2. 上海海洋大学水产与生命学院 上海 201306;
3. 青岛海洋科学与技术试点国家实验室 山东 青岛 266071;
4. 莱州明波水产有限公司 山东 莱州 261400
2. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306, China;
3. Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, Shandong 266071, China;
4. Laizhou Mingbo Aquatic Co. Ltd., Laizhou, Shandong 261400, China
溶解氧是影响鱼类等水生生物生长和存活的重要水环境因子之一,物理、化学或生物等因素的季节性或突发性改变,均可引起水体中溶解氧降低甚至缺氧,导致鱼类产生强烈的应激反应(Abdel-Tawwab et al, 2019; 吴碧银等, 2021)。为适应水环境溶解氧变化,鱼类在进化和繁衍过程中形成了不同应答模式和适应性调控机制,并通过一系列行为、生理和组织结构变化,如改变呼吸模式、增强血氧亲和力等,获取充足的氧气或者限制组织对氧气的需求,降低能量消耗,从而维持正常生理功能(张安杰, 2014; 钱辰颖, 2020; 王盼等, 2021)。临界氧分压(critical oxygen tension, Pcrit)和失去平衡点(loss of equilibrium, LOE)是被广泛应用于评价鱼类低氧耐受能力的有效指标(宋银都等, 2019)。Pcrit和LOE越低,则表明鱼类低氧耐受能力越强,反之则越弱。明确鱼类Pcrit和LOE,可为人工养殖条件下的水体溶解氧调控提供科学依据,从而减少损失。研究发现,低氧环境可导致鱼类的正常呼吸和生理代谢发生紊乱,从而影响鱼类的行为和生理生化等指标(Buentello et al, 2000; Thurston et al, 2011)。熊向英等(2016)研究发现,水中溶解氧含量降低,鲻鱼(Mugil cephalus)幼鱼生长缓慢,行动能力下降。低氧环境下鲤鱼(Cyprinus carpio)则可通过增加呼吸频率,获取更多氧气供应,适应低氧环境(Glass et al, 1990)。目前,国内外对鱼类低氧研究主要集中在不同种属鱼类的低氧耐受,而对不同规格的同一种属鱼类低氧耐受性报道不多。麻艳群等(2021)研究发现,体质量对斑点叉尾

斑石鲷(Oplegnathus punctatus)俗称“黑金鼓”、“花金鼓”,在中国东南沿海、朝鲜半岛和日本等海域均有分布,其肉质鲜美、营养丰富,深受广大消费者的喜爱和青睐。我国北方冬季严寒,斑石鲷不能在外海越冬,目前主要采用“陆海接力养殖”模式,即在工厂化车间完成大规格苗种培育工作,待到海水温度升高至适宜生长水温,将斑石鲷转运至大型工程化围栏进行接力养殖。研究表明,大型工程化围栏远离岸线,圈围水体面积大,水流交换充分,自然禀赋优良,斑石鲷生长速度快,同时经过自然环境下野化驯养,其生理代谢功能得到显著提升(Jia et al, 2021a)。围栏圈养水体溶解氧是影响斑石鲷养殖生产的重要环境因子之一,其含量伴随着水体温度变化产生剧烈波动,呈现显著的季节性变化,水温越高,水分子之间的间隙越小,水的溶解氧能力就越差(饶胡敏等, 2017)。此外,水体中营养盐含量(薛彬等, 2016)、自然气候改变(胡蓉等, 2014)、藻类和光合细菌的分布也显著影响水体中的溶解氧浓度。目前,国内外对斑石鲷的研究主要集中在饲料营养(Wang et al, 2021)、循环水养殖(张皓天等, 2020; 宋协法等, 2017)、运输应激(张宇雷等, 2017)、疾病诊断监测(Huang et al, 2021)和免疫调控(Liu et al, 2019)等方面。溶解氧作为重要环境因子,显著影响养殖鱼类的生长存活,而目前对斑石鲷低氧耐受的研究尚未有系统报道,同时,在低氧胁迫过程中相应生理生化反应尚不明确,基于此,本研究通过对比分析2种规格斑石鲷的Pcrit和LOE,观察其呼吸行为的变化,分析低氧胁迫和恢复溶解氧过程中的血液生理生化指标[皮质醇、葡萄糖含量、血红蛋白(Hb)浓度、红细胞(red blood cell, RBC)和白细胞(white blood cell, WBC)数,红细胞积压(hematocrit, HCT)]的变化,阐明斑石鲷低氧耐受能力和生理反应,相关结果可为斑石鲷陆海接力养殖提供基础性数据。
1 材料与方法 1.1 实验材料实验在山东莱州明波水产有限公司开展,实验材料均由该公司培育,斑石鲷体格规整、活力健康,平均体长分别为(9.80±1.10)和(20.00±4.20) cm,平均体质量分别为(50.10±0.70)和(200.30±4.20) g。实验开始前,将2种规格的斑石鲷置于12个实验桶(60 L)中暂养2周,每个桶放置15尾实验鱼,每天08:00和16:00各投喂1次(海童,中国潍坊三通生物工程有限公司),每次投喂量为鱼体体重的3%。保持水体中溶解氧浓度高于7.00 mg/L,水温为(23.0±0.5)℃,氨氮浓度 < 0.1 mg/L,亚硝酸盐浓度为0~0.05 mg/L,正常光照。
1.2 实验设计 1.2.1 低氧耐受能力实验实验参考封闭静水式方法(张安杰, 2014)并做改进。取6个实验桶,用塑料布将实验桶密封,实验开始时关闭循环水系统,做隔绝空气处理。通过溶氧仪(AZ86031型,中国台湾鑫恒)自动监测和记录水体中溶解氧浓度变化,根据体重和溶解氧变化计算实验鱼代谢率MO2 [mg/(kg·h)],公式如下:
| $ {\text{M}}{{\text{O}}_{\text{2}}} = ({[{O_{\text{2}}}]_{{t_1}}} - {[{O_{\text{2}}}]_{{t_2}}}) \times \frac{V}{t} \times \frac{1}{{{\text{BW}}}} $ |
式中,[O2]t1和[O2]t2 (mg/L)分别为监测时间点t1和t2时的溶解氧浓度;V为实验桶的总体积减去鱼的体积;t(h)为时间点t1和t2间的间隔;BW(kg)为实验鱼的体质量。
Pcrit值表示随溶氧水平下降,鱼类不能维持其代谢率时的水体溶氧水平,该值与鱼类低氧耐受能力呈反比,即Pcrit值越大,说明鱼类耐低氧能力越弱,反之则越强。将代谢率和溶氧水平作图,采用双线法求出MO2随溶解氧下降而出现显著下降拐点,即得到Pcrit值。
在监测记录水体溶解氧溶度变化的同时,参照Wu等(2016)的方法,观察实验鱼游泳和呼吸行为,随机挑选3尾实验鱼,记录其每分钟鳃盖闭合次数,统计呼吸频率(次/min),50 g斑石鲷每隔1 h统计1次,200 g斑石鲷每隔10 min统计1次。当实验鱼失去平衡时(LOE;侧翻,腹部朝上),记录此时溶解氧溶度并统计呼吸频率。
1.2.2 低氧胁迫和恢复实验明确了2种规格斑石鲷的Pcrit值和LOE值之后,每个规格斑石鲷分别设置1个对照组和3个处理组,对照组和处理组均为3个平行。实验开始时,关闭处理组循环水系统,用塑料布将实验桶密封,至水中溶解氧浓度到达Pcrit值和出现LOE现象[鱼体失去平衡、侧翻;50 g斑石鲷溶解氧为(0.93±0.11) mg/L,200 g斑石鲷溶解氧为(1.16±0.08) mg/L]时取样,每桶随机取3尾鱼。取完LOE点样品,立即打开循环水系统,去掉塑料布进行恢复溶解氧处理(20 min后溶解氧恢复至正常水平;7.00 mg/L),并在恢复24 h (R 24)后取样。实验期间,观察2种规格斑石鲷在低氧胁迫和恢复溶解氧过程中游泳和呼吸行为的变化,统计斑石鲷的呼吸频率,呼吸次数以鳃盖一张一合记为1次。
1.3 血液样本采集与处理从每个平行取3尾鱼(共9尾),迅速捞出置于MS-222海水溶液(200 mg/L)中。深度麻醉后,使用1 mL注射器从尾静脉取血,分为2份。1份直接用于检测血液红细胞(RBC)数、白细胞(WBC)数、血红蛋白(Hb)含量和红细胞积压(HCT);1份放于含有EDTAK2抗凝剂的抗凝管中,4℃静置1.5 h,4000 r/min条件下离心10 min制备血浆,存于–80℃冰箱中,检测葡萄糖和皮质醇含量。
1.4 血液样本分析采用迈瑞全自动血液细胞分析仪(BC-2800 vet)检测血液红细胞数、白细胞数、血红蛋白含量、平均红细胞积压。采用南京建成公司试剂盒检测葡萄糖含量,所用波长为563 nm。采用放射性免疫法检测血清中皮质醇含量,所用仪器为放射免疫ϒ计数器,详细步骤参见说明书。
1.5 数据分析实验数据用平均值±标准差(Mean±SD)表示,采用SPSS 16.0软件进行单因素方差分析(one-way ANOVA),并采用Tukey´s多重比较分析2种规格斑石鲷重要指标的差异,显著性差异用P < 0.05表示。
2 结果 2.1 斑石鲷低氧耐受能力如图 1所示,随着水体溶解氧浓度下降,2种规格斑石鲷的呼吸频率呈逐渐上升然后下降的变化趋势,50 g和200 g斑石鲷分别在溶解氧浓度下降至1.27 mg/L (420 min)和1.10 mg/L (90 min)时,呼吸频率达到最高(P < 0.05)。随后,随着溶解氧浓度的进一步降低,50 g和200 g斑石鲷分别在实验后480 min (1.16 mg/L)和110 min (0.93 mg/L)出现LOE现象(腹部向上)。

|
图 1 斑石鲷呼吸频率和溶解氧浓度 Fig.1 Dissolved oxygen consumption and respiratory rate of spotted knifejaw 不同字母表示显著性差异(P < 0.05)。下同。 The significant differences are shown in different letters (P < 0.05). The same as below. |
根据“双线法”作图统计发现,2种规格斑石鲷的Pcrit处水体溶解氧浓度分别为(3.15±0.12)和(4.05± 0.09) mg/L (图 2)。当水体溶解氧浓度降至2种规格斑石鲷的Pcrit值以下时,斑石鲷机体出现胁迫反应。
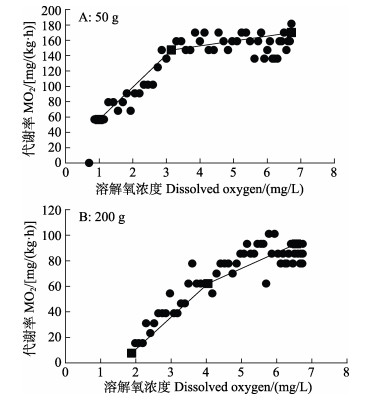
|
图 2 斑石鲷临界氧分压 Fig.2 Critical oxygen tension of spotted knifejaw |
如图 3所示,在低氧胁迫和恢复溶解氧过程中,2种规格斑石鲷的呼吸频率均呈先升高后降低的趋势,且50 g斑石鲷在Pcrit处的呼吸频率显著高于200 g斑石鲷(P < 0.05),LOE点处2种规格斑石鲷的呼吸频率无显著差异(P > 0.05),恢复正常溶解氧24 h后,2种规格斑石鲷的呼吸频率均逐渐恢复,与对照组相比无显著差异(P > 0.05)。
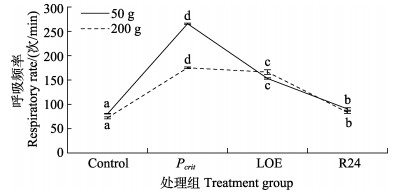
|
图 3 低氧胁迫和恢复溶氧过程中斑石鲷的呼吸频率 Fig.3 Respiratory rate of spotted knifejaw during hypoxia and re-oxygenation |
在低氧和恢复正常溶解氧过程中,2种规格斑石鲷的血浆皮质醇和葡萄糖浓度变化情况如图 4所示,其血浆葡萄糖(图 4A)和皮质醇浓度(图 4B)均随养殖环境溶解氧浓度的降低而升高,50 g斑石鲷的血浆葡萄糖和皮质醇浓度在Pcrit值和LOE值处均较对照组有显著差异,且都在LOE值处达到最高值(P < 0.05);200 g斑石鲷的血浆皮质醇浓度变化趋势与50 g斑石鲷相同。在恢复溶解氧阶段,血浆葡萄糖和皮质醇浓度逐渐降低,至恢复正常溶解氧24 h时,与对照组差异不显著(P > 0.05)。

|
图 4 低氧胁迫和恢复溶氧过程中斑石鲷的 Fig.4 Changes of plasma glucose and cortisol content in spotted knifejaw during hypoxia and re-oxygenation 血浆葡萄糖和皮质醇浓度 |
如表 1所示,在Pcrit值和LOE值处,50 g斑石鲷的白细胞数、红细胞积压、血液红细胞数、血红蛋白浓度均显著高于对照组(P < 0.05),且在Pcrit值处达到最高值;而200 g斑石鲷的这4个指标均较对照组显著升高(P < 0.05),其中,白细胞数、红细胞积压和血红蛋白浓度在LOE值时达到最大值,血液红细胞数在Pcrit值时达到最大值。恢复正常溶氧24 h后,2种规格斑石鲷的各项指标均与对照组无显著差异。
|
|
表 1 低氧和复氧过程中2种规格斑石鲷的血液生理指标变化 Tab.1 Changes of blood physiological indexes of spotted knifejaw during hypoxia and re-oxygenation |
鱼类低氧耐受能力决定了其对低氧环境的适应性,Pcrit和LOE值是反映低氧耐受能力的重要指标,Pcrit值越低,表明鱼体在低溶氧水平下维持稳定代谢的能力越强(Ultsch et al, 2019)。鱼类Pcrit值存在种属间差异,同时受体质量的影响。Candebat等(2020)研究发现,当水中溶解氧浓度降至(2.92±1.48) mg/L时,大规格黄条
环境胁迫可导致鱼类血浆皮质醇浓度升高,其可促进能量的重新分配,以确保鱼类维持机体平衡(Flik et al, 2006),低氧胁迫可以显著地影响血浆皮质醇浓度。王燚纬(2019)研究发现,随着低氧持续时间的增加,团头鲂血浆皮质醇浓度呈上升趋势,恢复正常溶解氧24 h后,血浆皮质醇浓度显著降低。本研究中,2种规格斑石鲷血浆皮质醇浓度在低氧和恢复溶解氧过程中呈先上升后下降的变化趋势,在Pcrit和LOE值处的血浆皮质醇浓度较对照组均升高,至LOE值处达到最高,说明斑石鲷的血浆皮质醇浓度显著受低氧影响(区又君等, 2014)。除皮质醇外,血浆血糖作为鱼体重要能量来源同样在低氧过程中发挥重要作用(Li et al, 2018)。研究表明,在鱼类正常代谢过程中,葡萄糖一部分进入肝脏中转化为糖原储存,一部分用于合成脂肪和氨基酸等,还有一部分进入血液成为血糖,为机体维持正常生理代谢供能(刘康, 2017)。当鱼类经历低氧等外界环境突变时,会代偿性升高血液葡萄糖浓度,应对环境刺激诱导的能量供应,同时葡萄糖浓度升高变化幅度同鱼类规格密切相关。黄建盛等(2019)和王维政等(2021)分别研究发现,体质量为(240.78±30.24)和(50.44±2.78) g的军曹鱼(Rachycentron canadum)低氧应激后血浆皮质醇浓度显著升高至20和10 mmol/L,这表明规格越大对低氧环境反应越敏感。本研究中,低氧条件下2种规格斑石鲷的血浆葡萄糖浓度均增加,但小规格斑石鲷的血浆葡萄糖浓度较大规格斑石雕上升幅度小,表现出更强的低氧耐受性。恢复正常溶解氧24 h后,2种规格斑石鲷的血浆葡萄糖浓度均回落至正常值,说明恢复正常溶解氧24 h可显著缓解低氧胁迫对斑石鲷的影响(郭志雄, 2020)。
RBC是鱼类血液重要组成成分,其主要生理功能是携带和运输氧气,Hb同样参与氧气运输,低氧条件可导致鱼类RBC和Hb显著增高(王晓雯等, 2016)。研究发现,急性低氧条件下鲫鱼(Carassius auratus)幼鱼RBC和Hb显著升高,表明其可通过增加RBC和Hb增强自身血液载氧能力,从而适应低氧胁迫(张曦, 2011)。本研究中,2种规格斑石鲷的RBC和Hb变化趋势相同,均在Pcrit和LOE值处升高,恢复24 h后回落至正常值。说明斑石鲷血液指标随水中溶解氧浓度的改变而变化。王志飞等(2019)研究同样发现,秀丽高原鳅(Triplophysa venusta)在低氧环境中血液RBC和Hb含量显著上调。此外,李欣茹(2018)研究发现,低氧胁迫环境可导致暗纹东方鲀血液HCT显著上升,在恢复正常溶解氧过程中该值回落,说明低氧条件下暗纹东方鲀可调节HCT,增加自身对低氧的适应性。本研究中,2种规格斑石鲷的血液HCT随水中溶解氧含量的下降显著上升,在恢复正常溶解氧24 h后恢复至正常值,表明低氧条件下,斑石鲷同样可以通过增加HCT降低自身对氧气的需求,从而增加自身低氧环境适应性。鱼体WBC主要起免疫和防御作用,保护鱼体抵抗侵害。沈凡等(2010)研究表明,黄颡鱼(Pelteobagrus fulvidraco)血液WBC在低氧环境下显著增加,鱼体应对低氧环境开启免疫和防御屏障。本研究中,2种规格斑石鲷的血液WBC随水中溶解氧浓度的下降均呈升高趋势,分别在Pcrit和LOE值处达到最高值,至恢复正常溶氧后WBC减少,说明斑石鲷在低氧环境下,提高机体免疫功能,应对低氧胁迫可能带来的损伤(常志成等, 2018)。此外,50 g斑石鲷的血液生理指标均在Pcrit值处达到最大值,而200 g斑石鲷的血液WBC、Hb和HCT都在LOE值处达到最大值,表明不同规格斑石鲷在应对低氧应激时,对低氧耐受能力不同,导致其启动生理反应的进程存在差异(丁彦文等, 2000; 孙耀等, 2001)。
综上,本研究发现,2种规格斑石鲷的低氧耐受能力存在显著差异,小规格斑石鲷对低氧环境具有更强的适应性,在低氧应激胁迫过程中可通过增加呼吸频率、提高血液RBC和Hb含量增加机体对O2的摄入,增加皮质醇和葡萄糖含量为机体提供能量,应对低氧应激诱导的生理变化。恢复正常溶解氧24 h后,2种规格斑石鲷逐渐恢复到正常状态。相关结果可以为斑石鲷陆海接力高效养殖提供理论依据,减少因环境溶解氧改变带来的经济损失。
ABDEL-TAWWAB M, MONIER M N, HOSEINIFAR S H, et al. Fish response to hypoxia stress: Growth, physiological, and immunological biomarkers. Fish Physiology and Biochemistry, 2019, 45(3): 997-1013 DOI:10.1007/s10695-019-00614-9 |
BUENTELLO J A, GATLIN Ⅲ D M, NEILL W H. Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture, 2000, 182(3/4): 339-352 |
CANDEBAT C L, BOOTH M, WILLIAMSON M J E, et al. The critical oxygen threshold of yellow tail kingfish (Seriola lalandi). Aquaculture, 2020, 516: 734519 DOI:10.1016/j.aquaculture.2019.734519 |
CHANG Z C, WEN H S, ZHANG M Z, et al. Effects of dissolved oxygen levels on oxidative stress response and energy utilization of juvenile Chinese sea bass (Lateolabrax maculatus) and associate physiological mechanisms. Periodical of Ocean University of China (Natural Science), 2018, 48(7): 20-28 [常志成, 温海深, 张美昭, 等. 溶解氧水平对花鲈幼鱼氧化应激与能量利用的影响及生理机制. 中国海洋大学学报(自然科学版), 2018, 48(7): 20-28 DOI:10.16441/j.cnki.hdxb.20170296] |
CUI K K, YOU H Z, DING Z Y, et al. Study on oxygen consumption rate and asphyxiation point of different size sea bass. Scientific Fish Farming, 2018(3): 57-58 [崔宽宽, 尤宏争, 丁子元, 等. 不同规格花鲈的耗氧率与窒息点研究. 科学养鱼, 2018(3): 57-58 DOI:10.14184/j.cnki.issn1004-843x.2018.03.032] |
DING Y W, LI J E. A preliminary study on the oxygen consumption of fry of flat bream Rhabdosargus sarba (Forskal). Journal of Zhanjiang Ocean University, 2000, 20(3): 8-12 [丁彦文, 李加儿. 平鲷Rhabdosargus sarba (Forskal)幼鱼耗氧率的初步研究. 湛江海洋大学学报, 2000, 20(3): 8-12 DOI:10.3969/j.issn.1673-9159.2000.03.002] |
FLIK G, KLAREN P H M, VAN DEN BURG E H, et al. CRF and stress in fish. General and Comparative Endocrinology, 2006, 146(1): 36-44 DOI:10.1016/j.ygcen.2005.11.005 |
GLASS M L, ANDERSEN N A, KRUHØFFER M, et al. Combined effects of environmental PO2 and temperature on ventilation and blood gases in the carp Cyprinus carpio L. Journal of Experimental Biology, 1990, 148(1): 1-17 |
GUO Z X. Effects of hypoxic environment on biochemical indexes, related gene expression and transcriptome analysis of cobia juveniles. Master´s Thesis of Guangdong Ocean University, 2020 [郭志雄. 低氧环境对军曹鱼幼鱼生化指标、相关基因表达的影响及其转录组学分析. 广东海洋大学硕士研究生学位论文, 2020]
|
HU R, WEI Y, MA F F. Comparison and analysis of two dissolved oxygen reduction events in the Dapeng Bay. Journal of Ocean Technology, 2014, 33(2): 24-28 [胡蓉, 魏艳, 马方方. 大鹏湾两次溶解氧骤降事件对比与分析. 海洋技术学报, 2014, 33(2): 24-28] |
HUANG J S, LU Z, CHEN G, et al. Acute hypoxia stress on blood biochemical indexes of large-sized juvenile cobia (Rachycentron canadum). Acta Oceanologica Sinica, 2019, 41(6): 76-84 [黄建盛, 陆枝, 陈刚, 等. 急性低氧胁迫对军曹鱼大规格幼鱼血液生化指标的影响. 海洋学报, 2019, 41(6): 76-84 DOI:10.3969/j.issn.0253-4193.2019.06.007] |
HUANG X H, WEI J G, ZHENG Q, et al. Isolation, identification and genomic analysis of an ISKNV-type megalocytivirus from spotted knifejaw (Oplegnathus punctatus). Aquaculture, 2021, 532: 736032 DOI:10.1016/j.aquaculture.2020.736032 |
JIA Y D, GAO Y T, GAO Y H, et al. Growth performance, hematological and biochemical parameters, and hepatic antioxidant status of spotted knifejaw Oplegnathus punctatus in an offshore aquaculture net pen. Aquaculture, 2021, a, 541: 736761 |
JIA Y D, WANG J W, GAO Y T, et al. Hypoxia tolerance, hematological, and biochemical response in juvenile turbot (Scophthalmus maximus L.). Aquaculture, 2021, b, 535: 736380 |
LI X M, WANG X D, QI C L, et al. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture, 2018, 495: 187-195 DOI:10.1016/j.aquaculture.2018.05.031 |
LI X R. Effects of hypoxia stress on energy metabolism, blood indexes and gene expression of Takifugu puffer. Master´s Thesis of Nanjing Normal University, 2018 [李欣茹. 低氧胁迫对暗纹东方鲀能量代谢、血液指标及基因表达的影响. 南京师范大学硕士研究生学位论文, 2018]
|
LIU J L, CHEN Z W, ZHANG J Q, et al. Studies on oxygen consumption rates and suffocation points of three sizes Pseudobagras ussuriensis. Journal of Tianjin Agricultural University, 2012, 19(3): 4-7 [刘金兰, 陈志伟, 张家全, 等. 不同规格乌苏里拟鲿耗氧率及窒息点研究. 天津农学院学报, 2012, 19(3): 4-7] |
LIU K. Comparative study of carbohydrate and lipid metabolism of juvenile golden pompano (Trachinotus ovatus) and juvenile largemouth bass (Micropterus salmoides). Doctoral Dissertation of Guangdong Ocean University, 2017 [刘康. 卵形鲳鲹(Trachinotus ovatus)幼鱼和大口黑鲈(Micropterus salmoides)幼鱼糖脂代谢比较研究. 广东海洋大学博士研究生学位论文, 2017]
|
LIU X B, LI X M, DU X X, et al. Spotted knifejaw (Oplegnathus punctatus) MyD88: Intracellular localization, signal transduction function and immune responses to bacterial infection. Fish and Shellfish Immunology, 2019, 89: 719-726 DOI:10.1016/j.fsi.2019.04.035 |
MA Y Q, SI N, QU Z Q, et al. Effect of body mass on the oxygen consumption rate of Ictalurus punctatus juveniles. Hebei Fisheries, 2021(5): 1–3, 40 [麻艳群, 司楠, 曲照球, 等. 体质量对斑点叉尾  幼鱼耗氧率的影响.幼鱼耗氧率的影响. 河北渔业, 2021(5): 1–3, 40] 幼鱼耗氧率的影响.幼鱼耗氧率的影响. 河北渔业, 2021(5): 1–3, 40] |
OU Y J, FAN C Y, LI J E, et al. Acute hypoxia stress on blood biochemical indexes in selective group of Tranchinotus ovatus. Acta Oceanologica Sinica, 2014, 36(4): 126-131 [区又君, 范春燕, 李加儿, 等. 急性低氧胁迫对卵形鲳鲹选育群体血液生化指标的影响. 海洋学报, 2014, 36(4): 126-131 DOI:10.3969/j.issn.0253-4193.2014.04.014] |
PICHAVANT K, MAXIME V, THÉBAULT M T, et al. Effects of hypoxia and subsequent recovery on turbot Scophthalmus maximus: Hormonal changes and anaerobic metabolism. Marine Ecology-Progress Series, 2002, 225: 275-285 DOI:10.3354/meps225275 |
QIAN C Y. Effects of hypoxia and hypoxia on the morphological changes of gill and enzyme activity of tissues in the new strain F5 of Megalobrama amblycephala. Master´s Thesis of Shanghai Ocean University, 2002 [钱辰颖. 低氧和高氧对团头鲂F5新品系鳃组织形态变化及各组织酶活性的影响. 上海海洋大学硕士研究生学位论文, 2020]
|
RAO H M, HUANG W Y. Discussion on influencing factor of content of dissolved oxygen in water. Journal of Salt Science and Chemical Industry, 2017, 46(3): 40-43 [饶胡敏, 黄旺银. 影响水体中溶解氧含量因素的探讨. 盐科学与化工, 2017, 46(3): 40-43] |
ROBIN B, HARRY K, CHRIS G C. Hypoxia tolerance and oxygen regulation in Atlantic salmon, Salmo salar from a Tasmanian population. Aquaculture, 2011, 318(3/4): 397-401 |
RUAN W, JI W, ZHENG L, et al. On hypoxia stress in fish and its nutritional regulation and response. Marine Fisheries, 2020, 42(6): 751-761 [阮雯, 纪炜炜, 郑亮, 等. 鱼类低氧胁迫及营养调控和应对研究进展. 海洋渔业, 2020, 42(6): 751-761 DOI:10.3969/j.issn.1004-2490.2020.06.011] |
SHEN F, FAN Q X, YANG K, et al. The immune responses of yellow catfish (Pelteobagrus fulvidraco) and its susceptibility to Aeromonas hydrophila at different dissolved oxygen levels. Freshwater Fisheries, 2010, 40(4): 44-49 [沈凡, 樊启学, 杨凯, 等. 不同溶氧条件下黄颡鱼免疫机能及抗病力的研究. 淡水渔业, 2010, 40(4): 44-49 DOI:10.3969/j.issn.1000-6907.2010.04.008] |
SONG X F, CHENG Y W, XING D C, et al. Influences of temperature and weight on oxygen consumption rate and ammonium excretion of Oplegnathus punctatus and their circadian rhythms. Periodical of Ocean University of China (Natural Science), 2017, 47(9): 21-29 [宋协法, 程亚伟, 邢道超, 等. 温度、体重对斑石鲷耗氧率、排氨率的影响及昼夜节律变化. 中国海洋大学学报(自然科学版), 2017, 47(9): 21-29] |
SONG Y D, TANG S J, ZHAO J L. A preliminary study on suffocation point and oxygen consumption rate of Siniperca chuatsi juveniles. Journal of Aquaculture, 2019, 40(3): 3-5 [宋银都, 唐首杰, 赵金良. 鳜幼鱼窒息点、耗氧率的初步研究. 水产养殖, 2019, 40(3): 3-5 DOI:10.3969/j.issn.1004-2091.2019.03.002] |
SUN Y, ZHANG B, TANG Q S. Effects of ration level and food species on energy budget of Sabastodes fuscescens. Marina Fisheries Research, 2001, 22(2): 32-37 [孙耀, 张波, 唐启升. 摄食水平和饵料种类对黑鲪能量收支的影响. 海洋水产研究, 2001, 22(2): 32-37] |
THURSTON R V, PHILLIPS G R, RUSSO R C, et al. Increased toxicity of ammonia to rainbow trout (Salmo gairdenri) resulting from reduced concentrations of dissolved oxygen. Canadian Journal of Fisheries and Aquatic Sciences, 2011, 38(8): 938-988 |
ULTSCH G R, REGAN M D. The utility and determination of Pcrit in fishes. Journal of Experimental Biology, 2019, 222(22): 203646 |
WANG J T, LIU T, ZHENG P Q, et al. Effect of dietary lipid levels on growth performance, body composition, and feed utilization of juvenile spotted knifejaw Oplegnathus punctatus. Aquaculture Reports, 2021, 21: 100797 |
WANG P, SHI W J, WAN X H, et al. Effects of hypoxia and reoxygenation on respiratory metabolism enzyme and antioxidant enzyme activities in Exopalaemon carinicauda. Progress in Fishery Sciences, 2021, 42(4): 106-115 [王盼, 史文军, 万夕和, 等. 低氧–复氧对脊尾白虾呼吸代谢和抗氧化酶活力的影响. 渔业科学进展, 2021, 42(4): 106-115] |
WANG W Z, ZENG Z Q, HUANG J S. Hypoxia stress on growth, serum biochemical and non-specific immune indexes of juvenile cobia (Rachycentron canadum). Haiyang Xuebao, 2021, 43(2): 49-58 [王维政, 曾泽乾, 黄建盛, 等. 低氧胁迫对军曹鱼幼鱼生长、血清生化和非特异性免疫指标的影响. 海洋学报, 2021, 43(2): 49-58] |
WANG X W, ZHU H, HU H X, et al. Effects of hypoxia on physiological status of Siberian sturgeon on Acipenser baeri juveniles. Fisheries Science, 2016, 35(5): 459-465 [王晓雯, 朱华, 胡红霞, 等. 低氧胁迫对西伯利亚鲟幼鱼生理状态的影响. 水产科学, 2016, 35(5): 459-465] |
WANG Y W. Effects of hypoxia and culture density on physiological and biochemical indexes and tissue structure of Megalobrama amblycephala. Master´s Thesis of Shanghai Ocean University, 2019 [王燚纬. 低氧、养殖密度对团头鲂生理生化指标和组织结构的影响. 上海海洋大学硕士研究生学位论文, 2019]
|
WANG Z F, ZUO P X, LENG Y, et al. Study of the asphyxiation point and oxygen consumption rate of juvenile Triplophysa venusta. Fisheries of Heilongjiang, 2019(2): 42-45 [王志飞, 左鹏翔, 冷云, 等. 秀丽高原鳅幼鱼窒息点与耗氧率的研究. 黑龙江水产, 2019(2): 42-45] |
WANNAMAKER C M, RICE J A. Effects of hypoxia on movements and behavior of selected estuarine organisms from the southeastern United States. Journal of Experimental Marine Biology and Ecology, 2000, 249(2): 145-163 |
WU B Y, XU J, CAO D C, et al. Genome-wide association analysis of hypoxia adaptation traits in common carp (Cyprinus carpio). Progress in Fishery Sciences, 2022, 43(2): 98-106 [吴碧银, 许建, 曹顶臣, 等. 鲤低氧适应性状的全基因组关联分析. 渔业科学进展, 2022, 43(2): 98-106] |
WU Z H, YOU F, WEN A Y, et al. Physiological and morphological effects of severe hypoxia, hypoxia and hyperoxia in juvenile turbot (Scophthalmus maximus L.). Aquaculture Research, 2016, 47(1): 219-227 |
XIONG X Y, HUANG G Q, PENG Y H, et al. Effect of hypoxia on growth performance, energy metabolism and oxidative stress of Mugil cephalus. Journal of Fisheries China, 2016, 40(1): 73-82 [熊向英, 黄国强, 彭银辉, 等. 低氧胁迫对鲻幼鱼生长、能量代谢和氧化应激的影响. 水产学报, 2016, 40(1): 73-82] |
XUE B, ZHU J P, LI T J, et al. Distribution of dissolved oxygen and nutrients and its relationship around Shengsi Islands of Zhejiang. Guangzhou Chemical Industry, 2016, 44(17): 150-151 [薛彬, 朱敬萍, 李铁军, 等. 嵊泗海域溶解氧与营养盐分布特征及其相关性研究. 广州化工, 2016, 44(17): 150-151] |
ZHANG A J. Comparison of hypoxia tolerance and locomotor performance in two cyprinids with incompletely overlapped habitat. Master´s Thesis of Chongqing Normal University, 2014 [张安杰. 两种生境不完全重叠的鲤科鱼类耐低氧及运动能力的比较. 重庆师范大学硕士研究生学位论文, 2014]
|
ZHANG H T, SONG X F, LIU X Z, et al. Dynamic response of microbial community to water quality change in biofilter for recirculating aquaculture system of Oplegnathus punctatus. Chinese Journal of Environmental Engineering, 2020, 14(8): 2176-2185 [张皓天, 宋协法, 刘兴震, 等. 斑石鲷循环水养殖系统生物滤池微生物群落对水质变化的动态响应. 环境工程学报, 2020, 14(8): 2176-2185] |
ZHANG X, FU S J, PENG J L, et al. The effect of acute hypoxia on blood parameters of juvenile crucian carp. Journal of Chongqing Normal University (Natural Science), 2011, 28(4): 19-22 [张曦, 付世建, 彭姜岚, 等. 急性低氧对鲫鱼幼鱼血液基础指标的影响. 重庆师范大学学报(自然科学版), 2011, 28(4): 19-22] |
ZHANG Y L, GUAN C W. Ship rocking affecting serum biochemical indexes of spotted knifejaw (Oplegnathus punctatus). Chinese Agricultural Science Bulletin, 2017, 33(29): 145-149 [张宇雷, 管崇武. 船载摇摆胁迫对斑石鲷血液生化指标的影响研究. 中国农学通报, 2017, 33(29): 145-149] |
ZHAO W W, CAO Z D, FU S J. The effects of dissolved oxygen level on the swimming performances of juvenile Parabramis pekinensis and Spinibarbus sinensis. Acta Hydrobiologica Sinica, 2013, 37(2): 314-320 [赵文文, 曹振东, 付世建. 溶氧水平对鳊鱼、中华倒刺鲃幼鱼游泳能力的影响. 水生生物学报, 2013, 37(2): 314-320] |



