2. 青岛海洋科学与技术试点国家实验室海洋生态 与环境科学功能实验室 山东 青岛 266071;
3. 潍坊渔业产业技术研究院 山东 潍坊 261108
2. Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, Shandong 266071, China;
3. Weifang Institute of Fishery Industrial Technology, Weifang, Shandong 261108, China
海洋渔业碳汇是海洋生物“蓝色碳汇”的重要组成部分,不仅包括处于食物网较低营养级的贝藻养殖等使用的碳,同时,还包括某些生物资源种类通过摄食和生长活动所使用的碳(唐启升等, 2016)。作为海洋生态系统中最主要的初级生产力之一,藻类等海洋植物是公认的高效固碳生物:通过光合作用直接吸收海水中的CO2,从而增加了海洋的碳汇,促进并加速了大气中的CO2向海水中扩散,有利于减少大气中的CO2 (唐启升等, 2016)。而大型海藻支撑了大量的海洋生物类群,端足类是其中最重要的类群之一(Cacabelos et al, 2010)。端足类不仅以大型海藻作为栖息地、庇护所和育幼场,更以大型海藻作为营养来源(Wessels et al, 2006)。这些藻栖动物在利用海藻的过程中又为其他海洋动物提供了重要的食物来源(Duffy et al, 2000; Jiménez-Prada et al, 2021),将大型海藻的初级生产力通过食物链传递到更高营养级,对整个生态系统的物质循环和能量流动产生重要影响(Tano et al, 2016)。海藻群落与栖息在其中的端足类之间的关系是该生态系统过程中的一个关键环节。也正因如此,大型海藻和藻栖动物之间的关系一直是海藻群落生态功能的研究热点之一(刘书荣, 2019),藻栖动物对大型海藻的选择性摄食即是其中的一个重要研究内容。
和许多无脊椎动物一样,端足类对大型海藻并非不加区分地摄食,研究显示,端足类会优先摄食“可口”的大型海藻,尤其是生长迅速的短生性大型海藻(如浒苔),它们的摄食往往可以减少这类大型海藻的生物量积累(郑新庆等, 2013; 薛素燕等, 2018),使大型海藻的群落结构向端足类不喜欢摄食的藻类占主导方向发展(Duffy, 1990),从而对大型海藻群落结构起到重要调控作用。
作为食物链中物质循环和能量传递的关键环节,端足类通过摄食作用,将大型海藻固定的碳传递转移到高级消费者,从而实现“碳封存”或“碳移出”,在海洋渔业碳汇过程中可能也发挥着不可忽视的作用。本文以山东半岛端足类中华原钩虾(Eogammarus possjeticus)为实验对象,研究了其对5种优势大型海藻的摄食选择性,并初步探讨了钩虾潜在的碳汇影响,以期为碳汇渔业的发展提供基础数据。
1 材料与方法 1.1 实验材料本研究的中华原钩虾采自山东潍坊下营浅海附近的养殖池塘,浒苔(Ulva prolifera)、肠浒苔 (U. intestinalis)、扁浒苔(U. compressa)、线形硬毛藻(Chaetomorpha linum)和丝毛藻(Cloniophora sp.)这 5种实验大型海藻采自山东青岛近岸海域及海水养殖池塘。中华原钩虾和海藻带回实验室后,在100 L的塑料圆形桶中暂养,暂养的海水来自青岛近岸海域,海水经砂滤过滤。暂养水温为15~18℃,盐度为29~31,pH为8.0~8.2,溶氧(DO)为6.7~7.5 mg/L。暂养桶中每天更新海水1/3~1/2。
1.2 摄食实验 1.2.1 大型海藻无选择性摄食实验实验前将中华原钩虾和各种大型海藻分离培养24 h,待胃排空后,将中华原钩虾转移至含2000 mL过滤海水的塑料方缸(20 cm×15 cm×10 cm)中,并持续充气。分别以 5种大型海藻投喂中华原钩虾,每个塑料方缸中放入规格较一致的中华原钩虾0.4 g (约100尾),每种鲜海藻的投喂量约为2 g,保证中华原钩虾充足的食物供应。48 h后分别收集残余海藻,用蒸馏水冲洗残余海藻表面,再用定性滤纸将残余海藻表面的水分吸干,分析天平(精度为0.000 1 g)称量投喂前后鲜海藻重量及中华原钩虾体重(湿重),计算中华原钩虾的摄食率。实验设置6个平行,4个对照(不放钩虾),摄食率计算公式如下:
| $ C=[C_{1}·(1+K)–C_{2}]/(W·t) $ |
式中,C表示摄食率,单位体重(湿重)中华原钩虾对海藻鲜藻体的日摄食量[g鲜重/(g·d)]或干藻体的日摄食量[g干重/(g·d)];C1和C2分别代表海藻初、末重量(g);K表示在t时间内海藻的生长系数(由对照组实验前后海藻重量的变化除以海藻初始重量计算获得),W表示中华原钩虾的体重(g)。
1.2.2 大型海藻选择性摄食实验在3000 mL的塑料方缸中(20 cm×20 cm×10 cm)放入中华原钩虾 50尾(湿重约0.2 g)和上述5种大型海藻共同培养,每种鲜海藻放入约1 g。为方便观察及残余海藻的收集,将5种海藻相互间隔3~5 cm呈2排随机放置,每12 h记录各海藻上面钩虾的个体数量,连续记录48 h,实验结束后收集残余海藻,计算中华原钩虾的摄食率。实验设置6个平行和4个对照(不放钩虾),其他操作同上。
1.3 指标测定与计算本研究以5种大型海藻的干湿比(DW/FW,平行样n=4)、总有机碳含量(TOC, n=4)、总氮含量(TN, n=4)和碳氮比(C/N)为基本营养成分指标。海藻湿重与干重测定时,先用蒸馏水冲洗海藻表面,再用定性滤纸将海藻表面水分吸干,分析天平称量鲜海藻重量(FW),然后再将海藻置于70℃烘箱中烘干至恒重(48 h),称量海藻干重(DW),计算DW/FW。干藻体经研磨、包埋后,采用Elementar EL型元素分析仪(德国Elementar公司)测定海藻TOC和TN,并计算C/N。
1.4 数据处理与分析所有实验数据经SPSS 23.0统计软件进行处理分析,采用单因素方差分析(one-way ANOVA),不同处理组间采用Turkey方法进行多重比较,相关性分析采用双变量相关分析(Bivariate),以P<0.05作为不同处理间差异显著标准。
2 结果 2.1 5种海藻的碳氮含量特征如表1所示,肠浒苔与浒苔的干湿比相当(分别为0.08和0.10),为5种绿藻中最低,丝毛藻的干湿比最高(为0.29),约为肠浒苔和浒苔的3倍、扁浒苔的2.4倍、线性硬毛藻的1.5倍(P<0.05)。TOC含量最高的是扁浒苔为37.72%,最低的是丝毛藻为25.44%,肠浒苔与浒苔的TOC含量相当,分别为33.28%和32.86%。线性硬毛藻有最高的TN含量为4.17%,C/N最低为6.66,肠浒苔则相反,TN含量最低为2.77%,C/N最高为11.95。
|
|
表 1 5种大型海藻的碳氮含量 Tab.1 Carbon and nitrogen contents of five species of macroalgae (Mean±SD) |
中华原钩虾对5种大型海藻鲜藻体(FW)单位体重日摄食率和干藻体(DW)单位体重日摄食率的变化趋势基本一致 (图1),对肠浒苔和浒苔的摄食率最高,分别为0.81和0.80 g鲜重/(g·d),而对线性硬毛藻和丝毛藻的摄食率最低,分别为0.19和0.21 g鲜重/(g·d),其中,对肠浒苔和浒苔鲜藻体的摄食率约为后二者的4倍,差异显著(P<0.05),同样地,用干藻体计算的摄食率以线性硬毛藻的最低,约为浒苔的1/2 (P<0.05)。
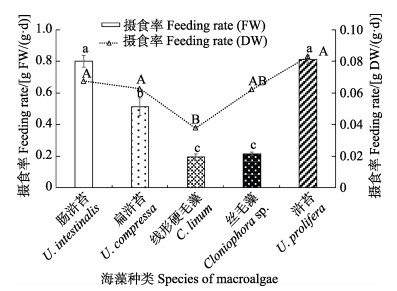
|
图 1 无选择实验中华原钩虾对5种海藻鲜藻体(FW)和干藻体(DW)的摄食率 Fig.1 The feeding rates of five kinds of macroalgae by E. possjeticus on fresh (FW) and dry algae (DW) in non-selective feeding experiments 不同字母表示组间差异显著(P<0.05),下同。 Different letters indicate significant difference between treatments (P<0.05), the same as below. |
在无选择性摄食实验中,中华原钩虾的摄食率与海藻TOC含量的变化趋势基本一致,其摄食率大致随海藻TOC含量的降低而降低(图2),而TN含量的变化趋势与中华原钩虾摄食率相反,即中华原钩虾摄食率随海藻TN含量的升高而呈降低趋势(图3);中华原钩虾摄食率的变化趋势与C/N基本一致(图4),而与干湿比呈负相关(图5)。经Bivariate相关性分析,中华原钩虾的摄食率与海藻TOC含量(P<0.05)、TN含量(P<0.05)、C/N(P<0.01)和干湿比(P<0.01)均显著相关。
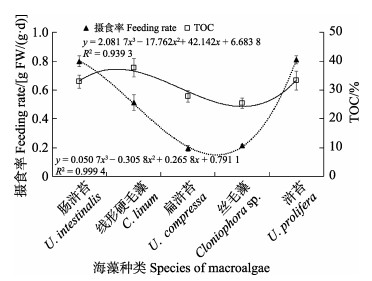
|
图 2 中华原钩虾摄食率与干海藻TOC含量关系 Fig.2 Relationship between feeding rate of E. possjeticus and TOC concentration of dry algae |

|
图 3 中华原钩虾摄食率与干海藻TN含量关系 Fig.3 Relationship between feeding rate of E. possjeticus and TN concentration of dry algae |

|
图 4 中华原钩虾摄食率与干海藻C/N关系 Fig.4 Relationship between feeding rate of E. possjeticus and C/N ratio of dry algae |
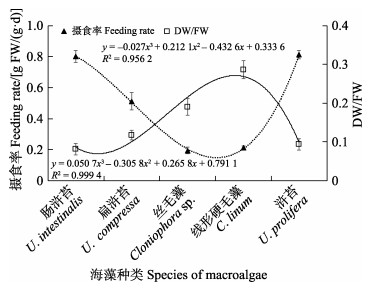
|
图 5 中华原钩虾摄食率与海藻干湿比 Fig.5 Relationship between feeding rate of E. possjeticus and DW/FW ratio |
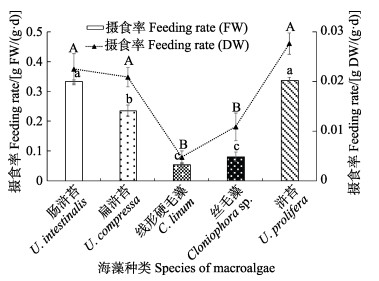
在同时投喂5种大型海藻后,中华原钩虾选择栖息于大型海藻的个体数量比例以丝毛藻最高,其次为肠浒苔和浒苔,比例最低的为扁浒苔(图6, P<0.05)。中华原钩虾对5种大型海藻的摄食率差异显著(图7, P<0.05),且与无选择性摄食实验的趋势相似,对肠浒苔和浒苔的摄食率最高,对扁浒苔的次之,对丝毛藻和线性硬毛藻的最小。
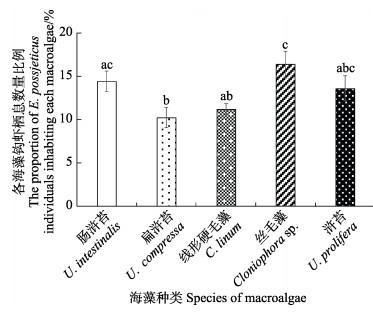
|
图 6 中华原钩虾在5种海藻栖息的个体数量比例 Fig.6 Proportion of E. possjeticus individuals inhabiting five species of macroalgae |
|
图 7 选择实验中华原钩虾对5种海藻鲜藻体(FW)和干藻体(DW)的摄食率 Fig.7 Feeding rates of five kinds of macroalgae by E. possjeticus on fresh (FW) and dry algae (DW) in a selective experiment |
本研究采用海藻的TOC、TN、C/N以及DW/FW表征海藻的基本营养特征。结果显示,中华原钩虾对高含水量、高TOC含量的扁浒苔、肠浒苔和浒苔摄食率较高,而对低含水量、低TOC含量的线性硬毛藻和丝毛藻摄食率较低。郑新庆等(2013)开展强壮藻钩虾(Ampithoe valida)对3种海藻的摄食实验,结果表明,强壮藻钩虾的摄食率与海藻的含水量呈正相关,与海藻的TOC含量呈负相关,对高含水量、低碳含量的藻类会通过高摄食率的补偿性摄食来满足自身代谢需求。与之相似的是,中华原钩虾也是通过补偿性摄食,获得高含水量藻类的能量补偿;不同的是,中华原钩虾和强壮藻钩虾对不同TOC含量海藻的摄食率有所差异。许多陆生动物和一些海洋植食性无脊椎动物,表现出对高氮含量植物的摄食偏好(Valentine et al, 2006),但中华原钩虾对高TN含量的线性硬毛藻和丝毛藻摄食率反而较低。相似地,强壮藻钩虾也未表现出对高TN藻类的摄食偏好,郑新庆等(2013)认为,钩虾对海藻的摄食选择性可能与其生活习性有关,如非管栖的漫游性钩虾更喜欢摄食高营养价值的食物,而管栖性钩虾对不同营养价值的食物没有明显的摄食选择性(Cruz-Rivera et al, 2000)。上述结论似乎不完全适应于中华原钩虾,中华原钩虾属于非管栖、营自由生活的端足类,本研究的5种海藻中,它对高TOC海藻摄食率高,而对高TN海藻摄食率低。除此之外,研究显示,大型海藻分泌的次生代谢物、形态特征等都会影响钩虾的摄食选择性(Duffy et al, 1991; Cruz-Rivera et al, 2001; Toth et al, 2005; Yun et al, 2007; Sotka, 2007; 郑新庆, 2008)。由此可见,钩虾对大型海藻的摄食选择性因物种不同而异。
另外,大型藻类形态结构的复杂性会影响钩虾的丰度和生物量。Huang等(2007)对南极半岛西部帕默站附近8种优势海藻上端足目生物进行调查,发现分枝数量最多的海藻中端足目丰度最高。Holmlund等(1990)也证实,与具有简单叶片形态的海藻上相比,端足类生物在高度分枝、形态复杂的藻类上,更不易被鱼类捕食。这也为本研究中丝毛藻、肠浒苔和浒苔上栖息的钩虾数量较多提供了解释和证明。相较于钩虾栖息数量最少的扁浒苔,丝毛藻、肠浒苔和浒苔的形态结构更复杂,细丝状的分枝更多且更密集,可为钩虾提供更为隐蔽的栖息环境。
3.2 钩虾选择性摄食对大型海藻的影响端足类被认为是海藻场中一类重要的植食性动物,有较小的觅食范围和较高的种群密度,是大型海藻的主要消费类群(Carpenter, 1986)。据Balducci等(2001)的调查发现,栖息在硬石莼(U. rigida)占优势的植物群落上的动物(<10 mm)中,钩虾(Gammarus aequicauda)的丰度占82.8%,它的日摄食量占海藻特定生长率(SGR)的15%。在中国筼筜湖藻场底栖动物群落中,端足类属于绝对优势类群,其丰度占底栖动物总丰度的85.8%~98.7%,其中,3月强壮藻钩虾丰度可达12 000 ind./m2 (郑新庆, 2008)。有着如此高密度的端足类,其选择性啃食对海藻的群落结构有着重要的影响(Valentine et al, 2006; Guidone et al, 2015)。Crawley等(2007)通过室内研究发现,端足类使得边花昆布(Ecklonia radiate)和马尾藻(Sargassum sp.)的生物量分别损失了69%~98%和64%。在美国北卡罗纳州沿岸,藻钩虾的摄食作用可抑制绿藻的生长,使原来的海藻群落逐渐演替为以红藻为优势种的海藻群落(Duffy et al, 2000)。还有研究显示,在某些富营养化水域,钩虾的摄食量甚至超过大型海藻的生长,它们的摄食压力甚至决定某些海藻的存在与否(Geertz-Hansen et al, 1993)。
研究中发现,利用端足类的选择性摄食作用,一方面可以控制一些养殖经济海藻上的附着生物,减少其覆盖率,使养殖海藻在对营养盐和光的竞争中占据优势(魏龑伟, 2014)。如Duffy等(1990)研究发现,藻钩虾(A. marcuzii)、麦秆虫(Caprella penantis)和镰形叶钩虾(Jassa falcate)能明显抑制马尾藻上附生藻类的生长;另一方面,端足类的摄食作用可控制藻类初级生产者的生物量和生产力,尤其针对一些可引起绿潮或赤潮暴发的海藻种类,从而维持水生态系统功能完整性(Poore et al, 2013)。如郑新庆等(2013)研究表明,强壮藻钩虾的啃食作用对孔石莼(U. pertusa)生物量的增加有明显抑制作用。本研究中,中华原钩虾对浒苔类绿藻也具有较高的摄食率,如对肠浒苔和浒苔的摄食率达到0.81和0.80 g鲜重/(g·d),即对鲜藻体的日摄食率可达80%以上,这与薛素燕等(2018)的研究结果高度一致(中华原钩虾对浒苔干藻体的日摄食率为7.6%,换算成鲜藻体的日摄食率约为82.8%),同时,薛素燕等(2018)也提出,在海水池塘这样的封闭半封闭水域中,可利用中华原钩虾高强度的啃食作用来抑制或控制浒苔等海藻。
3.3 钩虾在海洋渔业资源中潜在的碳汇影响大型海藻作为海域初级生产力的重要来源之一(何培民等, 2015),是高效的固碳生物,驱动着整个近岸海洋生态系统的生态过程(章守宇等, 2019)。端足类是大型海藻群落中最重要的消费类群之一,也是重要经济鱼类、甲壳类、头足类,甚至灰鲸(Eschrichtius robustus)等海洋动物的重要食物来源(Duffy et al, 2000; Moren et al, 2006; Rodkina et al, 2020; Jiménez-Prada et al, 2021; Xue et al, 2021),处于食物链的中间环节,在生态系统的物质循环和能量流动中起着承上启下的作用(Costa et al, 1999)。端足类对大型海藻的啃食作用使其承担了将初级生产者固定的“碳”向高级消费者传递转移的职责(图8),是实现“碳转移”的重要通道。碳转移的过程使得这些处于食物链顶端的海洋动物,通过生物泵的形式进行碳封存,一部分随着人类捕捞收获被移除海洋水体,实现“碳移出”,另一部分未被人类捕捞的海洋动物则继续进行“碳储存”和食物链传递(唐启升, 2011; 张波等, 2013、2022)。

|
图 8 大型海藻的“碳”通过钩虾向更高营养级传递转移 Fig.8 The carbon transfer from macroalgae to higher trophic level by gammarus |
总有机碳(TOC)含量是大型海藻碳汇能力的评价指标之一(刘耀谦等, 2019)。本研究中,肠浒苔、扁浒苔和浒苔的TOC含量均超过了30%,属于固碳能力较高的大型海藻,而浒苔类绿藻尤其受到钩虾的青睐,易被优先选择摄食。由于浒苔类绿藻属于生长迅速的短生性大型海藻(Goecker et al, 2003),通过钩虾的摄食,能够快速将其固定的碳向更高营养级转移,可能会加速海洋渔业碳汇进程。
综上可知,以钩虾为代表的端足类在海洋渔业碳汇中扮演着碳转移“通道”的角色,其转移的碳量中包括以其为主要营养来源的处于顶端营养级的海洋捕捞业的碳汇量和未捕捞的渔业资源量的碳汇量,在海洋渔业碳汇过程中发挥着重要的作用。
BALDUCCI C, SFRISO A, PAVONI B. Macrofarina impact on Ulva rigida C. Ag. production and relationship with environmental variables in the lagoon of Venice. Marine Environmental Research, 2001, 52: 27-49 |
CACABELOS E, OLABARRIA C, INCERA M, et al. Effects of habitat structure and tidal height on epifaunal assemblages associated with macroalgae. Estuarine, Coastal and Shelf Science, 2010, 89(1): 43-52 DOI:10.1016/j.ecss.2010.05.012 |
CARPENTER C R. Partitioning herbivory and its effects on coral reef algal communities. Ecological Monographs, 1986, 56(4): 345-363 DOI:10.2307/1942551 |
COSTA F O, COSTA M H. Life history of the amphipod Gammarus locusta in the Sado estuary (Portugal). Acta Oecologica, 1999, 20(4): 305-314 DOI:10.1016/S1146-609X(99)00136-8 |
CRAWLEY K R, HYNDES G A. The role of different types of detached macrophytes in the food and habitat choice of a surf-zone inhabiting amphipod. Marine Biology, 2007, 151(4): 1433-1443 DOI:10.1007/s00227-006-0581-0 |
CRUZ-RIVERA E, HAY M E. Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology, 2000, 81(1): 201-219 DOI:10.1890/0012-9658(2000)081[0201:CQRQFC]2.0.CO;2 |
CRUZ-RIVERA E, HAY M E. Macroalgal traits and the feeding and fitness of an herbivorous amphipod: The roles of selectivity, mixing, and compensation. Marine Ecology- Progress Series, 2001, 218: 249-266 DOI:10.3354/meps218249 |
DUFFY J E, HAY M E. Food and shelter as determinants of food choice by an herbivorous marine amphipod. Ecology, 1991, 72(4): 1286-1298 DOI:10.2307/1941102 |
DUFFY J E, HAY M E. Strong impacts of grazing amphipods on the organization of a benthic community. Ecological Monographs, 2000, 70(2): 237-263 DOI:10.1890/0012-9615(2000)070[0237:SIOGAO]2.0.CO;2 |
DUFFY J E. Amphipods on seaweeds: Partners or pests? Oecologia, 1990, 83: 267–276
|
GEERTZ-HANSEN O, SAND-JENSEN K, HANSEN D F, et al. Growth and grazing control of abundance of the marine macroalga, Ulva lactuca L. in a eutrophic Danish estuary. Aquatic Botany, 1993, 46(2): 101-109 |
GOECKER M E, KAIL S E. Grazing preferences of marine isopods and amphipods on three prominent algal species of the Baltic Sea. Journal of Sea Research, 2003, 50(4): 309-314 DOI:10.1016/j.seares.2003.04.003 |
GUIDONE M, THORNBER C S, VAN ALSTYNE K L. Herbivore impacts on two morphologically similar bloom-forming Ulva species in a eutrophic bay. Hydrobiologia, 2015, 753(1): 175-188 DOI:10.1007/s10750-015-2204-6 |
HE P M, LIU Y Y, ZHANG J W, et al. Research progress on the effects of macroalgae on carbon sink. Journal of Fishery Sciences of China, 2015, 22(3): 588-595 [何培民, 刘媛媛, 张建伟, 等. 大型海藻碳汇效应研究进展. 中国水产科学, 2015, 22(3): 588-595] |
HOLMLUND M B, PETERSON C H, HAY M E. Does algal morphology affect amphipod susceptibility to fish predation? Journal of Experimental Marine Biology and Ecology, 1990, 139(1/2): 65–83
|
HUANG Y M, AMSLER M O, MCCLINTOCK J B, et al. Patterns of gammaridean amphipod abundance and species composition associated with dominant subtidal macroalgae from the western Antarctic Peninsula. Polar Biology, 2007, 30(11): 1417-1430 DOI:10.1007/s00300-007-0303-1 |
JIMÉNEZ-PRADA P, HACHERO-CRUZADO I, GUERRA- GARCIA J M. Aquaculture waste as food for amphipods: The case of Gammarus insensibilis in marsh ponds from southern Spain. Aquaculture International, 2021, 29: 139-153 DOI:10.1007/s10499-020-00615-z |
LIU S R. The effects of seaweeds on two categories of representative seaweed-associated amphipods biological traits in mussel-rafts culture area. Master´s Thesis of Shanghai Ocean University, 2019 [刘书荣. 贻贝筏式养殖区大型海藻对两类代表性藻栖端足目生物特征的影响. 上海海洋大学硕士研究生学位论文, 2019]
|
LIU Y Q, ZHANG C X, SUN S L, et al. Carbon sequestration potential research of macroalage in the intertidal rocky zone in Naozhou Island. Journal of Guangdong Ocean University, 2019, 39(5): 78-84 [刘耀谦, 张才学, 孙省利, 等. 硇洲岛岩礁带大型海藻固碳潜能. 广东海洋大学学报, 2019, 39(5): 78-84] |
MOREN M, SUONTAMA J, HEMRE G I, et al. Element concentrations in meals from krill and amphipods— Possible alternative protein sources in complete diets for farmed fish. Aquaculture, 2006, 261(1): 174-181 DOI:10.1016/j.aquaculture.2006.06.022 |
POORE A G, GALLAGHER K M. Strong consequences of diet choice in a talitrid amphipod consuming seagrass and algal wrack. Hydrobiologia, 2013, 701(1): 117-127 DOI:10.1007/s10750-012-1263-1 |
RODKINA S A, KIYASHKO S I, DEMCHENKO N L. Trophic basis of dominant amphipods in the gray whale feeding grounds near northeastern Sakhalin Island (the Sea of Okhotsk) inferred from fatty acid and stable isotope analyses. Marine Environmental Research, 2020, 158: 104999 DOI:10.1016/j.marenvres.2020.104999 |
SOTKA E E. Restricted host use by the herbivorous amphipod Peramphithoe tea is motivated by food quality and abiotic refuge. Marine Biology, 2007, 151(5): 1831-1838 DOI:10.1007/s00227-007-0612-5 |
TANG Q S, LIU H. Strategy for carbon sink and its amplification in marine fisheries. Strategic Study of CAE, 2016, 18(3): 68-73 [唐启升, 刘慧. 海洋渔业碳汇及其扩增战略. 中国工程科学, 2016, 18(3): 68-73 DOI:10.3969/j.issn.1009-1742.2016.03.012] |
TANG Q S. Ways and scientific problems of marine biological carbon sink amplification. 399th Symposium of Xiangshan Science Conference, 2011, 10-15 [唐启升. 海洋生物碳汇扩增途径与科学问题. 香山科学会议第399次学术研讨会, 2011, 10-15] |
TANO S, EGGERTSEN M, WIKSTRÖM S A, et al. Tropical seaweed beds are important habitats for mobile invertebrate epifauna. Estuarine, Coastal and Shelf Science, 2016, 183: 1-12 DOI:10.1016/j.ecss.2016.10.010 |
TOTH G B, LANGHAMER O, PAVIA H. Inducible and constitutive defenses of valuable seaweed tissues: Consequences for herbivore fitness. Ecology, 2005, 86(3): 612-618 DOI:10.1890/04-0484 |
VALENTINE J F, DUFFY J E. The central role of grazing in seagrass ecology. In: Seagrasses: Biology, Ecologyand Conservation. Springer, 2007, 463-501 |
WEI Y W. Basic ecology research on biofouling Caprella acanthogaster in Sungo Bay. Master´s Thesis of Shanghai Ocean University, 2014 [魏龑伟. 桑沟湾污损生物—多棘麦杆虫的基础生态学研究. 上海海洋大学硕士研究生学位论文, 2014]
|
WESSELS H, HAGEN W, MOLIS M, et al. Intra- and interspecific differences in palatability of Arctic macroalgae from Kongsfjorden (Spitsbergen) for two benthic sympatric invertebrates. Journal of Experimental Marine Biology and Ecology, 2006, 329(1): 20-33 DOI:10.1016/j.jembe.2005.08.006 |
XUE S Y, DING J K, LI J Q, et al. Effects of live, artificial and mixed feeds on the growth and energy budget of Penaeus vannamei. Aquaculture Reports, 2021, 19: 100634 DOI:10.1016/j.aqrep.2021.100634 |
XUE S Y, MAO Y Z, DING J K, et al. Feeding of amphipod Eogammarus possjeticus on Ulva prolifera and other types of baits. Oceanologia et Limnologia Sinica, 2018, 49(5): 1109-1115 [薛素燕, 毛玉泽, 丁敬坤, 等. 中华原钩虾(Eogammarus possjeticus)对浒苔(Ulva prolifera)及其他类型饵料的摄食研究. 海洋与湖沼, 2018, 49(5): 1109-1115] |
YUN H Y, CRUZ J, TREITSCHKE M, et al. Testing for the induction of anti-herbivory defences in four Portuguese macroalgae by direct and water-borne cues of grazing amphipods. Helgoland Marine Research, 2007, 61(3): 203-209 DOI:10.1007/s10152-007-0067-6 |
ZHANG B, SUN S, TANG Q S. Carbon sink by marine fishing industry. Progress in Fishery Sciences, 2013, 34(1): 70-74 [张波, 孙珊, 唐启升. 海洋捕捞业的碳汇功能. 渔业科学进展, 2013, 34(1): 70-74 DOI:10.3969/j.issn.1000-7075.2013.01.011] |
ZHANG B, TANG Q S. Carbon sink assessment for capture stock in China coastal ocean. Progress in Fishery Sciences, 2022, 43(5): 126-131 [张波, 唐启升. 中国近海渔业生物捕捞群体碳汇评估. 渔业科学进展, 2022, 43(5): 126-131] |
ZHANG S Y, LIU S R, ZHOU X J, et al. Ecological function of seaweed-formed habitat and discussion of its application to sea ranching. Journal of Fisheries of China, 2019, 43(9): 2004-2014 [章守宇, 刘书荣, 周曦杰, 等. 大型海藻生境的生态功能及其在海洋牧场应用中的探讨. 水产学报, 2019, 43(9): 2004-2014] |
ZHENG X Q, HUANG L F, LI Y C, et al. The feeding selectivity of an herbivorous amphipod Ampithoe valida on three dominant macroalgal species of Yundang Lagoon. Acta Ecologica Sinica, 2013, 33(22): 7166-7172 [郑新庆, 黄凌风, 李元超, 等. 啃食性端足类强壮藻钩虾对筼筜湖三种大型海藻的摄食选择性. 生态学报, 2013, 33(22): 7166-7172] |
ZHENG X Q. A Preliminary study on the impacts of amphipods' grazing on the macroalgal community in Yundang Lagoon. Master´s Thesis of Xiamen University, 2008 [郑新庆. 端足类啃食作用对筼筜湖大型海藻群落影响的初步研究. 厦门大学硕士研究生学位论文, 2008]
|



