2. 山东省海洋资源与环境研究院 山东 烟台 264006;
3. 山东省农业交流合作中心 山东 济南 250199
2. Shandong Marine Resource and Environment Research Institute Yantai, Shandong 264006, China;
3. Shandong Agricultural Cooperation Service Center, Jinan, Shandong 250199, China
人类CO2排放引起的全球变暖已经危及到人类社会的可持续发展。为了应对全球变暖,联合国大会早在1992年制定了《联合国气候变化框架公约》,把控制CO2等温室气体排放、应对全球气候变暖给人类经济和社会带来不利影响纳入国际法框架(焦念志, 2012)。中国作为负责任大国,2020年9月在第75届联合国大会上提出了2030年实现碳达峰和2060年实现碳中和的承诺(焦念志, 2021)。但仅靠控制CO2排放实现碳中和有局限性,在减排的同时进行增汇才是最有效的方式(张继红等, 2021)。海洋作为全球最大的碳库,在调节全球气候变化过程中起到了极为重要的作用,增汇潜力巨大(Khatiwala et al, 2009)。在“十三五”时期,我国已将“建立增加海洋碳汇的有效机制”与“探索开展海洋等生态系统碳汇试点”列入《生态文明体制改革总体方案》(张永雨等, 2017)。
为实现海洋增汇,科学家提出了“蓝碳”策略:通过恢复与保护红树林、盐沼湿地、海草床等三类近海生态系统,以加强有机碳埋藏,进而减缓气候变暖(McLeod et al, 2011)。而大型海藻因大多生长在岩基环境,碳埋藏过程受阻,被排除在“蓝碳”系统之外。但大型海藻同样具有强烈的碳汇过程(Duarte et al, 2013; Ross et al, 2015)。海藻能将大量的光合作用产物以溶解有机碳(dissolved organic carbon, DOC)的形态释放到环境中,释放的DOC经微型生物碳泵(microbial carbon pump, MCP)的作用可转化为惰性溶解有机碳(recalcitrant dissolved organic carbon, RDOC) (Jiao et al, 2010)。由于RDOC化学性质稳定,可实现碳千年尺度上的封存,进而有效缓解全球变暖(Ogawa et al, 2001)。养殖活动人为可控性强,若能通过海藻养殖增汇,无疑可以实现经济效益与生态效益的双赢(唐启升等, 2016)。初步估算,我国养殖海藻每年DOC释放量达82.2×104~91.5×104 t C/a,养殖海藻释放的DOC经过MCP作用每年可生成60万t以上的RDOC,约为我国海岸带“蓝碳”储碳量的1.7倍,碳汇潜力巨大(Abdullah et al, 2004; Krause-Jensen et al, 2016)。
光照强度与营养盐是藻类生长的重要环境因素,其不仅影响藻类的初级生产力,还调控藻类的DOC释放过程(韩婷婷等, 2014; 袁艳敏等, 2020)。Wada等(2007)通过现场实验发现,日本欧拉湾的褐藻(Ecklonia cava)初级生产力与DOC释放均与光照强度正相关。Reed等(2015)对美国莫霍克礁巨藻(Macrocystis pyrifera)藻床的研究也发现了同一现象,尽管巨藻释放DOC季节规律不明显,但与海面光照显著正相关。但也有研究持相反观点,如Maranon等(2005)在研究凯尔特海域的浮游藻类时发现,藻类释放DOC与光照强度的变化没有相关性。Mueller等(2016)通过实验发现,氮磷加富后,黑暗条件与光照条件下珊瑚藻释放DOC的速率差异不显著。为此,藻类释放DOC的环境调控目前形成了两个截然相反的假说:“溢出”假说与“扩散”假说。“溢出”假说认为,藻类释放DOC与光照强度正相关,其成分以高分子量物质为主;“扩散”机制假说则认为,藻类释放DOC受控于营养盐,且以小分子量物质为主(Bjornsen, 1988; Maranon et al, 2005)。海带是我国最主要的大型养殖藻类,其年产量约为我国海藻养殖产量的63.98%,增汇潜力巨大(于秀娟等, 2020)。而光照和营养盐对海带释放DOC速率还没有相关文献的报道。基于此,本研究探究光照强度与营养盐对海带幼苗释放DOC的影响,以期为探明海带释放DOC的环境调控机制提供参考,为海带养殖增汇提供科学依据。
1 材料与方法 1.1 实验材料实验海带幼苗于2020年12月取自山东省荣成市东楮岛海带养殖区。所取海带幼苗平均体长为(34.26±8.99) cm,湿重为(5.25±1.86) g。海带幼苗取样后黑暗冷藏保存,4 h内运至实验室,在循环水养殖系统中暂养5 d后用于实验,暂养水温为(13.5±0.5)℃,光照强度为(63±9) μmol photons/(m2·s)。
1.2 实验方法 1.2.1 光照强度对海带幼苗释放DOC的影响实验期间为海带幼苗海上夹苗期,此时水下0~1 m处的光强为50~300 μmol photons/(m2·s) (王月, 2016)。为模拟海上条件,设置0、83、165和250 μmol photons/(m2·s)共4个光照强度,每个光照强度设置6个平行,每个平行样本在2 L酸洗玻璃瓶中放入未受损伤的单株海带幼苗,通过光照培养箱进行不同光强培养,培养时间为6~8 h。同时,每个光照处理组设置3个未放置海带幼苗的平行样,作为空白对照。培养水温为(14.0±0.5)℃,海水为自然海水。在培养前、后抽取100 mL水样2份,其中,1份用于DOC含量和吸收光谱测定,水样–20℃黑暗冷冻保存,直至分析;另1份用于溶解氧(DO)测定,水样经KI固定,并在12 h内经碘量法滴定完毕,具体操作见GB/T 12763.4-2007。
1.2.2 营养盐对海带幼苗释放DOC的影响设置氮加富、磷加富、氮磷加富和自然海水4个实验组。自然海水磷酸盐、无机氮浓度分别为3.5、97 μg/L,略低于同期桑沟湾营养盐水平,呈营养限制状态 (李凤雪等, 2020)。使用K2HPO4、KNO3分析纯试剂分别配制成100、1000 μg/L标准液用于加富,使各加富组目标营养盐提升10倍,使海水中营养盐的浓度达到藻类生长率饱和浓度(Wyatt et al, 2014)。加富后各组氮、磷营养盐摩尔比如表1所示。实验过程光照强度为250 μmol photons/(m2·s)。培养水温为(14.0± 0.5)℃。其余处理同1.2.1。
|
|
表 1 各实验组氮磷摩尔比 Tab.1 Molar ratio of N and P in experimental groups |
使用岛津TOC-LCPH总有机碳分析仪测定样品的DOC含量。DOC释放速率[RDOC, μmol/(g·h)]指单位质量(干重)海带幼苗在单位时间内引起的水体DOC含量的变化,其计算公式为:
| $ {R_{{\text{DOC}}}} = \frac{{({C_t} - {C_0}) \times V \times 1000}}{{{W_D} \times {M_C} \times t}} $ | (1) |
式中,Ct为实验结束时海带幼苗的DOC浓度(mg/L);C0为空白对照瓶中DOC的浓度(mg/L);V为养殖用海水体积(L);WD为实验海带幼苗的干质量(kg);t为实验处理时间(h),MC为碳的相对分子质量。
1.3.2 释放DOC占净初级生产力(net primary productivity, NPP)比重 海带幼苗氧气释放速率[
| $ \Delta {C_{{{\text{O}}_{\text{2}}}}} = \frac{{c{{({\text{O}})}_t} - c{{({\text{O}})}_0}}}{{{W_D} \times h \times 2}} $ | (2) |
式中,
释放DOC占NPP比重(P, %),是在假设碳固定与净产氧的摩尔比平衡(即1 mol C固定等于1 mol O2释放)的条件下,相同时间内海带幼苗释放DOC占净初级生产力比例,其计算公式为:
| $ P = \frac{{\Delta {C_{{\text{DOC}}}}}}{{\Delta {C_{{{\text{O}}_{\text{2}}}}}}} $ | (3) |
式中,∆CDOC为单位时间内的DOC浓度变化[μmol/(g·h)];
使用UV-5100B紫外可见分光光度计测定过滤水样的紫外可见吸收光谱。以超纯水为空白对照,使用10 mm石英比色皿在200~700 nm范围内扫描,扫描间隔为1 nm。波长λ的吸收系数[a(λ),m–1]公式为:
| $ {\text{a}}(\lambda ) = \frac{{2.303 \times A(\lambda )}}{{\text{b}}} $ | (4) |
式中,A(λ)为吸光度,b为光程路径(m)。
S275~295反映DOC相对分子质量与光反应活性,值越小,相对分子质量越大(Wada et al, 2007),光谱斜率S的计算公式为:
| $ {\text{a}}(\lambda ) = {\text{a}}({\lambda _0}) \times {\text{exp}}[S \times ({\lambda _0} - \lambda )] $ | (5) |
式中,a(λ)为DOM吸收系数(m–1);λ为波长(nm);λ0为参照波长(nm)。
1.4 数据分析采用Excel 2003软件对实验数据进行整理,采用SPSS 26.0进行单因素方差分析(其中,P<0.05为差异显著,P<0.01为差异极显著),用LSD法进行多重比较,使用Origin 2021绘图。
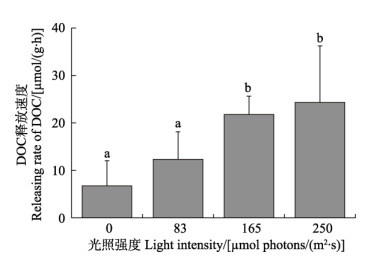
2 结果 2.1 不同光照条件下海带幼苗的处理结果 2.1.1 不同光照强度下的DOC释放速率研究结果显示,光照强度显著影响海带幼苗DOC的释放速率,DOC释放速率随光照强度增加呈上升趋势(图1)。黑暗条件下[0 μmol photons/(m2·s)],海带幼苗的DOC释放速率最低,为(6.73±5.30) μmol/(g·h)。光照强度为250 μmol photons/(m2·s)时,海带幼苗的DOC释放速率最高,为(24.31±5.84) μmol/(g·h),约为黑暗条件下的4倍左右。
|
图 1 不同光照强度下海带幼苗DOC释放速率 Fig.1 DOC releasing rate of S. japonica young seedling under different light intensities 字母不同代表组间有显著差异(P<0.05)。下同。 Treatment with different letters are significantly different at P<0.05. The same as below. |
研究结果显示,光照强度显著影响了海带幼苗的氧气释放速率,氧气释放速率随光照强度增加而呈现上升的趋势(图2)。在黑暗条件下,海带幼苗氧气净释放速率最低,为(66.70±12.23) μmol/(g·h)。在光照强度为250 μmol photons/(m2·s)条件下,海带幼苗氧气净释放速率最高,为(161.08±10.00) μmol/(g·h)。

|
图 2 不同光照处理强度下的海带幼苗释放氧气产量 Fig.2 Oxygen production of S. japonica young seedling under different light intensities |
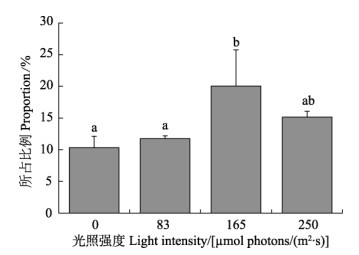
研究结果显示,光照强度显著影响了海带幼苗释放DOC占NPP比重,DOC释放量占净产氧比例呈现先上升后下降的趋势(图3)。在黑暗条件下,释放DOC占NPP比例最低,为(10.31±1.81)%。在光照强度为165 μmol photons/(m2·s)时,占比例最高,达(20.06±5.70)%。
|
图 3 不同光照强度下DOC释放量占净产氧比例 Fig.3 Proportion of DOC release in oxygen production under different light intensities |
光照强度显著影响了海带幼苗释放DOC的光谱斜率,S275~295均值随光照强度上升呈下降趋势,表明释放DOC的分子量随光照强度增加而呈现增大的趋势(表2)。0、83 μmol photons/(m2·s)光照条件下S275~295均值组间差异不显著。
|
|
表 2 不同光强下海带幼苗释放DOC的S275~295值 Tab.2 S275~295 values of DOC released from S. japonica young seedling under different light intensities |
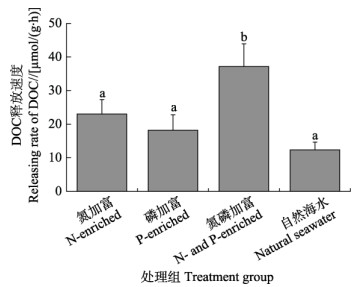
相较于自然海水,单一氮加富或磷加富没有显著提升DOC释放速率,氮磷共同加富则显著提升了DOC释放速率(图4)。自然海水组的海带幼苗的DOC释放速率最低,为(12.34±2.30) μmol/(g·h);氮磷加富组,海带幼苗的DOC释放速率最高,为(37.15±6.77) μmol/(g·h),约为自然海水组的4倍。
|
图 4 不同营养条件下海带幼苗DOC释放速率 Fig.4 DOC release rate of S. japonica young seedling under different nutritional conditions |
营养盐加富显著降低了海带氧气释放速率,氮加富、磷加富及氮磷共同加富条件下海带氧气释放速率均低于自然海水(图5)。氮加富、磷加富及氮磷共同加富条件下海带氧气释放速率组间差异不显著,分别较自然海水组下降了38.63%、43.85%和37.76%。

|
图 5 不同营养盐处理下的海带幼苗氧气净释放速率 Fig.5 Oxygen releasing rate of S. japonica young seedling under different nutritional conditions |
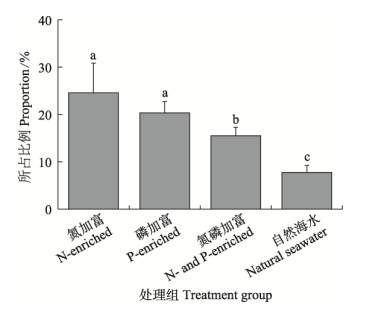
营养盐显著影响了海带幼苗释放DOC占NPP比重(图6)。在磷加富组中,所占比例最高,达(25.55±6.25)%;在自然海水中,所占比例最低,为(7.85±1.49)%。方差分析表明,单一营养盐加富的实验组显著高于氮磷加富组或自然海水组(P<0.05)。
|
图 6 不同营养盐处理下DOC释放量占净产氧比例 Fig.6 Proportion of DOC release in oxygen production under different nutritional conditions |
营养盐显著影响了海带幼苗释放DOC的光谱斜率,S275~295均值随着营养盐加富而呈现增大的趋势,表明释放DOC的分子量随营养盐加富而呈现减小的趋势(表3)。氮加富、磷加富条件下,S275~295均值组间差异不显著(P>0.05)。
|
|
表 3 不同营养条件下海带幼苗释放DOC的S275~295值 Tab.3 S275~295 values of DOC released from S. japonica young seedling under different nutritional conditions |
本研究发现,在自然海水条件下,海带幼苗释放DOC的速率与光照强度正相关。在光照强度为250 μmol photons/(m2·s)条件下,海带幼苗的DOC释放速率最高,约为黑暗条件的4倍左右(图2)。通过吸收光谱发现,250 μmol photons/(m2·s)条件下,S275~295显著低于黑暗条件,表明光照促进了溶解态大分子物质的释放(表2)。以上结果表明,在寡营养条件下,海带幼苗释放DOC受“溢出”机制调控。
通过营养加富实验发现,海带幼苗释放DOC受营养盐调控,氮磷加富条件下海带幼苗释放速率约为自然海水条件下的4倍(图4)。通过吸收光谱研究发现,在氮磷加富条件下,S275~295显著高于自然海水条件,表明营养盐加富促进了溶解态小分子物质的释放(表3)。以上结果表明,在营养加富条件下,海带幼苗释放DOC受“扩散”机制调控。
本研究发现,海带幼苗释放DOC同时存在“溢出”与“扩散”2种调控机制。何种机制占主导取决于营养条件。在自然海水的寡营养条件下,表现为“溢出”机制占主导;在氮磷营养盐充足条件下,表现为“扩散”机制占主导。浮游藻类、羽毛藻(Caulerpa sertularoides f. Longipes)、底栖珊瑚藻(Coralline algae)以及大型海藻释放DOC大多是在海水中营养盐较为贫瘠的条件下发生的,受“溢出”机制调控(Haas et al, 2010; Naumann et al, 2010; Barrón et al, 2014; Cherrier et al, 2015)。而在营养盐较为充足的条件下,藻类释放DOC的速率与光照强度无相关性,表明“溢出”调控机制失效(Marañón et al, 2005; Mueller et al, 2016; Wyatt et al, 2014)。Mueller等(2016)通过光照与营养盐交叉实验发现,在寡营养盐条件下,珊瑚藻释放DOC的速率与光照强度正相关。但添加氮磷营养盐后,在不同光强下藻类释放DOC的速率差异不显著,表明养分的添加导致DOC释放速率与光强的正相关性消失,营养限制决定了“溢出”机制何时起作用。原因可能是藻类光合作用受光照调节,而细胞的生长则受无机营养盐限制。在氮磷营养盐限制条件下,随着光照强度升高,细胞光合作用产出将超过细胞生长对有机质的需求(Fogg, 2009)。此时,细胞光合固定有机碳的速率将超过氮磷供给速率,从而导致细胞内碳元素大量富集,细胞内C∶N∶P比上升,生成的有机质主要以高分子量物质为主。此时表现为光强的增加促进有机质生成,从而加剧DOM的释放,“溢出”机制占主导(Mueller et al, 2016)。当环境中氮磷营养盐富集时,细胞内C∶N∶P比下降,因此,生成的有机质主要以低分子量物质为主。低分子量的DOM跨膜运输受细胞膜内外浓度差控制,表现为“扩散”机制占主导(Wyatt et al, 2014)。
海带幼苗光饱和点大约为100 μmol photons/(m2·s) (梁洲瑞等, 2020)。当光照达到或超过光饱和点时,藻类光合作用速率基本已无变化,DOC释放速率也应无变化。本研究设置的165、250 μmol photons/(m2·s)光照均已超过光饱和点,但仍表现出DOC释放速率随光照上升而上升。Reed等(2015)通过现场实验也发现类似现象,即使现场光照远远超过藻类光饱和点,藻类释放DOC速率仍然与光强正相关。具体是何原因导致这一现象的发生,尚需进一步研究。
大型藻释放的DOC中既有易于生物降解的活性溶解有机碳(labile dissolved organic carbon, LDOC)成分,如类蛋白质物质;又有难于降解的RDOC成分,如类腐殖质物质与芳香族类物质(Wada et al, 2007; Zhang et al, 2017; Chen et al, 2020)。RDOC的存在使大型藻类释放DOC的降解周期远超过浮游植物释放DOC的降解周期。如Wada等(2008)研究发现,褐藻苷苔释放DOC的降解周期为24~172 d,Watanabe等(2020)发现,马尾藻释放DOC的降解周期为111~238 d,均远超过浮游植物释放DOC的降解周期(2.8~40 d)。光照与营养条件的改变,均显著改变了其释放DOC相对分子量大小(表2和表3),表明其释放DOC的组成成分均发生了改变,其生物可利用性也发生了改变。Wyatt等(2014)也发现,氮磷营养盐加富后,底栖藻类释放DOC的生物可利用性更高。在未来研究中,尚需进一步探明光照与营养盐对海带释放DOC成分的影响,与异养细菌存在怎样的互作过程,何种养殖操作方式更有利于海带DOC的释放与RDOC的积累,才能将海带养殖增汇推入实际应用阶段。
ABDULLAH M I, FREDRIKSEN S. Production, respiration and exudation of dissolved organic matter by the kelp Laminaria hyperborea along the west coast of Norway. Journal of the Marine Biological Association of the UK, 2004, 84(5): 887-894 DOI:10.1017/S002531540401015Xh |
BARRÓN C, APOSTOLAKI E T, DUARTE C M. Dissolved organic carbon fluxes by seagrass meadows and macroalgal beds. Frontiers in Marine Science, 2014, 1: 42 |
BJORNSEN P K. Phytoplankton exudation of organic matter: Why do healthy cells do it?. Limnology and Oceanography, 1988, 33(1): 151-154 DOI:10.4319/lo.1988.33.1.0151 |
CHERRIER J, VALENTINE S, HAMILL B, et al. Light-mediated release of dissolved organic carbon by phytoplankton. Journal of Marine Systems, 2015, 147: 45-51 DOI:10.1016/j.jmarsys.2014.02.008 |
CHEN Y, XU C. Exploring new blue carbon plants for sustainable ecosystems. Trends in Plant Science, 2020, 25(3): 1067-1070 |
DUARTE C M, LOSADA I J, HENDRIKS I E, et al. The role of coastal plant communities for climate change mitigation and adaptation. Nature Climate Change, 2013, 3(11): 961-968 DOI:10.1038/nclimate1970 |
FOGG G E. The ecological significance of extracellular products of phytoplankton photosynthesis. Botanica Marina, 2009, 26(1): 3-14 |
HAAS A F, NAUMANN R S, STRUCK R, et al. Organic matter release by coral reef associated benthic algae in the Northern Red Sea. Journal of Experimental Marine Biology and Ecology, 2010, 389(1/2): 53-60 |
HAN T T, JIANG Z J, ZHANG J H, et al. Effects of DIN enrichment on inorganic carbon system of Saccharina japonica culture water. Progress in Fishery Sciences, 2014, 35(1): 18-25 [韩婷婷, 蒋增杰, 张继红, 等. 溶解无机氮加富对海带养殖水体无机碳体系的影响. 渔业科学进展, 2014, 35(1): 18-25 DOI:10.3969/j.issn.1000-7075.2014.01.003] |
JIAO N, HERNDL G J, HANSELL D A, et al. Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nature Reviews Microbiology, 2010, 8: 593-599 DOI:10.1038/nrmicro2386 |
JIAO N Z. Carbon fixation and sequestration in the ocean, with special reference to the microbial carbon pump. Scientia Sinica Terrae, 2012, 42(10): 1473-1486 [焦念志. 海洋固碳与储碳——并论微型生物在其中的重要作用. 中国科学:地球科学, 2012, 42(10): 1473-1486] |
JIAO N Z. Develop marine "negative emission" technology to support national "carbon neutral" demand. China Academic Journal Electronnic Publishing House, 2021, 36(2): 179-187 [焦念志. 研发海洋“负排放”技术支撑国家“碳中和”需求. 中国科学院院刊, 2021, 36(2): 179-187] |
KHATIWALA S, PRIMEAU F, HALL T. Reconstruction of the history of anthropogenic CO2 concentrations in the ocean. Nature:International Weekly Journal of Science, 2009, 462: 346-349 |
KRAUSE-JENSEN D, DUARTE C M. Substantial role of macroalgae in marine carbon sequestration. Nature Geoscience, 2016, 9: 737-742 DOI:10.1038/ngeo2790 |
LIANG Z R, LIU F L, DU X X, et al. Effects of light intensities on the growth and biochemical characteristics of Laminaria hyperborea young seedling. Progress in Fishery Sciences, 2019, 40(4): 115-122 [梁洲瑞, 刘福利, 杜欣欣, 等. 光强对极北海带幼苗生长和生化特性的影响. 渔业科学进展, 2019, 40(4): 115-122] |
LI F X, DU M R, GAO Y P, et al. Temporal and spatial distribution variation of picoplankton and environmental impact factors in Sanggou Bay. Journal of Fisheries of China, 2020, 44(7): 1100-1111 [李凤雪, 杜美荣, 高亚平, 等. 桑沟湾养殖水域微微型浮游生物的时空分布特征及环境影响因素. 水产学报, 2020, 44(7): 1100-1111] |
MARAÑÓN E, CERMEÑO P, PÉREZ V. Continuity in the photosynthetic production of dissolved organic carbon from eutrophic to oligotrophic waters. Marine Ecology Progress Series, 2005, 299: 7-17 DOI:10.3354/meps299007 |
MCLEOD E, CHMURA G L, BOUILLON S, et al. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment, 2011, 9(10): 552-560 DOI:10.1890/110004 |
MUELLER B, HAAN J D, VISSER P M, et al. Effect of light and nutrient availability on the release of dissolved organic carbon (DOC) by Caribbean turf algae. Scientific Reports, 2016, 6: 23248 DOI:10.1038/srep23248 |
NAUMANN M S, HAAS A, STRUCK U, et al. Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs, 2010, 29(3): 649-659 DOI:10.1007/s00338-010-0612-7 |
OGAWA H, AMAGAI Y, KOIKE I, et al. Production of refractory dissolved organic matter by bacteria. Science, 2001, 292(5518): 917-920 DOI:10.1126/science.1057627 |
REED D C, CARLSON C A, HALEWOOD E R, et al. Patterns and controls of reef-scale production of dissolved organic carbon by giant kelp Macrocystis pyrifera. Limnology and Oceanography, 2015, 60(6): 1996-2008 DOI:10.1002/lno.10154 |
ROSS H, ALECIA B, MACREADIE P I, et al. Can macroalgae contribute to blue carbon?. An Australian perspective, Limnology and Oceanography, 2015, 60(5): 1689-1706 |
TANG Q S, LIU H. Strategy for carbon sink and its amplification in marine fisheries. Strategic Study of ACE, 2016, 18(3): 68-73 [唐启升, 刘慧. 海洋渔业碳汇及其扩增战略. 中国工程科学, 2016, 18(3): 68-73 DOI:10.3969/j.issn.1009-1742.2016.03.012] |
WADA S, AOKI M N, TSUCHIYA Y, et al. Quantitative and qualitative analyses of dissolved organic matter released from Ecklonia cava Kjellman, in Oura Bay, Shimoda, Izu Peninsula, Japan. Journal of Experimental Marine Biology and Ecology, 2007, 349: 344-358 DOI:10.1016/j.jembe.2007.05.024 |
WADA S, AOKI M N, MIKAMI A, et al. Bioavailability of macroalgal dissolved organic matter in seawater. Marine Ecology Progress Series, 2008, 370: 33-44 DOI:10.3354/meps07645 |
WANG Y. The study of the relationship of the marine primary productivity and the environmental factors in the artificial reef area around the Dalian Islands. Master´s Thesis of Dalian Ocean University, 2016 [王月. 褡裢岛周边海域初级生产力与环境因子关系的研究. 大连海洋大学硕士研究生学位论文, 2016]
|
WATANABE K, YOSHIDA G, HORI M, et al. Macroalgal metabolism and lateral carbon flows can create significant carbon sinks. Biogeosciences, 2020, 17: 2425-2440 DOI:10.5194/bg-17-2425-2020 |
WYATT K H, TELLEZ E, WOODKE R L, et al. Effects of nutrient limitation on the release and use of dissolved organic carbon from benthic algae in Lake Michigan. Freshwater Science, 2014, 33(2): 557-567 DOI:10.1086/675453 |
YUAN Y M, LIU F L, DU X X, et al. Study on the assimilation of nitrogen and phosphorus and on the sorption of heavy metals in Laminaria hyperborean. Progress in Fishery Sciences, 2020, 41(3): 25-31 [袁艳敏, 刘福利, 杜欣欣, 等. 极北海带对氮、磷吸收和砷、镉、铅吸附的研究. 渔业科学进展, 2020, 41(3): 25-31] |
YU X J, XU L J, WU F X, et al. China fisheries statistical yearbook. Beijing: China Agricultural Press, 2020 [于秀娟, 徐乐俊, 吴反修, 等. 中国渔业统计年鉴. 北京: 中国农业出版社, 2020]
|
ZHANG J H, LIU J H, ZHANG Y Y, et al. Strategic approach for mariculture to practice “ocean negative carbon emission”. Bulletin of the Chinese Academy of Sciences, 2021, 36(3): 252-258 [张继红, 刘纪化, 张永雨, 等. 海水养殖践行“海洋负排放”的途径. 中国科学院院刊, 2021, 36(3): 252-258] |
ZHANG Y Y, ZHANG J H, LIANG Y T, et al. Carbon sequestration processes and mechanisms in coastal mariculture environments in China. Science China Earth Sciences, 2017, 47(12): 1414-1424 [张永雨, 张继红, 梁彦韬, 等. 中国近海养殖环境碳汇形成过程与机制. 中国科学:地球科学, 2017, 47(12): 1414-1424] |



