichthys multiradiatus in central Mexico. Journal of Parasitology, 2012, 98(6): 1105-1108 DOI:10.1645/GE-3053.1 |
Shaw RW, Kent ML, Brown AM, et al. Experimental and natural host specificity of Loma salmonae (Microsporidia). Diseases of Aquatic Organisms, 2000, 40(2): 131-136 |
Sterud E, Forseth T, Ugedal O, et al. Severe mortality in wild Atlantic salmon Salmo salar due to proliferative kidney disease (PKD) caused by Tetracapsuloides bryosalmonae (Myxozoa). Diseases of Aquatic Organisms, 2007, 77(3): 191-198 |
Stoltze K, Buchmann K. Effect of Gyrodactylus derjavini infections on cortisol production in rainbow trout fry. Journal of Helminthology, 2001, 75(3): 291-294 |
Tai RY, Xu J, Jiang YL, et al. Development of a universal 96 single nucleotide polymorphism array for salmonid fishes. Progress in Fishery Sciences, 2019, 40(1): 53-61 |
邰如玉, 许建, 江炎亮, 等. 鲑鳟通用型低通量单核苷酸多态性芯片的开发. 渔业科学进展, 2019, 40(1): 53-61 |
Taylor K, Jones S. An enzyme linked immunosorbent assay for the detection of Kudoa thyrsites in Atlantic salmon Salmo salar. Aquaculture, 2005, 250(1–2): 8-15 |
Thomassen JM. Hydrogen peroxide as a delousing agent for Atlantic salmon. In: Boxshall GA, Defaye D. Pathogens of wild and farmed fish: Sea lice. Ellis Horwood Limited Chichester UK, New York, USA. 1993, 290–295
|
Wagner EJ, Smith M, Arndt R, et al. Physical and chemical effects on viability of the Myxobolus cerebralis triactinomyxon. Diseases of Aquatic Organisms, 2003, 53(2): 133-142 |
Wahli T, Knuesel R, Bernet D, et al. Proliferative kidney disease in Switzerland: Current state of knowledge. Journal of Fish Diseases, 2002, 25(8): 491-500 DOI:10.1046/j.1365-2761.2002.00401.x |
Whipple MJ, Gannam AL, Bartholomew JL. Inability to control ceratomyxosis in rainbow trout and steelhead with oral treatments of glucan immunostimulant or the fumagillin analog TNP-470. North American Journal of Aquaculture, 2002, 64(1): 1-9 DOI:10.1577/1548-8454(2002)064<0001:ITCCIR>2.0.CO;2 |
Yao JY, Xu Y, Yuan XM, et al. Synthesis of 1-methyl-1, 2, 3, 4- tetrahydroisoquinoline derivatives and its antiparasitic activity against Ichthyophthirius multifiliis in vitro. Progress in Fishery Sciences, 2019, 40(1): 141-146 |
姚嘉赟, 徐洋, 袁雪梅, 等. 四氢异喹啉衍生物的合成及体外抗多子小瓜虫活性的研究. 渔业科学进展, 2019, 40(1): 141-146 |
You P, Yuan B, Yang J, et al. Pathogenic infections of Gyrodactylus brachymystacis (Monogenea) on Oncorhynchus mykiss (Walbaum) at a fish farm in the Qinling Mountain region of China. Journal of Fish Diseases, 2006, 29(5): 313-316 DOI:10.1111/j.1365-2761.2006.00711.x |
Young ND, Dyková I, Crosbie PB, et al. Support for the coevolution of Neoparamoeba and their endosymbionts, Perkinsela amoebae-like organisms. European Journal of Protistology, 2014, 50(5): 509-523 DOI:10.1016/j.ejop.2014.07.004 |
Zatti SA, Atkinson SD, Bartholomew JL, et al. Amazonian waters harbour an ancient freshwater Ceratomyxa lineage (Cnidaria: Myxosporea). Acta Tropica, 2017, 169: 100-106 DOI:10.1016/j.actatropica.2017.02.006 |
Zhang QZ, Zhang ZH, Chen DL, et al. Influence of water temperature on hatchability and activity power of theront of Ichthyophthirius multifiliis. Ecological Science, 2010, 29(2): 116-120 |
张其中, 张占会, 陈达丽, 等. 水温对多子小瓜虫孵化及幼虫活力的影响. 生态科学, 2010, 29(2): 116-120 |
Zilberg D, Gross A, Munday BL. Production of salmonid amoebic gill disease by exposure to Paramoebasp. harvested from the gills of infected fish. Journal of Fish Diseases, 2001, 24(2): 79-82 |



