2. 浙江海洋大学海洋科学与技术学院 浙江 舟山 316022;
3. 中国水产科学研究院黄海水产研究所 农业农村部海洋渔业可持续发展重点实验室 山东 青岛 266071
2. Marine Science and Technology College, Zhejiang Ocean University, Zhoushan, Zhejiang 316022, China;
3. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture and Rural Affairs, Qingdao, Shandong 266071, China
在过去的几十年里,我国对虾养殖迅速发展,2019年,对虾总产量为145.02万t,占甲壳类总产量的83.16% (农业农村部渔业渔政管理局等, 2020)。凡纳滨对虾(Litopenaeus vannamei)是对虾主要养殖品种,2019年其产量占对虾总产量的78.91% (农业农村部渔业渔政管理局等, 2020)。研究发现,在高密度对虾养殖过程中,饵料中仅有20%~30%的氮、10%~20%的磷被对虾利用(臧维玲等, 2009; Chen et al, 2018),随着养殖的进行,养殖水体中氮磷营养盐的增加,造成水体富营养化,促进藻类生长,甚至会引发有害藻华(常孟阳等, 2019),水质恶化,最终会影响对虾的生长,引发病害(Tseng et al, 2004)。
浮游植物是养殖生态系统的重要组成部分,可以通过光合作用吸收利用环境中的氮、磷等营养盐,对养殖生态系统内物质循环和能量流动起重要作用。利用浮游植物净化和调控养殖水质不仅减轻了养殖引起的负面环境影响(黄翔鹄等, 2005; Ramli et al, 2017),还为养殖生物提供直接或间接饵料(刘瑞卿等, 2021),是一项成本低、能耗少、效益高、开发潜力大的环保工程。作为一种绿色、健康和低成本的养殖环境水质改良方法,微藻定向培育技术已在水产养殖业中得到广泛的关注和应用(黄翔鹄等, 2021)。黄翔鹄等(2005)研究了固定化微藻对改善凡纳滨对虾养殖水质和对虾抗病能力的影响,发现引入固定化波吉卵囊藻(Oocystis borgei)和微绿球藻(Nannochloris oculata)均能改善养殖水质,提高对虾抗病能力;波吉卵囊藻对氨氮的吸收能力较强,而微绿球藻对亚硝酸盐的吸收能力较强。Ramli等(2017)研究了固定化毛枝藻(Stigeoclonium nanum)对循环水养殖系统中氨氮和硝酸盐的去除能力,发现该藻对氨氮的去除能力强于对硝酸盐的去除能力。
用于调控养殖环境水质的微藻是否能适应本地的温度、光照、水环境等条件是选择定向培育微藻藻种的首要考虑因素,因此,从原位环境中分离筛选藻种,更有利于其在调控水质方面发挥正常生态功能(黄翔鹄等, 2021)。2019年,凡纳滨对虾工厂化养殖池中,一株硅藻在养殖中后期长期占优势,因其个体较小且细胞外壳覆盖一层硅质膜,难以用光学显微镜准确鉴定其分类地位,在以该藻为优势种的养殖池中,对虾生长状况良好(Qiao et al, 2020)。本研究以从凡纳滨对虾工厂化养殖水体中分离纯化出的一株微藻为研究对象,利用形态学结合分子生物学手段,对该藻进行鉴定,进一步研究该藻在不同浓度氨氮、硝态氮条件下的生长响应,以期为定向培育该藻并应用于对虾工厂化养殖水环境水质调控提供参考。
1 材料与方法 1.1 藻种分离与培养从山东省海阳黄海水产有限公司凡纳滨对虾工厂化养殖池(36°40′23″N,121°09′00″E)中取500 mL养殖水体,置于冰袋上带回实验室。将水样置于载玻片上,在显微镜下(Olympus CKX41, 日本)用毛细吸管吸取单细胞,于含有f/2培养基的48孔细胞培养板中,将该细胞培养板放入培养箱培养,经扩大培养后,利用平板涂布法通过添加抗生素去除培养液中的细菌,得到纯净的藻液。
分离纯化后的藻株编号为HY01,培养在250 mL三角瓶中,培养温度为28℃,培养光照度为2000 lx,光暗比12 h︰12 h。
1.2 分离藻株HY01的鉴定 1.2.1 光学显微镜鉴定取1 mL处于指数生长期的藻液在光学显微镜(Olympus CKX41, 日本)下观察其形态特征。
1.2.2 电子显微镜鉴定取50 mL处于指数生长期的藻液,4000 r/min离心10 min,弃上清液,保留约20 mL的浓缩藻液,加入等体积97%的浓硫酸,于60℃水浴加热20 min,冷却后加入同体积65%的浓硝酸,继续水浴加热,直至沉淀变为白色。将处理后的样品4000 r/min离心5 min,弃上清液,之后用蒸馏水清洗沉淀数次,直至中性。干燥后镀金,用扫描电子显微镜观察,拍照。
1.2.3 分子生物学鉴定取100 mL处于指数生长期的藻液,8000 r/min离心10 min,弃上清液。用DNA提取试剂盒(TIANGEN,北京)提取总DNA。以上述DNA为模板,用真核藻类18S rDNA通用引物Euk1A (5′-CTGGTTGATCCTGCCAG-3′)和Euk516r (5′-ACCAGACTTGCCCTC-3′)进行PCR扩增,反应产物用1%的琼脂糖凝胶电泳检测,将通过验证的阳性PCR产物送北京睿博兴科生物技术有限公司测序。将测序所得基因序列在NCBI上用BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi)进行同源分析,下载相关序列,应用MEGA 5.05软件,以邻接法(Neighbor- Joining method)构建系统发育树。
1.3 分离藻株HY01对不同氮源的响应实验开始前,将处于指数生长期的藻细胞分别接种至氨氮和硝态氮为氮源的培养液中驯化培养2~3个生长周期。实验所用氨氮氮源为NH4Cl,浓度梯度为50、100、200、400、600和882 μmol/L,硝态氮氮源为NaNO3,浓度梯度为100、200、400、600和882 μmol/L,每个浓度设置3个平行。将驯化培养后处于指数生长期的藻细胞接种至不同浓度的氨氮和硝态氮为氮源的培养液中,每天取样,测定藻细胞密度,共培养10 d。
1.3.1 藻细胞生长测定取指数生长期的藻细胞,在400~800 nm之间,以培养液为参比调零建立基线,对用培养液稀释的不同浓度藻液进行连续扫描,确定分光光度法的最佳测试波长为676 nm。将处于指数生长期的藻细胞用培养液按比例稀释成各种浓度,用光学显微镜确定藻数量,以培养液为参比,将相应浓度藻液用分光光度计测定最佳测试波长下的吸光度,确定藻细胞浓度与藻液吸光度之间的线性关系,绘制藻细胞密度和吸光度值间的标准曲线(瞿唯钢等, 2013)。藻细胞密度(y)与吸光值(x)之间线性关系式为y=2×109x–7×106 (R2=0.999 2)。由此可见,该藻的细胞密度与藻液吸光值之间呈线性相关,可通过测定藻液的吸光值反映该藻的生长情况。
每天定时取样,利用分光光度计在最佳测试波长下测定吸光度值,利用上述标准曲线计算藻细胞密度,绘制分离藻株HY01的生长曲线。
分离藻株HY01的平均比生长速率计算公式如下:
| $ μ = ({\rm{ln}}N_{t} – {\rm{ln}}N_{0})/(T_{t}–T_{0}) $ |
式中,Nt为收获时藻细胞密度,N0为起始藻细胞密度,Tt为藻细胞收获时间,T0为藻细胞起始培养时间。
1.3.2 藻细胞蛋白含量测定在培养第10天,收集藻细胞,放入超低温冰箱冷冻至结冰,然后用真空冷冻干燥机冻干,冻干藻粉用于蛋白含量测定。蛋白含量按考马斯亮蓝法测定(黄俊远, 2014)。
1.3.3 数据处理与统计分析每组实验设置3个平行,测得数据进行平均值与标准差分析,数据汇总后利用Origin 9.2对数据进行整理作图。采用SPSS 20软件进行单因素方差分析(one-way ANOVA)和独立样本T检验(independent-sample T test),显著性水平为P < 0.05。
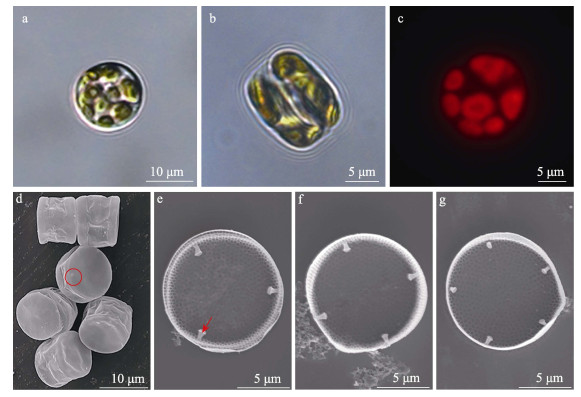
2 结果与分析 2.1 分离藻株HY01的鉴定分离藻株HY01的光镜和扫描电镜下的形态特征见图 1。分离藻株HY01细胞呈圆柱形,细胞直径为10.5~12.9 μm,平均直径约(11.4±1.0) μm。细胞外壳覆盖一层硅质膜(图 1d),难以用光学显微镜直接准确鉴定其分类地位。经酸处理后,在电子显微镜下观察发现,其壳面上有很多小孔,且壳中央的孔密度较壳边缘稀疏(图 1e~g),壳中央每10 μm内有20~26个小孔,壳边缘每10 μm内约有31~44个小孔(图 1e~g)。细胞壳面边缘处有眼斑(图 1d),在光学显微镜下不可见。细胞壳边缘处还有3~5个唇形突(图 1e~g)。根据该藻株HY01的形态学特征,判断其为微小辐环藻(Actinocyclus exiguus HY01)。
|
图 1 分离藻株HY01的形态特征 Fig.1 Morphological characterization of separated microalga HY01 a、b为光学显微镜观察;c为荧光显微镜观察;d~g为电子显微镜观察;d图中圆圈部分为眼斑;e图箭头指向唇形突。 a and b are pictures taken under light microscope; c is a picture taken under fluorescence microscope; d~g are pictures taken under scanning electron microscope; the circle part in figure d is pseudonodulus, the arrow points to labiate process in figure e. |
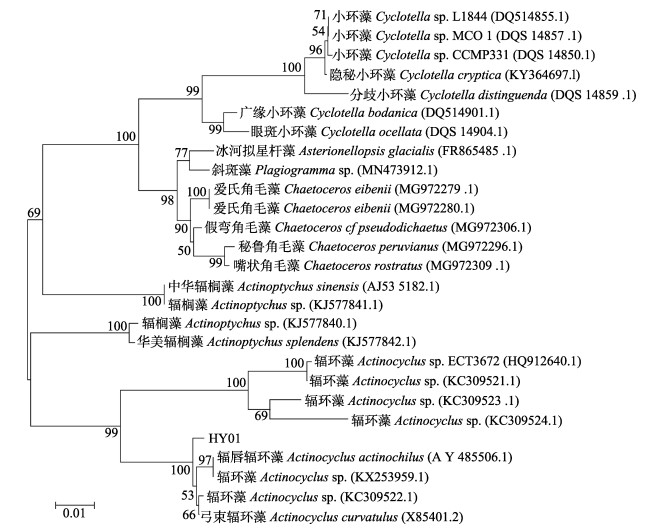
为进一步确定分离藻株HY01的分类地位,对分离藻株HY01进行18S rDNA扩增并测序,测序所得序列已提交到GenBank (https://www.ncbi.nlm.nih.gov/genbank/),序列号为MW915791.1。下载GenBank中与扩增序列相似性高的序列,利用MEGA5.05以邻接法构建系统发育树(图 2),结果显示该藻属于辐环藻属,与A. actinochilus (AY485506.1)、Actinocyclus sp. (KX253959.1、KC309522.1)和A. curvatulus (X85401.2)具有较高的相似性。
|
图 2 基于18S rRNA基因序列构建分离藻HY01与相似种的系统发育树 Fig.2 Phylogenetic tree based on 18S rDNA gene sequence between separated microalga HY01 and its similar species using Neighbor-Joining method |
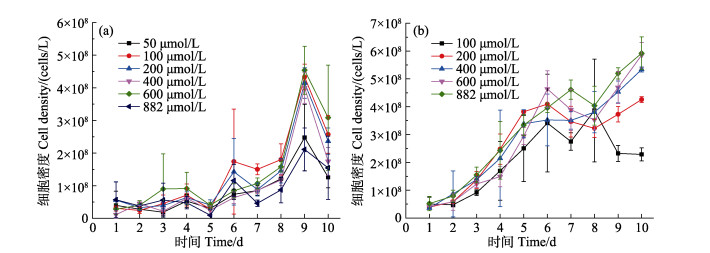
微小辐环藻HY01在不同浓度氨氮和硝态氮为氮源的培养条件下的生长曲线见图 3。如图 3所示,以氨氮为氮源培养微小辐环藻HY01,6种氨氮浓度下的细胞密度均在第9天达到峰值,其中,氨氮浓度为600 μmol/L时,藻细胞密度最高,达到4.54×108个/L,其次为100 μmol/L (4.33×108个/L);在氨氮浓度为882 μmol/L的培养液中,微小辐环藻HY01的细胞密度最低(2.11×108个/L) (图 3a)。以硝态氮为氮源培养微小辐环藻HY01,硝态氮浓度为100 μmol/L培养液中,藻细胞密度在培养第8天达到峰值,为3.86×108个/L;硝态氮浓度为200~882 μmol/L时,随着培养天数增加,藻细胞密度增加,第10天硝态氮浓度为882 μmol/L的培养液中藻细胞密度最高(5.92×108个/L),其次为600 μmol/L (5.85×108个/L) (图 3b)。
|
图 3 微小辐环藻HY01在不同浓度氨氮(a)和硝态氮(b)培养条件下的生长曲线 Fig.3 Growth curves of A. exiguus HY01 under different ammonia (a) and nitrate (b) concentrations |
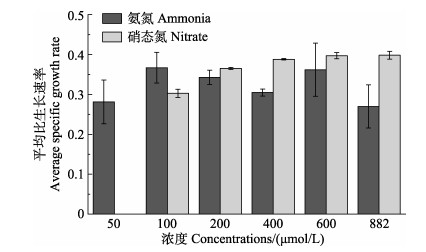
当氮源为氨氮时,微小辐环藻HY01的平均比生长速率在氨氮浓度为100和600 μmol/L时最高,分别为0.37±0.04和0.36±0.07,氨氮浓度为882 μmol/L时,平均比生长速率最低,为0.27±0.05;当氮源为硝态氮时,微小辐环藻HY01的平均比生长速率随着硝态氮浓度的升高呈显著升高趋势(P < 0.05),硝态氮浓度为600和882 μmol/L时,平均比生长速率最高,均为0.40±0.01 (图 4)。以氨氮为氮源时,微小辐环藻HY01的最高比生长速率显著低于以硝态氮为氮源时(P < 0.05)。
|
图 4 微小辐环藻HY01在不同浓度氨氮和硝态氮条件下的平均比生长速率 Fig.4 Average specific growth rate of A. exiguus HY01 under different ammonia and nitrate concentrations |
微小辐环藻HY01在不同浓度氨氮和硝态氮条件下的蛋白含量如图 5所示。当氮源为氨氮时,微小辐环藻HY01的蛋白含量在氨氮浓度为600 μmol/L时最高,为4.45 mg/g,其次是100 μmol/L时,为3.28 mg/g,氨氮浓度为50 μmol/L时,藻内蛋白含量最低,为1.38 mg/g;当氮源为硝态氮时,微小辐环藻HY01蛋白含量随着氮浓度的增加显著升高(P < 0.05),即硝态氮浓度为882 μmol/L时藻内蛋白含量最高,为11.97 mg/g,硝态氮浓度为100 μmol/L时最低,为4.55 mg/g (图 5)。以氨氮为氮源时,微小辐环藻HY01的蛋白含量显著低于以硝态氮为氮源时的蛋白含量(P < 0.05)。
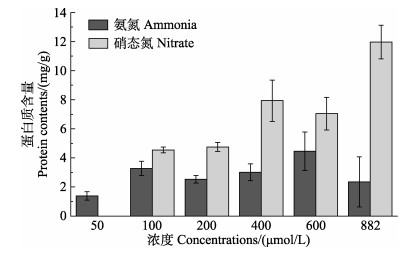
|
图 5 微小辐环藻HY01在不同浓度氨氮和硝态氮条件下的蛋白含量 Fig.5 Protein contents of A. exiguus HY01 under different ammonia and nitrate concentrations |
根据光镜和扫描电镜下的形态学特征,以及基于18S rDNA测序的分子生物学方法,判断该藻为微小辐环藻。微小辐环藻属于硅藻门(Bacillariophyta)、圆筛藻纲(Coscinodiscophyceae)、圆筛藻目(Coscinodiscales)、半盘藻科(Hemidiscaceae)、辐环藻属(Actinocyclus),该藻与辐环藻属其他种的主要区别在于细胞直径和壳面孔密度。目前已报道的辐环藻细胞直径和壳面孔密度见表 1 (Fryxell et al, 1981; 郭晓慧等, 2018)。调查发现,目前微小辐环藻是辐环藻属中个体最小的物种,其细胞直径为6.4~13 μm (Fryxell et al, 1981; 郭晓慧等, 2018),本研究分离出的微小辐环藻HY01细胞直径约为(11.4±1.0) μm;在壳面孔密度方面,微小辐环藻HY01壳面每10 μm孔的个数较其他辐环藻个数多,且边缘部分比中央部分明显密集。本研究中,微小辐环藻HY01壳面边缘唇形突数量为3~5个(图 1e~g),高于海南省海口市红城湖分离出的微小辐环藻唇形突数(2~3个) (郭晓慧等, 2018),而南大西洋样品中微小辐环藻有2~5个唇形突(Fryxell et al, 1981)。综上所述,本研究从凡纳滨对虾工厂化养殖水体中分离出的一株硅藻为微小辐环藻HY01,该藻细胞个体较小,是目前已知辐环藻属物种中最小的,用光学显微镜难以辨别;该藻壳面上有很多小孔,与其他辐环藻不同的是,微小辐环藻壳面孔纹排列相对紧密,且壳中央的孔密度较壳边缘稀疏,壳边缘具有眼斑结构,有3~5个唇形突。
|
|
表 1 辐环藻的细胞直径和壳面孔密度 Tab.1 Diameters and numbers of areolae per 10 μm on mantle of Actinocyclus |
本研究中微小辐环藻HY01分离自凡纳滨对虾工厂化养殖池,该藻在养殖中后期(4~5月)均为优势种,最高细胞密度达到2.77×107个/L,占了总细胞密度的75% (Qiao et al, 2020)。由辐环藻引发的赤潮报道较少,目前仅有2次,一次是在大西洋(48°49′51.6″N,16°30′36″W),细胞密度为4×105个/L,赤潮持续了5 d (Smythe-Wright et al, 2010);另一次发生在海南省海口市红城湖,持续时间为6 d,期间微小辐环藻的细胞密度保持在107~108个/L,致使红城湖40余吨花鰶(Clupanodon thrissa)死亡(郭晓慧等, 2018)。该藻没有毒性,导致鱼类死亡的原因可能与水体盐度剧烈变化造成应激反应有关(郭晓慧等, 2018)。
赤潮的暴发往往与水体的环境因素有关(张善发等, 2020)。温度是一切酶促反应的限制性因子,水温的变化直接影响浮游植物的生长代谢(窦勇等, 2020)。营养盐是浮游植物生长必需的营养元素,大部分赤潮的暴发都与水域的富营养化有关(徐宁等, 2005; Glibert et al, 2008; 张善发等, 2020)。海南红城湖微小辐环藻赤潮暴发期间,水体温度较高,平均约为31℃;水体总无机氮浓度为3.12 mg/L,其中,氨氮是总无机氮的主要组成部分,平均贡献率为84.8%;活性磷酸盐浓度为0.11 mg/L,氮磷比范围为13.1~66.8,平均值为31.4;水体中总无机氮和活性磷酸盐均超过四类水质,为高度富营养化(郭晓慧等, 2018)。因此,红城湖微小辐环藻赤潮的暴发可能与水体近年逐渐富营养化以及较高的水温有关(郭晓慧等, 2018)。凡纳滨对虾工厂化养殖池中,微小辐环藻HY01为优势种期间,养殖水体温度平均为28℃;水体总无机氮平均浓度为5.07 mg/L,其中,氨氮平均浓度为1.53 mg/L,分别占总无机氮的30%;活性磷酸盐的平均浓度为0.06 mg/L,氮磷比范围为27.6~38.6,平均值为32% (Qiao et al, 2020)。因此,微小辐环藻HY01可能更喜欢在温度较高、营养物质丰富的水域生长。
氮作为浮游植物生长代谢过程中不可缺少的元素之一,是参与细胞内核酸、蛋白质及叶绿素构成的主要成分(Robertson et al, 2009)。氨氮和硝态氮是养殖水体中无机氮的2种主要形态(Chen et al, 2018; Qiao et al, 2020),可被微藻直接吸收利用。氮营养盐的添加在一定程度上能够促进微藻的生长,但并非氮营养盐浓度越高微藻生物量就高,过高的氨氮不利于藻类生长,甚至影响其他氮源的吸收和转化(Collos et al, 2014; Gutierrez et al, 2016)。本研究分别以不同浓度氨氮和硝态氮为氮源培养微小辐环藻HY01,发现该藻均能利用氨氮和硝态氮进行生长,最适宜生长的氨氮浓度为600 μmol/L,最适宜生长的硝态氮浓度为882 μmol/L,但以氨氮为氮源时微小辐环藻HY01的最大细胞密度、最高比生长速率以及蛋白含量均低于以硝态氮为氮源(图 3~图 5)。通常认为浮游植物会优先利用氨氮,因为氨氮可以直接被利用,而硝态氮需要先被还原才能被利用(Dortch, 1990; Berges et al, 2008; Mulholland et al, 2008)。但不同藻类对不同形态氮的选择性不同,研究发现,硅藻更偏好硝态氮丰富的水域环境,而甲藻更倾向于高氨氮或尿素的水环境(Lomas et al, 1999; 欧美姗等, 2006; Mulholland et al, 2008)。因此,本研究分离出的微小辐环藻HY01可能更喜欢利用硝态氮,但对较高浓度的氨氮有一定的耐受性,这可能是微小辐环藻HY01在养殖中、后期长时间占优势的原因之一。
4 结论根据光镜和扫描电镜下的形态学特征,结合分子生物学技术,鉴定该分离藻株为微小辐环藻HY01。藻细胞个体较小,大小约为(11.4±1.0) μm,壳面上有很多小孔,光学显微镜下不可见,且壳中央的孔密度较壳边缘稀疏,壳边缘具有眼斑结构,有3~5个唇形突。以不同浓度氨氮和硝态氮为氮源培养微小辐环藻HY01,结果表明,微小辐环藻HY01可能更喜欢利用硝态氮,但对较高浓度的氨氮有一定的耐受性。
BERGES J A, MULHOLLAND M R. Enzymes and nitrogen cycling. Nitrogen in the Marine Environment (Second Edition), 2008, 1385-1444 |
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2020. Beijing: China Agriculture Press, 2020 [农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2020中国渔业统计年鉴. 北京: 中国农业出版社, 2020]
|
CHANG M Y, LI C L, DONG J, et al. Dynamic changes of phytoplankton composition during cyanobacteria blooms in aquaculture ponds. Progress in Fishery Sciences, 2019, 40(1): 36-45 [常孟阳, 李晨露, 董静, 等. 蓝藻水华暴发期间养殖池塘浮游藻类动态变化. 渔业科学进展, 2019, 40(1): 36-45 DOI:10.19663/j.issn2095-9869.20171227002] |
CHEN Z, GE H, CHANG Z, et al. Nitrogen budget in recirculating aquaculture and water exchange systems for culturing Litopenaeus vannamei. Journal of Ocean University of China, 2018, 17(4): 905-912 DOI:10.1007/s11802-018-3584-9 |
COLLOS Y, HARRISON P J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Marine Pollution Bulletin, 2014, 80(1/2): 8-23 |
DORTCH Q. The interaction between ammonium and nitrate uptake in phytoplankton. Marine Ecology Progress Series, 1990, 61: 183-201 DOI:10.3354/meps061183 |
DOU Y, SHANG J P, SHAO P, et al. Frequency of red tides in Bohai Sea and the influence of environmental factors (2000– 2016). Journal of Hydroecology, 2020, 41(6): 141-148 [窦勇, 尚金沛, 邵蓬, 等. 2000–2016年渤海赤潮发生规律及影响因素研究. 水生态学杂志, 2020, 41(6): 141-148] |
FRYXELL G A, SEMINA H J. Actinocyclus exiguus sp. nov. from the southern parts of the Indian and Atlantic Oceans. British Phycological Journal, 1981, 16(4): 441-448 |
GLIBERT P M, BURKHOLDER J, GRANELI E, et al. Advances and insights in the complex relationships between eutrophication and HABs: Preface to the special issue. Harmful Algae, 2008, 8(1): 1-2 DOI:10.1016/j.hal.2008.08.020 |
GUO X, TU Z G, CHEN D D, et al. Actinocyclus exiguus, a newly discovered diatom bloom-causative species in China. Journal of Tropical Oceanography, 2018, 37(4): 24-28 [郭晓慧, 涂志刚, 陈丹丹, 等. 微小辐环藻, 我国硅藻藻华的新记录种. 热带海洋学报, 2018, 37(4): 24-28] |
GUTIERREZ J, KWAN T A, ZIMMERMAN J B, et al. Ammonia inhibition in oleaginous microalgae. Algal Research, 2016, 19: 123-127 DOI:10.1016/j.algal.2016.07.016 |
HUANG J Y. Study of lipid, protein and polysaccharide extraction from Chlorella. Master´s Thesis of Beijing University of Chemical Technology, 2014 [黄俊远. 从小球藻中提取油脂、蛋白质和多糖的研究. 北京化工大学硕士研究生学位论文, 2014]
|
HUANG X H, LI C L, ZHENG L, et al. Studies on immobilized microalgae improving culture water quality and strengthening anti-disease ability of prawn. Marine Science Bulletin, 2005, 24(2): 57-62 [黄翔鹄, 李长玲, 郑莲, 等. 固定化微藻对改善养殖水质和增强对虾抗病力的研究. 海洋通报, 2005, 24(2): 57-62 DOI:10.3969/j.issn.1001-6392.2005.02.009] |
HUANG X H, LI F. Directional cultivation of microalgae in prawn pond. Journal of Biology, 2021, 38(2): 18-21 [黄翔鹄, 李峰. 虾池微藻的定向培育进展. 生物学杂志, 2021, 38(2): 18-21] |
LIU R Q, HE M L, WANG C H. Progress in research on application of microalgae bait in echinoderm culture. Progress in Fishery Sciences, 2021, 42(3): 17-25 [刘瑞卿, 何梅琳, 王长海. 微藻饵料在经济棘皮动物养殖中的应用及研究进展. 渔业科学进展, 2021, 42(3): 17-25] |
LOMAS M W, GLIBERT P M. Temperature regulation of nitrate uptake: A novel hypothesis about nitrate uptake and reduction in cool-water diatoms. Limnology and Oceanography, 1999, 44(3): 556-572 DOI:10.4319/lo.1999.44.3.0556 |
MULHOLLAND M R, LOMAS M W. Nitrogen uptake and assimilation. Nitrogen in the Marine Environment (2nd Edition), 2008, 303-384 |
OU M S, LÜ S H. Effects of different inorganic nitrogen sources on the growth of Prorocentrum donghaiense. Ecologic Science, 2006, 25(1): 28-31 [欧美珊, 吕颂辉. 不同无机氮源对东海原甲藻生长的影响. 生态科学, 2006, 25(1): 28-31] |
QIAO L, CHANG Z Q, LI J, et al. Phytoplankton community succession in relation to water quality changes in the indoor industrial aquaculture system for Litopenaeus vannamei. Aquaculture, 2020, 527: 735441 DOI:10.1016/j.aquaculture.2020.735441 |
QU W G, WEI F L, LIU M F, et al. Comparative study on algae cells counting methods of alga growth inhibition test. Agrochemicals, 2013(7): 497-502 [瞿唯钢, 魏方林, 刘梅凤, 等. 藻类生长抑制试验中藻细胞计数方法比较. 农药, 2013(7): 497-502] |
RAMLI N M, VERDEGEM M C J, YUSOFF F M, et al. Removal of ammonium and nitrate in recirculating aquaculture systems by the epiphyte Stigeoclonium nanum immobilized in alginate beads. Aquaculture Environment Interactions, 2017, 9: 213-222 |
ROBERTSON G P, VITOUSEK P M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annual Review of Environment and Resources, 2009, 34(1): 97-125 |
SMYTHE-WRIGHT D, BOSWELL S, KIM Y N, et al. Spatio- temporal changes in the distribution of phytopigments and phytoplanktonic groups at the Porcupine Abyssal Plain (PAP) site. Deep Sea Research Part Ⅱ Topical Studies in Oceanography, 2010, 57(15): 1324-1335 |
TSENG I T, CHEN J C. The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus under nitrite stress. Fish and Shellfish Immunology, 2004, 17(4): 325-333 |
XU N, DUAN S S, LI A F, et al. The relationships between the occurrences of red tides (HABs) and eutrophication in coastal waters. Acta Ecologica Sinica, 2005, 25(7): 1782-1787 [徐宁, 段舜山, 李爱芬, 等. 沿岸海域富营养化与赤潮发生的关系. 生态学报, 2005, 25(7): 1782-1787] |
ZANG W L, YANG M, DAI X L, et al. Experimental research on change of water quality and nitrogen budgets for indoor closed culture ponds of Litopenaeus vannamei. Journal of Agro-Environment Science, 2009, 28(5): 1019-1024 [臧维玲, 杨明, 戴习林, 等. 凡纳滨对虾室内封闭式养殖水质变化与氮收支的试验研究. 农业环境科学学报, 2009, 28(5): 1019-1024] |
ZHANG S F, WANG Q, GUAN C Y, et al. Study on the occurrence law of red tide and its influencing factors in the offshore waters of China from 2001 to 2017. Acta Scientiarum Naturalium Universitatis Pekinensis, 2020, 56(6): 1129-1140 [张善发, 王茜, 关淳雅, 等. 2001—2017年中国近海水域赤潮发生规律及其影响因素. 北京大学学报(自然科学版), 2020, 56(6): 1129-1140] |



