2. 上海海洋大学 水产种质资源发掘与利用教育部重点实验室 上海 201306;
3. 南方海洋科学与工程广东省实验室 广东 广州 511458;
4. 浙江省嵊泗县海洋科技研究所 浙江 舟山 202450
2. Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Shanghai Ocean University, Shanghai 201306, China;
3. Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory, Guangzhou, Guangdong 511458, China;
4. Shengsi Institute of Marine Science and Technology in Zhejiang Province, Zhoushan, Zhejiang 202450, China
厚壳贻贝(Mytilus coruscus)为一种具备较高营养的经济贝类(Wang et al, 2012; 徐嘉康等, 2017),常见于东海、黄海和渤海沿岸海域,主产区为浙江舟山东海海域,其养殖生产已发展了十几年,年度平均养殖数目高达50~60亿粒以上(王如才等, 1998; 罗友声等, 2003)。但近年来,由于贻贝养殖区域划分不规范、各级管理存在缺陷、贻贝灾害频发等问题,造成厚壳贻贝资源衰退(王靖陶等, 2010)。为了恢复和发展厚壳贻贝的野生资源,陆地工厂化人工育苗、海区增殖放流、贻贝海洋牧场养殖示范区建设等技术与产业迅速发展起来(张义浩等, 2003; 常抗美, 2007; 罗海忠等, 2016)。厚壳贻贝在其生活史中必须经历从浮游生活阶段过渡到附着生活阶段的变态发育过程,才可长成成贝(Wang et al, 2012)。附着变态后的贻贝也可通过切断其足丝的方式,寻找新的适宜环境进行二次附着(李太武, 2013)。因此,如何提高厚壳贻贝幼虫的附着变态率就成为其增养殖相关产业发展的核心技术问题。
研究表明,由海洋细菌所形成的生物被膜可以实现对大部分海洋无脊椎动物幼虫附着变态情况的调节(杨金龙等, 2012)。例如,假交替单胞菌属(Pseudoalteromonas)的某些细菌(Uhlinger et al, 1983; Holmström et al, 1999)可形成对厚壳贻贝(Yang et al, 2013)和华美盘管虫(Hydroides elegans) (Shikuma et al, 2014)等幼虫附着变态具有显著诱导作用的生物被膜。生物被膜的形成受许多因素的影响(Yang et al, 2014、2016a、2016b)。其中,对生物机体生理生化有影响的钙、铁离子等营养素会导致海洋细菌形成的生物被膜的形态结构、分布和蛋白含量具有差异,并影响厚壳贻贝的附着(孙俊杰等, 2016; 常睿珩等, 2020)。
B族维生素作为一类有机物质,广泛存在于自然环境中,是维持生物体生命活动的重要活性物质(Degnan et al, 2014)。它能够调控大多数细菌的生理和生化反应,例如糖代谢、脂代谢、蛋白代谢和DNA合成等,从而影响生物体生长发育(Woods et al, 1953; 王春华等, 2011; 王雅平等, 2019; 周莹等, 2020)。研究表明,B族维生素通过影响布鲁杆菌属(Brucella)和沙门菌属(Salmonella)细菌的主要营养物质代谢等生理生化反应进而调控细菌生物量(Matras, 1973);B族维生素可显著促进海蠕虫(Capitella teleta)幼虫附着变态(Burns et al, 2018)。其中,维生素B7(VB7)和B12(VB12)以辅基或辅酶的形式参与机体的生理生化反应,调节新陈代谢并维持细胞、器官和组织结构和功能的完整,确保生命活动正常运行(Brown, 1989; Knowles et al, 1989; Ekhard et al, 1998)。但B族维生素对海洋细菌生物被膜形成及海洋贝类幼虫变态的影响尚不清楚。
本研究通过将厚壳贻贝眼点幼虫直接暴露于VB7和VB12中,以及在对厚壳贻贝幼虫附着变态具有高诱导活性的海假交替单胞菌(Pseudoalteromonas marina)生物被膜形成过程中添加VB7和VB12,明确其对厚壳贻贝幼虫附着变态的影响,探索B族维生素、海洋细菌生物被膜形成和海洋贝类幼虫变态三者间的相互关系,为解析厚壳贻贝附着变态分子机制提供新思路和理论依据。同时,也为B族维生素应用于海洋牧场建设中人工鱼礁礁体构造和厚壳贻贝人工养殖行业技术的完善提供科学依据。
1 材料与方法 1.1 实验菌株实验所用的海假交替单胞菌分离自浙江省舟山市嵊泗县海域(30°72′N、122°76′E)挂板形成的自然生物被膜,并储存于–80℃超低温冰箱中(Peng et al, 2020)。
1.2 实验材料实验所用的VB7和VB12购于西格玛奥德里奇(上海)贸易有限公司。实验中使用的厚壳贻贝幼虫在2019年中旬在浙江省某县(30°69′N、122°46′E)采集。在实验室环境中,以10 ind./mL密度于盐度30的自然海水中暂养,每2 d换水,每天投喂1×104 cells/mL湛江等鞭金藻(Isochrysis zhanjiangensis)藻液,避光18℃充气培养7 d后进行实验(梁箫等, 2020a)。
1.2.1 VB7和VB12直接诱导将VB7、VB12和通过杀菌处理过的海水相溶(autoclaved filtered seawater, AFSW),并在此基础上形成pH=7.6的母液,将母液加入无菌培养皿(规格为64 mm×19 mm)中,并加适量AFSW定容至20 mL,设立空白对照组和B族维生素终浓度为0.02、0.2、2和20 mmol/L的不同实验组,每组设置9个生物学重复。将20只眼点幼虫添加到无菌培养皿中,置于18℃避光环境中培养96 h。记录12、24、48及96 h的幼虫附着变态数量,通过运算获得幼虫附着变态率。
1.2.2 VB7和VB12处理后海假交替单胞菌生长曲线绘制参照朱艳蕾等(2016)方法测定处理后海假交替单胞菌生长曲线,取菌液(OD600 nm约为1),按1%接种量转接至盛有300 mL 2216E液体培养基的圆底烧瓶内连续培养,封瓶膜封口,不同培养时间取培养液,立即测定OD600 nm值,每个时间点设置3个生物学重复,以OD值为纵坐标、培养时间为横坐标,绘制生长曲线。
1.2.3 VB7和VB12处理后海假交替单胞菌生物被膜制备参照杨金龙等(2015)方法制备生物被膜,取储存的海假交替单胞菌划线于2216E平板上,25℃培养12 h后,挑取单菌落接种到2216E液体培养基中,置于25℃避光条件下扩大培养。以3500 r/min离心15 min,去除上清液,用AFSW洗涤细菌沉淀3次,最后定容至50 mL制成细菌悬浊液,取1 mL稀释(细菌悬浊液∶AFSW=1∶99)后的菌液过滤至0.22 μm滤膜上,0.1%吖啶橙染色5 min,然后在荧光倒置显微镜(Olympus BX51)下观察,计算细菌浓度。在装有无菌载玻片的无菌培养皿中分别加入适量的菌液与VB7和VB12的混合物,加适量AFSW定容至20 mL,并使细菌的初始浓度为5×108 cells/mL,VB7和VB12最终浓度为0 (对照)和0.02 mmol/L,每组设9个生物学重复,避光18℃下培养48 h,以制备生物被膜。
1.2.4 幼虫附着变态实验将附有生物被膜的载玻片转移至20 mL AFSW无菌培养皿中,并向培养皿中添加20只眼点幼虫,该实验由空白(Blank, 无菌玻片)、肾上腺素(Epinephrine, EPI)、自然生物被膜(Bacterial biofilm, BF)这3种对照组和实验组(生物被膜)组成(Satuito et al, 1999; Yang et al, 2008)。每组设置9个生物学重复,避光18℃条件下记录12、24、48和96 h幼虫附着变态率(梁箫等, 2020b)。
1.2.5 生物被膜细菌密度计数借鉴杨娜等(2017)提出的方法,记录细菌密度值,并把生物被膜在5%的福尔马林试剂内浸泡48 h,0.1%吖啶橙染色5 min,之后放到荧光显微镜(Olympus BX51)下计数细菌密度,每片生物被膜中随机选择10个视野,每组设置3个生物学重复。
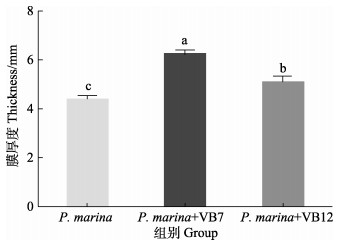
1.2.6 生物被膜膜厚度分析参照杨娜等(2017)的方法分析膜厚度,将生物被膜在5%的甲醛溶液中固定24 h,避光条件下5 μg/mL碘化丙啶(PI)浸染处理20 min,之后通过1×PBS进行多次清洗。并借助激光共聚焦显微镜(confocal laser scanning microscopy, CLSM)展开详细观察,针对每组设置3个生物学重复,并自由挑选出10个视野进行成像分析,以确定生物被膜膜厚度。
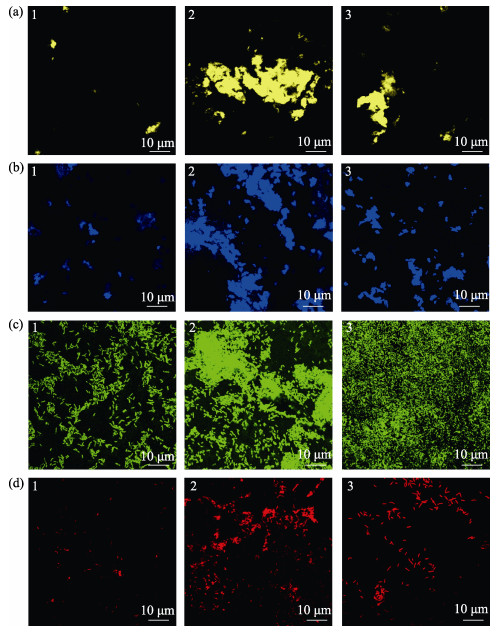
1.2.7 生物被膜胞外产物分析参照González-Machado等(2018)方法分析胞外产物。用0.9%生理盐水清洗培养好的生物被膜3次,通过相关试剂(表 1)进行染色处理,该步骤需要在没有光线的环境下操作20 min。之后通过0.9%的盐水进行漂洗,并放在同样没有光线的环境中,待其干燥之后通过CLSM显微镜详细观察,并自由挑选出10个视野对其成像效果予以分析。每组设置3个生物学重复。
|
|
表 1 生物被膜胞外产物染色试剂 Tab.1 Staining reagent of biofilm EPS composition |
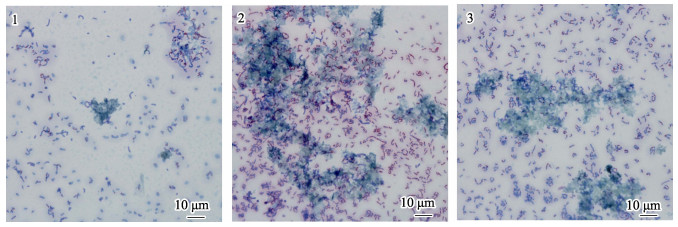
参照杨金龙等(2015)的方法制备生物被膜,从载玻片刮下生物被膜,涂布在玻璃片上,然后风干、染色。可拉酸染色参考Ren等(2016)的方法,将0.3 g碱性品红、90 mL含5%苯酚和10 mL 95%乙醇混合制成品红溶液,将玻璃片染色3 min。用媒染剂溶液[2.0 g/L KAl(SO4)2∶0.3 g/L FeCl3∶1.5 g/L丹宁酸=5∶2∶2]染色3 min。最后,1%亚甲基蓝溶液染色30 s。在每次染色之前,蒸馏水冲洗生物被膜以去除上一种染色液。细菌细胞染成红色,可拉酸染成蓝色。每组设置3个生物学重复。
1.2.9 可拉酸定量参考Obadia等(2007)的方法定量可拉酸。从12个生物被膜中收集细菌细胞,并将其悬浮在1 mL蒸馏水中。100℃煮沸细菌10 min,13, 000 g离心15 min,弃去沉淀,收集上清液,定量可拉酸。加入4.5 mL H2SO4/H2O溶液(6∶1),于1 mL上清液中制成混合液,100℃反应20 min。冷却至25℃,将2 mL混合液用于检测396 nm (A396co)和427 nm (A427co)吸光度。将100 μL的3%(m/v)半胱氨酸盐酸溶液与剩余混合液在25℃下避光孵育1 h后,在396 nm (A396cy)和427 nm (A427cy)测吸光度。将(A396cy–A396co)–(A427cy–A427co)的值代入L-岩藻糖浓度标准曲线(10~100 μg/mL)计算可拉酸浓度。
1.3 数据统计与分析使用JMP软件(ver.10.0.0)进行统计分析和相关性检验(Liang et al, 2020)。将幼虫的变态率百分比转化为反正弦以观察正态分布情况,如果符合该现象,则需要通过单因素方法对其中的差异进行分析。反之,便需要进行Kruskal-Wallis检验分析。Spearman多元分析方法用于幼虫变态率与B族维生素浓度及细菌密度之间的相关性分析,采用P为检验值,r为相关系数,显著性水平设置为0.05。生物被膜胞外产物含量分析使用image软件。
2 结果 2.1 VB7和VB12对幼虫变态的直接影响图 1显示,0.02、0.2 mmol/L VB7诱导幼虫变态率分别为(28.33±0.83)%和(11.67±0.83)%,VB12诱导幼虫变态率分别为(13.89±1.39)%和(1.67±0.83)%,与空白对照组相比,均可显著提高诱导厚壳贻贝幼虫变态(P < 0.05),其中以0.02 mmol/L的诱导效果最好。而2、20 mmol/L VB7的变态率分别为(5.56±1.30)%和(1.67±0.83)%,VB12的变态率分别为(1.67±0.83)%和0%,与空白对照组相比,均出现显著抑制作用(P < 0.05),其中以20 mmol/L浓度的抑制作用最强。
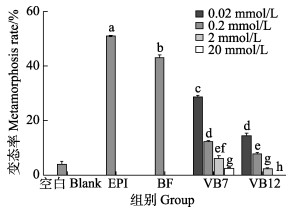
|
图 1 VB7和VB12对厚壳贻贝幼虫变态的影响(72 h) Fig.1 Effects of VB7 and VB12 on larval metamorphosis in the mussel M. coruscus at 72 h 不同字母表示差异显著(P < 0.05)。下同 Different letters show significant difference (P < 0.05). The same as below |
对照组和处理组海假交替单胞菌在培养1 h后进入生长对数期,6 h后进入稳定期(图 2)。培养6 h时,OD600 nm值分别为OD(P. marina)=0.9543、OD(P. marina+ 0.02 mmol/L VB7)=1.0607和OD(P. marina+0.02 mmol/L VB12)=0.9923,相比于单一细菌的对照组,0.02 mmol/L的VB7和VB12在生长对数期对细菌生长均具有显著影响(P < 0.05)。其中,VB7对细菌生长的促进作用持续至稳定期,而VB12在细菌生长9 h后无明显作用。
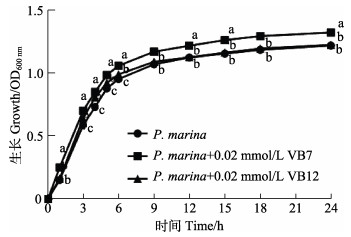
|
图 2 VB7和VB12处理后的海假交替单胞菌生长曲线 Fig.2 Growth curve of P. marina after treatment with VB7 or VB12 |
图 3显示,由VB7和VB12与海假交替单胞菌形成的生物被膜诱导的幼虫附着变态率显著高于细菌单一生物被膜(P < 0.05)。0.02 mmol/L浓度VB7处理后,海假交替单胞菌生物被膜诱导幼虫附着变态率为(71.0±3.9)%;0.02 mmol/L浓度VB12处理后的幼虫附着变态率为(60.1±3.3)%,二者均显著高于细菌单一生物被膜附着变态率(38.8±3.09)% (P < 0.05)。
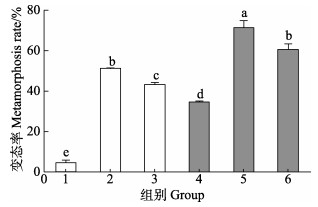
|
图 3 VB7和VB12处理后的海假交替单胞菌生物被膜对厚壳贻贝幼虫变态的影响(48 h) Fig.3 Effects on metamorphosis of post-larvae on the P. marina biofilms after treatment with VB7 or VB12 at 48 h 1. 空白;2. 肾上腺素;3. 自然微生物被膜;4. 细菌;5. 细菌+VB7;6. 细菌+VB12 1. Blank; 2. EPI; 3. BF; 4. P. marina; 5. P. marina+VB7; 6. P. marina+VB12 |
图 4显示,初始浓度为5×108 cells/mL的海假交替单胞菌分别与VB7和VB12共同形成生物被膜,其细菌密度明显高于细菌单一生物被膜(P < 0.05),VB7和VB12处理后的生物被细菌聚集性增强(图 5),且膜厚度显著提高(P < 0.05)(图 6)。相关分析结果显示,生物被膜所具备的细菌密度和膜厚度均与诱导活性具有极显著相关性(P < 0.05)(表 2)。

|
图 4 VB7和VB12处理后的海假交替单胞菌生物被膜细菌密度 Fig.4 Density of P. marina biofilms after treatment with VB7 or VB12 数据为9个生物学重复的平均值±标准误。下同 Data were Mean±SE of 9 duplicates. The same as below |
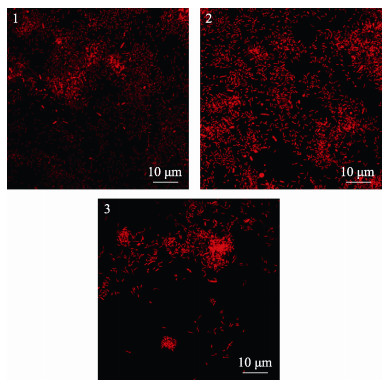
|
图 5 激光共聚焦扫描电镜下VB7和VB12处理后的海假交替单胞菌生物被膜形态 Fig.5 CLSM reveals morphology of P. marina biofilms after VB7 or VB12 treatment 1. P. marina; 2. P. marina+VB7; 3. P. marina+VB12 |
|
图 6 激光共聚焦扫描电镜下VB7和VB12处理后的海假交替单胞菌生物被膜厚度 Fig.6 CLSM reveals thickness of P. marina biofilms after treatment with VB7 or VB12 |
|
|
表 2 生物被膜厚度和细菌密度与诱导活性的相关性分析 Tab.2 Correlation analysis between biofilm thickness, bacterial density and inducing activity |
荧光染色显示(图 7),海假交替单胞菌单一生物被膜细菌呈颗粒状分布,胞外产物分布较为均匀,而VB7和VB12处理后的生物被膜细菌多聚集状态,胞外产物含量明显升高,且多呈块状分布。
|
图 7 激光共聚焦扫描电镜下VB7和VB12处理后的海假交替单胞菌生物被膜胞外产物
Fig.7 CLSM reveals extracellular products of P. marina bacterial biofilms after VB7 or VB12 treatment
1. P. marina; 2. P. marina+VB7; 3. P. marina+VB12 a:α多糖;b:β多糖;c:蛋白;d:脂质 a: α-Polysaccharide; b: β-Polysaccharide; c: Protein; d: Lipids |
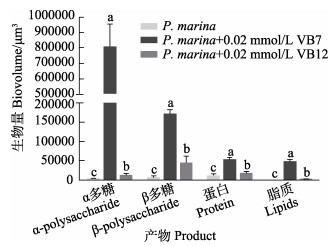
3组生物被膜α多糖、β多糖、蛋白质和脂质含量见图 8。与单一生物被膜相比,VB7处理后具有更高含量的α多糖、β多糖、蛋白质和脂质。其中,单一生物被膜的α多糖含量仅为(1536.50±57.36) μm3,而VB7处理后生物被膜的α多糖含量为(800, 590.25± 46, 499.65) μm3,上调了520倍(P < 0.05)(图 8)。
|
图 8 激光共聚焦扫描电镜下VB7和VB12处理后的P. marina生物被膜胞外产物含量 Fig.8 CLSM reveals extracellular products of P. marina biofilms after VB7 or VB12 treatment 浓度数据为9个重复的平均值±标准误 Data for concentration were Mean±SE of nine replicates |
染色结果显示,相比于单一细菌生物被膜,VB7和VB12处理后生物被膜可拉酸分布更密集(图 8),且含量明显提高(图 9)。单一细菌生物被膜可拉酸含量为(30.46±6.03) μg/mL,而VB7和VB12处理后含量分别为(215.78±24.18)和(158.27±23.27) μg/mL,升高了7.08倍和5.20倍(P < 0.05) (图 9)。
|
图 9 媒染剂染色生物被膜光学显微镜的观察
Fig.9 Light microscopic observation of mordant-stained biofilms
1. P. marina; 2. P. marina+VB7; 3. P. marina+VB12 细菌被染成红色,可拉酸被染成蓝色 The strains were stained red and the colonic acid was stained blue |
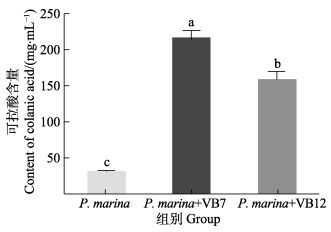
|
图 10 VB7和VB12处理后的P. marina生物被膜可拉酸含量 Fig.10 Colanic acid production in P. marina biofilms after VB7 or VB12 treatment 浓度数据为4个重复的平均值±标准误 Data for concentration were Mean±SE of four replicates |
B族维生素作为生长辅助因子,以辅基或辅酶的形式参与机体的生理生化反应,调节新陈代谢,并维持细胞、器官和组织结构和功能的完整,是确保所有生命活动正常运行的重要营养物质(Brown et al, 1989; Knowles et al, 1989; Ekhard et al, 1998)。本研究的结果显示,0.02 mmol/L浓度下VB7和VB12与初始细菌浓度为5×108 cells/mL的P. marina共同形成生物被膜,与P. marina单一生物被膜相比,细菌密度和膜厚度显著增加,细菌成聚集状态,并产生大量的多糖等胞外产物。Matras(1973)研究表明,B族维生素可通过影响布鲁杆菌属和沙门菌属细菌的主要营养物质代谢等生理生化反应来调节细菌生物量。因此,推测B族维生素的加入可以提高生物被膜的生物量和胞外产物含量,进而促进生物被膜的形成。
3.2 B族维生素对幼虫变态的影响 3.2.1 B族维生素对幼虫变态的直接影响许多海洋无脊椎动物在发育为成体前处于浮游幼虫阶段。幼虫的浮游阶段可以短则几分钟,也可以长达几个月。通常,海洋无脊椎动物幼虫的附着变态主要受外界环境和内源性因素控制(Crisp, 1974; Pawlik, 1992),特别是外界环境因子对于幼虫的附着变态至关重要(Morse, 1990; McClintock et al, 2001)。B族维生素广泛存在于自然环境中,并作为营养物质参与幼虫的附着变态过程。Burns等(2018)研究表明,B族维生素可通过影响海蠕虫幼虫的主要营养物质代谢水平进而提高幼虫附着变态率。然而,关于B族维生素与海洋贝类附着二者间的相互作用关系仍有待研究。
研究结果显示,0.02、0.2 mmol/L浓度下VB7和VB12的孵育均可以显著诱导厚壳贻贝幼虫变态。其中以0.02 mmol/L浓度下的诱导作用较强,但2、20 mmol/L浓度下VB7和VB12对幼虫变态起抑制作用。因此,推测在一定浓度的条件下,B族维生素可能通过增强幼虫糖类、脂类和蛋白质代谢及免疫系统功能进而促进厚壳贻贝等海洋贝类幼虫的附着变态。
3.2.2 生物被膜与幼虫变态的关系生物被膜生物量和胞外产物(胞外多糖、蛋白、脂质等)含量是影响海洋无脊椎动物附着的关键因素(Kirchman et al, 1981; Hadfield et al, 2011)。研究表明,厚壳贻贝幼虫附着变态和稚贝附着都受到细菌密度的影响(Wang et al, 2012; 杨金龙等, 2013; Yang et al, 2014)。厚壳贻贝与生物被膜相关性研究证实,细菌密度与附着变态呈显著相关;自然生物被膜和胞外产物的群落结构对合浦珠母贝(Pinctada fucata)幼虫附着具有十分关键的诱导功能(Yu et al, 2010);海洋假单胞菌Strain S9所对应的生物被膜胞外多糖大幅度加快了海鞘(Ciona intestinalis)幼虫附着变态(Szewzyk et al, 1991)。因此,推测厚壳贻贝幼虫附着变态除了与B族维生素的直接诱导作用有关外,还可能与生物被膜的生物量和胞外产物含量有关。研究结果显示,VB7和VB12处理后的生物被膜显著提高了厚壳贻贝幼虫的附着变态率,其生物被膜的生物量和胞外产物含量显著高于P. marina单一生物被膜,其中,胞外多糖含量升高520倍。前期研究发现,多糖能够诱导许多海洋无脊椎动物幼虫附着变态(Kirchman et al, 1982; Szewzyk et al, 1991; Matsumura et al, 1998; Khandeparker et al, 2003; Bao et al, 2007; Zeng et al, 2015),其中,最具代表性的是由生物学重复单元(葡萄糖、岩藻糖、半乳糖和葡萄糖醛酸)与α键和β键相连所组成的可拉酸(Whitfield, 2006; Schmid et al, 2015),可拉酸能在细菌周围产生高度负电荷胶囊(Goebel, 1963; Allen et al, 1987),并将吸附细菌周围的矿物质和营养物质(Ophir et al, 1994),促进具有诱导海洋无脊椎动物幼虫附着变态活性的附着基的形成。实验证明,P. marina细菌多糖相关基因缺失后,可拉酸的产生量增多,细胞内c-di-GMP水平升高,c-di-GMP水平的升高导致细菌运动能力降低,进而促进细菌粘附和生物被膜的形成,从而提高厚壳贻贝幼虫的附着变态率(Whiteley et al, 2015; Pérez-Mendoza et al, 2016; Peng et al, 2020)。本研究结果显示,VB7和VB12处理后生物被膜的生物量和胞外产物(可拉酸)含量均显著升高,并均对厚壳贻贝幼虫的附着变态存在显著诱导作用。因此,推测B族维生素可能通过协同c-di-GMP调节可拉酸的产生,从而正调控生物被膜的形成和厚壳贻贝幼虫的附着变态,但B族维生素对c-di-GMP水平的影响尚需进一步研究。这一发现为阐明生物被膜与幼虫之间的相互作用提供了新的视角。
本研究首次发现,VB7、VB12两种B族维生素对厚壳贻贝幼虫变态具有直接诱导作用。同时,VB7和VB12通过促进P. marina生物被膜的形成,增加生物被膜的细菌密度和膜厚度,提高生物被膜胞外产物含量,从而间接促进厚壳贻贝幼虫的附着变态。本研究为探究厚壳贻贝幼虫附着变态的分子机制提供了新的理论依据和创新思路,同时,为B族维生素在提高厚壳贻贝人工育苗技术、改善厚壳贻贝养殖产业问题和海洋牧场建设等生态修复方面的应用提供了理论基础。
ALLEN P M, FISHER D, SAUNDERS J R, et al. The role of capsular polysaccharide K21b of Klebsiella and of the structurally related colanic-acid polysaccharide of Escherichia coli in resistance to phagocytosis and serum killing. Journal of Medical Microbiology, 1987, 24(4): 363-370 DOI:10.1099/00222615-24-4-363 |
BAO W Y, YANG J L, SATUITO C G, et al. Larval metamorphosis of the mussel Mytilus galloprovincialis in response to Alteromonas sp. 1: Evidence for two chemical cues?. Marine Biology, 2007, 152(3): 657-666 DOI:10.1007/s00227-007-0720-2 |
BROWN M L. Present knowledge in nutrition. 6th ed. International Life Sciences Institute Press, Washington DC, 1989
|
BURNS R T, PECHENIK J A, BIGGERS W J, et al. The B vitamins nicotinamide (B3) and riboflavin (B2) stimulate metamorphosis in larvae of the deposit-feeding polychaete Capitella teleta: Implications for a sensory ligand-gated ion channel. PLoS One, 2018, 9(11): e109535 |
CHANG K M. On the sustainable development of mussel farming industry. China Fisheries, 2007(4): 17-18 [常抗美. 论贻贝养殖产业的可持续发展. 中国水产, 2007(4): 17-18 DOI:10.3969/j.issn.1002-6681.2007.04.010] |
CHANG R H, XU K H, CAI Y S, et al. Effects of the calcium ion on Pseudoalteromonas marina biofilm formation and plantigrade settlement of Mytilus coruscus. Journal of Dalian Ocean University,, 2020, 35(6): 893-900 [常睿珩, 许康豪, 蔡雨珊, 等. 钙离子对海假交替单胞菌生物被膜的形成及厚壳贻贝附着的影响. 大连海洋大学学报, 2020, 35(6): 893-900] |
CRISP D J. Chemoreception in marine organisms. London: Academic Press, 1974, 177-265 |
DEGNAN P H, TAGA M E, GOODMAN A L. Vitamin B 12 as a modulator of gut microbial ecology. Cell Metabolism, 2014, 20(5): 769-778 DOI:10.1016/j.cmet.2014.10.002 |
EKHARD E Z. Present knowledge in nutrition. 7th ed. International Life Sciences Institute Press, Washington DC, 1998, 154-199 |
GOEBEL W F. Colanic acid. Proceeding of National Academy, USA, 1963, 49(4): 464-471 DOI:10.1073/pnas.49.4.464 |
GONZÁLEZ-MACHADO C, ROSA C, RIESCO-PELÁEZ F, et al. Visualization and quantification of the cellular and extracellular components of Salmonella agona biofilms at different stages of development. PLoS One, 2018, 13(7): e0200011 DOI:10.1371/journal.pone.0200011 |
HADFIELD M G. Biofilms and marine invertebrate larvae: What bacteria produce that larvae use to choose settlement sites. Annual Review of Marine Science, 2011, 3: 453-470 DOI:10.1146/annurev-marine-120709-142753 |
HOLMSTRÖM C, KJELLEBERG S. Marine P. marina species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiology Ecology, 1999, 30(4): 285-293 DOI:10.1111/j.1574-6941.1999.tb00656.x |
KHANDEPARKER L, ANIL A C, RAGHUKUMAR S. Barnacle larval destination: Piloting possibilities by bacteria and lectin interaction. Journal of Experimental Marine Biology and Ecology, 2003, 289(1): 1-13 DOI:10.1016/S0022-0981(03)00024-8 |
KIRCHMAN D L, GRAHAM S, REISH D, et al. Bacteria induce settlement and metamorphosis of Janua (Dexiospira) brasiliensis Grube (Polychaeta: Spirprbidae). Mitchell Ralph, 1981, 56(2/3): 153-163 |
KIRCHMAN D, GRAHAM S, REISH D, et al. Lectins may mediate in the settlement and metamorphosis of Janua (Dexiospira) brasiliensis Grube. Marine Biology Letters, 1982, 3(3): 131-142 |
KNOWLES R J. The mechanism of biotin-dependent enzymes. Annual Review of Biochemistry, 1989, 58(1): 195-221 DOI:10.1146/annurev.bi.58.070189.001211 |
LI T W. Marine biology. Beijing: China Ocean Press, 2013 [李太武. 海洋生物学. 北京: 海洋出版社, 2013]
|
LIANG X, TONG H, PENG L H, et al. Regulation of formation of biofilms and larval settlement and metamorphosis of mussel Mytilus coruscus by cellulose. Journal of Dalian Ocean University, 2020a, 35(1): 75-82 [梁箫, 童欢, 彭莉华, 等. 纤维素对海洋细菌生物被膜形成及厚壳贻贝幼虫附着变态的调控. 大连海洋大学学报, 2020a, 35(1): 75-82] |
LIANG X, YANG L T, PENG L H, et al. Effects of biofilms of deep-sea bacteria under varying temperatures on larval metamorphosis of Mytilus coruscus. Journal of Fisheries of China, 2020b, 44(10): 1728-1741 [梁箫, 杨丽婷, 彭莉华, 等. 不同温度下形成的深海菌膜对厚壳贻贝幼虫变态的影响. 水产学报, 2020b, 44(10): 1728-1741] |
LIANG Y, ZHANG Y, PENG G D, et al. The flagellar gene regulates biofilm formation and mussel larval settlement and metamorphosis. International Journal of Molecular Sciences, 2020, 21(3): 710 DOI:10.3390/ijms21030710 |
LUO H Z, TAO L, WU Y T, et al. Thinking about the sustainable development of mussel industry in Luxian County Zhejiang Province. China Fisheries, 2016(12): 29-33 [罗海忠, 陶蕾, 吴一挺, 等. 浙江省嵊泗县贻贝产业可持续发展之思索. 中国水产, 2016(12): 29-33] |
LUO Y S. Mussel farming technology. Shanghai: Shanghai Scientific and Technical Publishers, 2003: 1-16 [罗有声. 贻贝养殖技术. 上海: 上海科学技术出版社, 2003: 1-16]
|
MATRAS J. Effect of group B vitamins on the multiplication rate of Salmonella and Brucella. Medycyna dowiadczalna i mikrobiologia, 1973, 25(1): 43-50 |
MATSUMURA K, NAGANO M, FUSETANI N. Purification of a larval settlement-inducing protein complex (SIPC) of the barnacle, Balanus amphitrite. Journal of Experimental Zoology, 1998, 281(1): 12-20 DOI:10.1002/(SICI)1097-010X(19980501)281:1<12::AID-JEZ3>3.0.CO;2-F |
MCCLINTOCK J B, BAKER J B. Marine chemical ecology. Boca Raton: CRC Press, 2001, 431-461 |
MORSE D E. Recent progress in larval settlement and metamorphosis: Closing the gaps between molecular biology and ecology. Bulletin of Marine Science, 1990, 46(2): 465-483 |
OBADIA B, LACOUR S, DOUBLET P, et al. Influence of tyrosine- kinase Wzc activity on colanic acid production in Escherichia coli K12 cells. Journal of Molecular Biology, 2007, 367(1): 42-53 DOI:10.1016/j.jmb.2006.12.048 |
OPHIR T, GUTNICK D L. A role of exopolysaccharides in the protection of microorganisms from desiccation. Applied and Environmental Microbiology, 1994, 60(2): 740-745 DOI:10.1128/aem.60.2.740-745.1994 |
PAWLIK J R. Chemical ecology of the settlement benthic marine invertebrates. Oceanography and Marine Biology: An Annual Review, 1992, 30: 273-335 |
PENG L H, LIANG X, XU J K, et al. Monospecific biofilms of P. marina promote larval settlement and metamorphosis of Mytilus coruscus. Scientific Reports, 2020, 10: 2577 DOI:10.1038/s41598-020-59506-1 |
PÉREZ-MENDOZA D, SANJUÁN J. Exploiting the commons: Cyclic diguanylate regulation of bacterial exopolysaccharide production. Current Opinion in Microbiology, 2016, 30: 36-43 DOI:10.1016/j.mib.2015.12.004 |
REN G, WANG Z, LI Y, et al. Effects of lipopolysaccharide core sugar deficiency on colanic acid biosynthesis in Escherichia coli. Journal of Bacteriology, 2016, 198: 1576-1584 DOI:10.1128/JB.00094-16 |
SATUITO C G, NATOYAMA K, YAMAZAKI M, et al. Induction of metamorphosis in the Pediveliger larvae of the mussel Mytilus galloprovincialis by neuroactive compounds. Fish Science, 1999, 65: 384-389 DOI:10.2331/fishsci.65.384 |
SCHMID J, SIEBER V, REHM B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Frontiers in Microbiology, 2015, 6: 496 |
SHIKUMA N J, PILHOFER M, WEISS G L, et al. Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science, 2014, 343(6170): 529-533 DOI:10.1126/science.1246794 |
SUN J J, LIANG X, GUO X P, et al. Effects of culture media on the biofilm formation and subsequent settlement of Mytilus coruscus. Journal of Fisheries of China, 2016, 40(8): 1229-1238 [孙俊杰, 梁箫, 郭行磐, 等. 培养基对微生物被膜形成和厚壳贻贝附着的影响. 水产学报, 2016, 40(8): 1229-1238] |
SZEWZYK U, HOLMSTROM C, WRANGSTADH M, et al. Relevance of the exopolysaccharide of marine Pseudomonas sp. strain S9 for the attachment of Ciona intestinalis larvae. Marine Ecology Progress Series, 1991, 75(2/3): 259-265 |
UHLINGER D J, WHITE D C. Relationship between physiological status and formation of extracellular polysaccharide glycocalyx in Pseudomonas atlantica. Applied and Environmental Microbiology, 1983, 45(1): 64-70 DOI:10.1128/aem.45.1.64-70.1983 |
WANG C H, ZHENG J W, SUN Y. The effects of B vitamins on the number of culture bacteria in the synthetic medium of pig dantovirus vaccine synthesis were tested. Modern Journal of Animal Husbandry and Veterinary Medicine, 2011(9): 48-49 [王春华, 郑建稳, 孙勇. 猪丹毒活疫苗合成培养基中B族维生素对培养菌数影响试验. 现代畜牧兽医, 2011(9): 48-49 DOI:10.3969/j.issn.1672-9692.2011.09.025] |
WANG C, BAO W Y, GU Z Q, et al. Larval settlement and metamorphosis of the mussel Mytilus coruscus in response to natural biofilms. Biofouling, 2012, 28(3): 249-256 DOI:10.1080/08927014.2012.671303 |
WANG J T, MU Y T. Management situation and suggestions of shellfish's culture in China. Chinese Fisheries Economics, 2010, 28(3): 43-47 [王靖陶, 慕永通. 我国贝类养殖管理现状及建议. 中国渔业经济, 2010, 28(3): 43-47 DOI:10.3969/j.issn.1009-590X.2010.03.010] |
WANG R C, WANG Z P, ZHANG J Z. Marine shellfish farming. Qingdao: China Ocean University Press, 1998, 124-140 [王如才, 王昭萍, 张建中. 海水贝类养殖学. 青岛: 中国海洋大学出版社, 1998, 124-140] |
WANG Y P, LI B S, WANG J Y, et al. Study on the optimum dietary pantothenic requirement of juvenile turbot (Scophthalmus maximus L.). Progress in Fishery Sciences, 2019, 40(6): 66-75 [王雅平, 李宝山, 王际英, 等. 大菱鲆幼鱼对饲料中泛酸最适需求量的研究. 渔业科学进展, 2019, 40(6): 66-75] |
WHITELEY C G, LEE D J. Bacterial diguanylate cyclases: Structure, function and mechanism in exopolysaccharide biofilm development. Biotechnology Advances, 2015, 33(1): 124-141 DOI:10.1016/j.biotechadv.2014.11.010 |
WHITFIELD C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annual Review of Biochemistry, 2006, 39-68 |
WOODS D D. Vitamin-B-group substances, especially folic acid and B12, in the metabolism of micro-organisms. Bulletin of the World Health Organization, 1953, 6(1/2): 35-57 |
XU J K, PENG L H, GAO W, et al. Effects of light intensity, water temperature and density on aggregation of juvenile mussel Mytilus coruscus. Journal of Dalian Ocean University, 2017, 32(3): 275-279 [徐嘉康, 彭莉华, 高伟, 等. 光照、水温和密度对厚壳贻贝稚贝群聚性的影响. 大连海洋大学学报, 2017, 32(3): 275-279] |
YANG J L, GUO X P, CHEN Y R, et al. Effects of bacterial biofilms formed on middle wettability surfaces on settlement of plantigrades of the mussel Mytilus coruscus. Journal of Fisheries of China, 2015, 39(3): 421-428 [杨金龙, 郭行磐, 陈芋如, 等. 中湿度表面的海洋细菌对厚壳贻贝稚贝附着的影响. 水产学报, 2015, 39(3): 421-428] |
YANG J L, LI X, LIANG X, et al. Effects of natural biofilms on settlement of plantigrades of the mussel Mytilus coruscus. Aquaculture, 2014, 424/425: 228-233 DOI:10.1016/j.aquaculture.2014.01.007 |
YANG J L, LI X, WANG C, et al. Settlement of the mussel Mytilus coruscus plantigrades in response to biofilms formed on low salinities. Marine Sciences, 2013, 37(8): 107-113 [杨金龙, 李响, 王冲, 等. 低盐度形成的微生物膜对厚壳贻贝稚贝附着的影响. 海洋科学, 2013, 37(8): 107-113] |
YANG J L, LI Y F, GUO X P, et al. The effect of carbon nanotubes and titanium dioxide incorporated in PDMS on biofilm community composition and subsequent mussel plantigrade settlement. Biofouling, 2016a, 32(7): 763-777 DOI:10.1080/08927014.2016.1197210 |
YANG J L, LI Y F, LIANG X, et al. Silver nanoparticles impact biofilm communities and mussel settlement. Scientific Reports, 2016b, 6: 37406 DOI:10.1038/srep37406 |
YANG J L, SATUITO C G, BAO W Y, et al. Induction of metamorphosis of pediveliger larvae of the mussel Mytilus galloprovincialis Lamarck, 1819 using neuroactive compounds, KCl, NH4Cl and organic solvents. Biofouling, 2008, 24(6): 461-470 DOI:10.1080/08927010802340309 |
YANG J L, SHEN P J, LIANG X, et al. Larval settlement and metamorphosis of the mussel Mytilus coruscus in response to monospecific bacterial biofilms. Biofouling, 2013, 29(3): 247-259 DOI:10.1080/08927014.2013.764412 |
YANG J L, SHEN P J, WANG C, et al. Effects of biofilms on settlement of plantigrades of the mussel Mytilus coruscus. Journal of Fisheries of China, 2013, 37(6): 904-909 [杨金龙, 慎佩晶, 王冲, 等. 微生物膜对厚壳贻贝稚贝附着的影响. 水产学报, 2013, 37(6): 904-909] |
YANG J L, WANG C, GU Z Q, et al. A review on the role of marine biofilms on larval settlement and metamorphosis of marine invertebrate. Marine Sciences, 2012, 36(8): 116-121 [杨金龙, 王冲, 顾忠旗, 等. 微生物膜对海洋无脊椎动物幼体附着变态的影响研究. 海洋科学, 2012, 36(8): 116-121] |
YANG J L, ZHOU X, LI Y F, et al. Plantigrade settlement of the mussel Mytilus coruscus in response to natural biofilms on different surfaces. Journal of the Marine Biological Association of the United Kingdom, 2014, 94(8): 1639-1649 DOI:10.1017/S0025315414001039 |
YANG N, LIANG X, PENG L H, et al. Effects of gut bacteria on the settlement of spats of Mytilus coruscus. Marine Sciences, 2017, 41(11): 45-54 [杨娜, 梁箫, 彭莉华, 等. 肠道细菌对厚壳贻贝稚贝附着的作用研究. 海洋科学, 2017, 41(11): 45-54 DOI:10.11759/hykx20170626001] |
YU X J, HE W H, LI H X, et al. Larval settlement and metamorphosis of the pearl oyster Pinctada fucata in response to biofilms. Aquaculture, 2010, 306(1): 334-337 |
ZENG Z S, GUO X P, LI B Y, et al. Characterization of self-generated variants in Pseudoalteromonas lipolytica biofilm with increased antifouling activities. Applied Microbiology and Biotechnology, 2015, 99: 10127-10139 DOI:10.1007/s00253-015-6865-x |
ZHANG Y H, ZHAO S L. Mussel species and growth developing investigation around Shengshan Archipelago. Journal of Zhejiang Ocean University (Natural Sciences), 2003(1): 67-73 [张义浩, 赵盛龙. 嵊山列岛贻贝养殖种类生长发育调查. 浙江海洋学院学报(自然科学版), 2003(1): 67-73 DOI:10.3969/j.issn.1008-830X.2003.01.015] |
ZHOU Y, WANG J Y, LI B S, et al. Dietary vitamin B6 requirements of juvenile rockfish (Sebastes schlegeli). Progress in Fishery Sciences, 2020, 41(6): 37-48 [周莹, 王际英, 李宝山, 等. 许氏平鲉幼鱼对饲料中维生素B6需求量的研究. 渔业科学进展, 2020, 41(6): 37-48] |
ZHU Y L. Experimental method of bacteria growth curve determination. Journal of Microbiology, 2016, 36(5): 108-112 [朱艳蕾. 细菌生长曲线测定实验方法的研究. 微生物学杂志, 2016, 36(5): 108-112] |



