2. 中国水产科学研究院黄海水产研究所 山东 青岛 266071;
3. 青岛海洋科学与技术试点国家实验室海洋药物与生物制品功能实验室 山东 青岛 266235
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao, Shandong 266071, China;
3. Laboratory for Marine Drugs and Bioproducts, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, Shandong 266235, China
海参属于棘皮动物门(Echinodermata)、海参纲(Holothuroidea),全世界海参约900多种,其中40种可供食用(廖玉麟, 2001)。中国是世界上海参养殖产量最大的国家,2020年产量达到196 564 t (农业农村部渔业渔政管理局, 2021),养殖种类为仿刺参(Apostichopus japonicus),养殖区域集中在辽宁、山东、福建等地。仿刺参是我国药食同源的典型品种,具有极高的食用和经济价值。
海参具有自溶的特点,活海参采捕上岸后,必须立即进行加工,加工过程会产生30%左右的副产物,如海参肠、性腺、呼吸树等组织,目前,对这些副产物加工利用的程度很低,有些甚至被直接丢弃,造成环境污染和资源浪费(Mamelona et al, 2010)。海参性腺富含优质蛋白(Sun et al, 2017),可用于开发膳食补充剂型产品。海参性腺还含有海参多糖、海参皂苷、活性脂质等多种活性成分(Yang et al, 2020; 袁文鹏等, 2010; 张健等, 2013)。Mamelona等(2007)发现,大西洋海参性腺中酚类和黄酮类物质含量较高,是潜在的天然抗氧化剂来源。太平洋一些地区,如新西兰和库克群岛的居民定期从海参内脏中收集性腺,用于烹饪美食或作为传统膳食的蛋白质来源(Drumm et al, 2005)。在日本,海参性腺被加工成丸剂和粉剂功能性产品,价格昂贵(曹荣等, 2020),而我国对海参性腺的开发利用程度还很低。
海参性腺蛋白含量高,多种成分具有生理活性(刘昕等, 2016),极具开发潜力。生物酶法是提高蛋白质利用率的有效手段,可以将大分子蛋白降解为易溶解且有利于人体吸收的小分子肽类和氨基酸,同时,在一定程度上缓解原料腥味重的问题(Wang et al, 2018; Li et al, 2016)。本研究以仿刺参性腺为原料,采用前期研究确立的酶解条件(曹荣等, 2012)对仿刺参性腺进行酶解,对酶解过程中可溶性蛋白、氨基酸态氮含量变化进行检测分析,对风味变化进行研究,以期为仿刺参性腺相关产品的开发提供参考。
1 材料与方法 1.1 实验材料与设备仿刺参性腺由山东青岛海滨食品有限公司提供,系当年春季采捕底播仿刺参时现场分离得到;中性蛋白酶(20万U/g)、牛血清白蛋白、硫酸铜、酒石酸钠钾、三氯乙酸、冰乙酸、乙酸钠、甲醛、乙酰丙酮和硫酸铵等均为国产分析纯,购自国药集团化学试剂有限公司。
SHA-B型恒温震荡水浴锅(江苏常州国华电器有限公司);Centrifuge 5804r型冷冻离心机(上海富众生物科学有限公司);UV-2800型紫外可见分光光度计(上海尤尼克柯仪器有限公司);PEN3便携式电子鼻(AIRSENSE公司,德国);L-8900型氨基酸自动分析仪(日立高新技术公司,日本);7980A/5975C型气相色谱–质谱联用仪(Agilent公司,美国)。
1.2 实验方法 1.2.1 仿刺参性腺酶解液的制备取新鲜仿刺参性腺匀浆,按料液比1∶3 (m/v)混匀,加入中性蛋白酶2000 U/g,在45℃下酶解2 h (曹荣等, 2012),酶解过程每30 min取一次样,进行各项指标测定。
1.2.2 可溶性蛋白含量测定取5.0 mL酶解液加入等体积的TCA溶液(质量分数为15%),静置20 min后,10 000 r/min离心10 min。上清液定容至10 mL,取1 mL待测液,采用双缩脲比色法(王永华, 2010)测定可溶性蛋白质含量。
1.2.3 氨基酸态氮含量测定参照GB/T 5009.39- 2003,采用甲醛比色法测定。
1.2.4 水解度计算水解度DH (degree of hydrolysis)按照下式(曹荣等, 2014)进行计算:
| $ {\rm{DH}}(\% ) = \frac{{{P_2} - {P_1} + \left( {{N_2} - {N_1}} \right) \times 9.14}}{{{N_0} - {P_1} - {N_1} \times 9.14}} \times 100 $ |
式中,P2为酶解液中可溶性蛋白质含量(mg/g);P1为水解前匀浆液中可溶性蛋白质含量(mg/g);N2为酶解液中氨基酸态氮含量(mg/g);N1为水解前匀浆液中氨基酸态氮含量(mg/g);N0为蛋白质总含量(mg/g);9.14为氨基酸态氮与氨基酸之间的换算系数,以20种氨基酸平均分子量128 Da计。
1.2.5 游离氨基酸组成测定采用氨基酸自动分析仪测定游离氨基酸组成。
1.2.6 挥发性成分测定采用PEN3电子鼻分析酶解液的气味特征。取1 mL酶解液置于顶空瓶中,孵育15 min,准备时间10 s,检测时间60 s,清洗时间60 s。电子鼻测定结果利用WinMuster进行线性判别分析(Linear Discriminant Analysis, LDA)。
采用GC-MS对酶解过程挥发性成分的相对含量进行进一步分析。气相色谱条件:HP-INNOWAX毛细管色谱柱(30.00 m×250.00 μm×0.25 μm),325℃,载气为He,分流进样模式,分流比5∶1,分流流量为5 mL/min,恒压为48 kPa,进样量为20 μL,起始温度为40℃,保持5 min,以8℃/min升到250℃,保持5 min。质谱条件:离子源温度为230℃,四极杆温度150℃,质量扫描范围30~400 u,电子能量为70 eV。通过计算机检索谱库NIST 08进行定性分析,按照峰面积归一化法计算相对含量。
1.2.7 感官评价将样品随机编号,由6位接受过感官评定培训的人员组成评定小组,按表 1标准分别对海参性腺酶解液风味进行评分,结果以平均值表示。
|
|
表 1 酶解液的风味感官评价标准 Tab.1 Sensory evaluation standard for flavor of enzymatic hydrolysate |
实验重复2次,每次设3个平行样品,结果以平均值±标准差(Mean±SD)表示,使用Origin软件对数据进行统计学处理,采用t-test进行组间差异显著性分析,P < 0.05则差异显著。
2 结果与分析 2.1 仿刺参性腺酶解过程可溶性蛋白质、氨基酸态氮含量与水解度的变化仿刺参性腺酶解过程可溶性蛋白质、氨基酸态氮含量变化如图 1a所示。匀浆液中可溶性蛋白质初始含量为1.14 g/100 g,在前30 min内迅速增加至4.80 g/100 g左右,之后基本保持不变。氨基酸态氮含量在前90 min内随时间延长而逐渐增加,初始匀浆液中氨基酸态氮含量为0.15 g/100 g,90 min时达到0.50 g/100 g,之后虽略有下降,但差异并不显著(P > 0.05)。水解度是衡量蛋白质水解程度的指标,可通过酶解过程中可溶性蛋白质和氨基酸态氮的含量变化表示。如图 1b所示,水解度随酶解时间延长呈上升趋势,60 min后趋于稳定,90 min时水解度达43.66%,120 min时水解度略有下降,但与酶解60 min和90 min的水解度相比无显著差异(P > 0.05)。苏明月等(2019)分析了牡蛎肉的酶解过程,发现可溶性蛋白质和水解度都呈先增加后基本保持不变的趋势,本研究结果与之基本一致。
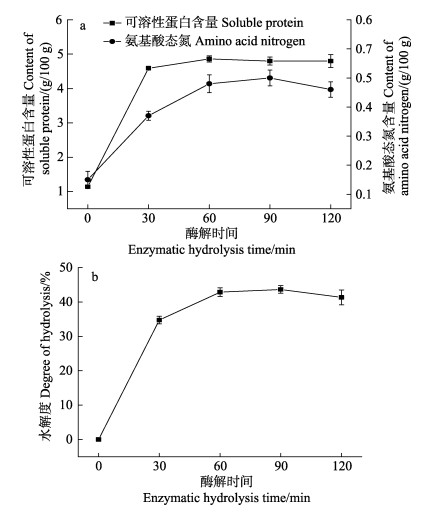
|
图 1 仿刺参性腺酶解过程可溶性蛋白质、氨基酸态氮含量(a)与水解度(b)变化情况 Fig.1 Changes in soluble protein, amino acid nitrogen (a) and degree of hydrolysis (b) of A. japonicus gonads during enzymatic hydrolysis |
以水产品加工副产物为原料进行酶解,得到的酶解产物往往风味较差(李利敏等, 2014)。随着酶解的进行,大分子蛋白质被降解为小分子肽类以及氨基酸。一些特定的肽类和氨基酸具有呈味特性,从而影响酶解液的风味特征。与此同时,伴随酶解过程,会生成一些小分子挥发性化合物(王红丽等, 2018),影响酶解液的气味。
仿刺参性腺酶解液的感官评分结果如图 2所示。随着酶解时间延长,酶解液的鲜味有所增强,可能与蛋白质降解生成了较多呈鲜味的氨基酸有关,且酶解液咸味突出,可能会与鲜味产生相乘作用。赵阳等(2015)研究紫贻贝(Mytilus edulis)蛋白酶解过程中呈味物质释放规律时发现,酶解过程中产生的肽类物质对酶解液风味有一定影响,主要是酶解过程中产生的寡肽与其他呈味物质发生相乘作用,使酶解液风味饱满、口感协调。随着酶解时间的延长,酶解液的腥味有所减弱,表明生物酶解在脱除不良风味方面有一定作用。另外,甜味和苦味也略有增强。
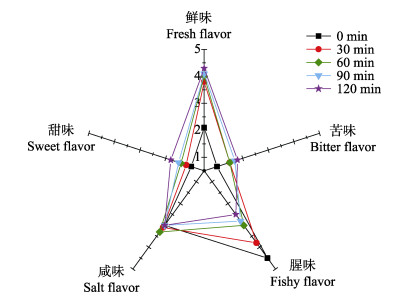
|
图 2 仿刺参性腺酶解过程感官评分 Fig.2 Sensory evaluation of A. japonicus gonad during enzymatic hydrolysis |
游离氨基酸是重要的滋味成分。仿刺参性腺酶解过程游离氨基酸的变化情况见表 2。随酶解时间的延长,游离氨基酸含量逐渐增多。初始匀浆液中游离氨基酸总量为(725.07±33.12) mg/L,其中谷氨酸含量最高,为(270.56±9.78) mg/L;酶解60 min时,游离氨基酸含量为(2221.04±89.91) mg/L,其中谷氨酸含量仍为最高,甘氨酸和丙氨酸含量次之;酶解120 min时,游离氨基酸含量达(3277.49±131.02) mg/L,谷氨酸含量为(711.97±34.12) mg/L,其次为甘氨酸和丙氨酸。
|
|
表 2 仿刺参性腺酶解过程中游离氨基酸含量的变化 Tab.2 Changes in free amino acids of A. japonicus gonad during enzymatic hydrolysis |
谷氨酸是典型的鲜味氨基酸(Je et al, 2005),酶解120 min时滋味活度值(Taste activity values, TAV)由初始的5.41增加至14.24,对酶解液风味有重要贡献。赵谋明等(2006)对低值鱼酶解液中游离氨基酸进行了测定,认为谷氨酸、天冬氨酸与各种盐、有机酸的相乘作用可能对酶解液的呈味产生积极作用。仿刺参性腺酶解液中除谷氨酸外,其他种类氨基酸的TAV均小于1,对滋味的贡献小。其中,丙氨酸的TAV值相对较高,达0.77,赋予酶解液轻微甜味。在整个酶解过程中,蛋氨酸、亮氨酸和苯丙氨酸等苦味氨基酸尽管含量显著增加,但由于其阈值较高,对酶解液的滋味贡献值较低,说明酶解液中苦味并非由氨基酸产生,推测可能是酶解过程中生成了一些苦味肽。
2.4 仿刺参性腺酶解过程电子鼻分析结果采用电子鼻分析仿刺参性腺酶解过程气味变化,结果见图 3。横坐标第一主成分的贡献率为84.64%,纵坐标第二主成分的贡献率为12.53%,总贡献率为97.17%,基本涵盖了样本的信息。LDA是将样品信号数据通过运算法则投影到某一方向,进而依据距离远近分辨组间气味差异大小。由图 3可以看出,不同酶解时间的仿刺参性腺酶解液气味有明显差异,表明酶解过程伴随小分子挥发性化合物的生成或反应。
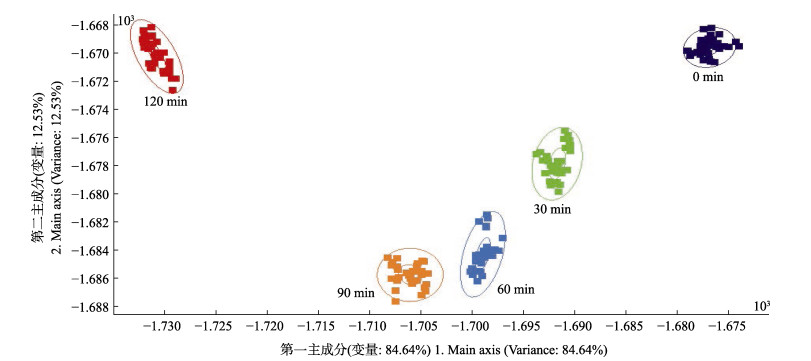
|
图 3 仿刺参性腺酶解液气味组成LDA结果 Fig.3 The LDA results of odor composition in A. japonicus gonad enzymatic hydrolysate |
采用GC-MS对仿刺参性腺酶解过程挥发性成分进行分析,结果见表 3。从仿刺参性腺酶解液中共鉴定出挥发性物质43种,其中,烃类17种、醇类9种、醛类7种、酮类4种以及其他6种。酶解0、60和120 min时,3组样品中均含有的挥发性成分有10种,包括烃类4种、醇类2种、醛类2种、酮类1种和二甲基硫醚。初始匀浆液中共鉴定出24种挥发性成分化合物,其中,二甲基硫醚、1-辛醇和1, 5-二甲基-6-亚甲基螺环[2.4]庚烷含量相对较高。酶解60 min时,1-辛醇、1, 5-二甲基-6-亚甲基螺环[2.4]庚烷比例增加,而二甲基硫醚相对含量显著降低,此时出现了一些新的挥发性成分,如环癸烷、环辛烯、苯乙醇、3-甲基-1-丁醇、乙酸。酶解120 min时,检测出对二甲苯、2-十二烯醛、苯乙醛、3-甲基丁醛、庚醇、Z-2-十八烯-1-醇、3-乙烯基-环辛烯、3-庚烯、1-甲基-4-亚甲基环己烷等挥发性成分,烃类相对含量从31.77%增加到48.49%。
|
|
表 3 仿刺参性腺酶解过程挥发性成分分析结果 Tab.3 Analysis results of volatile components in A. japonicus gonad during enzymatic hydrolysis |
部分烷烃类源于脂肪酸降解,芳香烃则主要由带芳香基的游离氨基酸氧化产生(Wang et al, 2020; Xie et al, 2008)。烷类和烃类风味特征为清香香甜型,但由于烷烃类阈值较高,对风味贡献不大(Martins et al, 2000)。烯烃类化合物具有较低的阈值,且大多带有果香味(赵玲等, 2021),酶解120 min后烯烃含量有所增加,赋予酶解液一定的清新和香甜味。
醇类物质通常是由多不饱和脂肪酸氧化产生。饱和醇类化合物阈值较高,对风味贡献不大。不饱和的烯醇式结构化合物阈值较低,具有独特的清香、果香、花香和甜味等令人愉悦的风味,对酶解液风味有正面作用(Fratini et al, 2012)。1-辛醇的相对含量由初始的19.78%增加至29.35%,为酶解液增添了柑橘和玫瑰气息(步营等, 2020)。1-辛烯-3-醇是一种亚油酸的分解产物,表现出类似蘑菇的香气(Wu et al, 2014; Zhang et al, 2019)。乙醇是食品中重要的挥发性成分,主要来源于多不饱和脂肪酸的脂氧合反应(Radovcic et al, 2016),其相对含量在酶解过程中逐渐降低。酶解前60 min醇类相对含量升高,主要与多不饱和脂肪酸的氧化有关;酶解120 min后,醇类含量有所降低,而醛类物质含量增多,可能与醇类物质发生氧化有关(Zou et al, 2018)。
酮类物质生成与氨基酸分解、多不饱和脂肪氧化降解等有关(徐永霞等, 2021)。酮类化合物的阈值一般比较高,对风味贡献不大。酮类物质中3-羟基-2-丁酮变化最为明显,相对含量随酶解时间延长而增加。
醛类在高浓度时气味令人不悦,而低浓度时往往呈现出香气(高瑞昌等, 2013)。醛类物质主要来源于脂肪的氧化或氨基酸的Strecker降解反应,其中,6~9个碳原子的醛一般具有清香、果香和脂肪香味,且阈值较低又具有叠加效应(周益奇等, 2006),往往在食品风味中起重要作用。酶解后产生的苯乙醛可产生青草香或花香(Zhao et al, 2017),3-甲基丁醛有轻微甜桔香或脂肪香气,主要衍生自脂质的温和氧化并可能赋予酶解液麦芽或坚果味(李学鹏等, 2020)。辛醛主要由油酸的氧化产生(Zhao et al, 2020)。壬醛则具有鱼腥味、生油脂的味道(刘奇等, 2012)。
杂环类化合物一般阈值很低,2-戊基呋喃具有类似火腿的香味,对酶解液有一定的增香作用(赵静等, 2016)。二甲基硫醚是海鲜腥味的主要来源之一,酶解前的相对含量高达22.59%,随着酶解的进行,二甲基硫醚含量显著降低,这与酶解液腥味减弱的现象一致。
3 结论仿刺参性腺酶解过程中,蛋白质水解成小分子肽及游离氨基酸,一方面使酶解液成分发生了改变,另一方面使其呈现出不同于原液的风味。酶解过程中,可溶性蛋白质含量在前30 min内迅速增加,氨基酸态氮含量在90 min时达到最高,此时水解度为43.66%。随着酶解时间延长,酶解液的鲜味和甜味有所增强,这与游离氨基酸中谷氨酸、甘氨酸和丙氨酸含量高的结果一致。电子鼻分析结果表明,仿刺参性腺酶解后气味发生显著变化。通过进一步的GC-MS分析发现,酶解后以二甲基硫醚为代表的腥味物质含量显著降低,而一些烃类、醇类、醛类物质的产生赋予了酶解液令人愉悦的气味。
BU Y, ZHU L W, HE W, et al. Study on the flavor improving effect of blue clam cooking liquid and enzymatic hydrolysate. Modern Food Science and Technology, 2020, 36(1): 253-261 [步营, 祝伦伟, 何玮, 等. 蓝蛤蒸煮液与酶解液的风味改善作用研究. 现代食品科技, 2020, 36(1): 253-261] |
CAO R, LIU Q, YIN B Z. Response surface methodology to optimize the enzymatic hydrolysis of sea cucumber gonads. Food Science, 2012, 33(2): 29-33 [曹荣, 刘淇, 殷邦忠. 响应面法优化海参性腺酶解工艺. 食品科学, 2012, 33(2): 29-33] |
CAO R, WANG S Y, ZHAO L, et al. Analysis and evaluation of nutritional composition of male and female gonads of sea cucumber. China Fishery Quality and Standards, 2020, 10(2): 23-30 [曹荣, 王善宇, 赵玲, 等. 仿刺参雄、雌性腺营养组成分析与评价. 中国渔业质量与标准, 2020, 10(2): 23-30 DOI:10.3969/j.issn.2095-1833.2020.02.003] |
CAO R, ZHANG Y, LIU Q, et al. Application of response surface methodology on enzymatic hydrolysis of digestive gland and gonad of Asterias amurensis. Food Research and Development, 2014, 35(9): 76-80 [曹荣, 张媛, 刘淇, 等. 响应面法优化多棘海盘车消化腺、生殖腺酶解工艺. 食品研究与开发, 2014, 35(9): 76-80] |
DRUMM D J, LONERAGAN N R. Reproductive biology of Holothuria leucospilota in the Cook Islands and the implications of traditional fishing of gonads on the population. New Zealand Journal of Marine and Freshwater Research, 2005, 39(1): 141-156 DOI:10.1080/00288330.2005.9517297 |
Fishey and Fishery Administration of the Ministry of Agriculture. China fisheries statistical yearbook. Beijing: China Agricultural Publishing House, 2021, 6 [农业农村部渔业渔政管理局. 2021中国渔业统计年鉴. 北京: 中国农业出版社, 2021, 6] |
FRATINI G, LOIS S, PAZOS M, et al. Volatile profile of Atlantic shellfish species by HS-SPME GC/MS. Food Research International, 2012, 48(2): 856-865 DOI:10.1016/j.foodres.2012.06.033 |
GAO R C, SU L, HUANG X Y, et al. Research progress of flavor substances in aquatic products. Fishery Science, 2013, 32(1): 59-62 [高瑞昌, 苏丽, 黄星奕, 等. 水产品风味物质的研究进展. 水产科学, 2013, 32(1): 59-62] |
JE J Y, PARK P J, JUNG W K, et al. Amino acid changes in fermented oyster (Crassostrea gigas) sauce with different fermentation periods. Food Chemistry, 2005, 91(1): 15-18 DOI:10.1016/j.foodchem.2004.05.061 |
LI L M, YANG P, ZHANG G Z, et al. Activated carbon adsorption of tilapia scraps proteolysis solution and its effect on flavor components. Food Research and Development, 2014, 35(11): 5-9 [李利敏, 杨萍, 张冠洲, 等. 活性炭吸附罗非鱼下脚料蛋白酶解液及对风味成分的影响. 食品研究与开发, 2014, 35(11): 5-9] |
LI X P, LIU Y W, XIE X X, et al. Effect of thermal pretreatment on enzymatic hydrolysis of blue clam and the taste characteristics of enzymatic hydrolysate. Food Science, 2020, 41(2): 133-140 [李学鹏, 刘晏玮, 谢晓霞, 等. 热预处理对蓝蛤酶解及酶解液呈味特性的影响. 食品科学, 2020, 41(2): 133-140] |
LI Y Y, SHANG J, JIANG Z Z, et al. Regulation mechanism of peptides derived from sea cucumber (Apostichopus japonicas) for modulation of learning and memory. Food Science and Biotechnology, 2016, 25(1): 241-246 DOI:10.1007/s10068-016-0035-5 |
LIAO Y L. Chinese sea cucumber. Bulletin of Biology, 2001, 9: 1-3 [廖玉麟. 我国的海参. 生物学通报, 2001, 9: 1-3] |
LIU Q, HAO S X, LI L H, et al. Analysis of volatile components in different parts of sturgeon. Food Science, 2012, 33(16): 142-145 [刘奇, 郝淑贤, 李来好, 等. 鲟鱼不同部位挥发性成分分析. 食品科学, 2012, 33(16): 142-145] |
LIU X, LIU J X, ZHANG J, et al. Separation and purification of sea cucumber egg polysaccharide and its anti-tumor activity in vitro. Food Science, 2016, 37(23): 105-110 [刘昕, 刘京熙, 张健, 等. 仿刺参卵多糖的分离纯化及体外抗肿瘤活性. 食品科学, 2016, 37(23): 105-110 DOI:10.7506/spkx1002-6630-201623018] |
MAMELONA J, LOUIS R S, PELLETIER É. Nutritional composition and antioxidant properties of protein hydrolysates prepared from echinoderm byproducts. International Journal of Food Science and Technology, 2010, 45(1): 147-154 |
MAMELONA J, PELLETIER É, GIRARD-LALANCETTE K, et al. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chemistry, 2007, 104(3): 1040-1047 |
MARTINS S I, JONGEN W M, VAN BOEKEL M A. A review of Maillard reaction in food and implications to kinetic modelling. Trends in Food Science and Technology, 2000, 11(9): 364-373 |
RADOVCIC N M, VIDACEK S, JANCI T, et al. Characterization of volatile compounds, physico-chemical and sensory characteristics of smoked dry-cured ham. Journal of Food Science and Technology-Mysore, 2016, 53(11): 4093-4105 |
SU M Y, YUE M, MAO Z J, et al. Study on the proteolytic characteristics and flavor characteristics of cooked oyster meat. Food Technology, 2019, 44(5): 128-134 [苏明月, 岳敏, 毛振杰, 等. 蒸煮牡蛎肉的蛋白酶解特性及风味特征的研究. 食品科技, 2019, 44(5): 128-134] |
SUN N, CUI P, JIN Z, et al. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates. Food Chemistry, 2017, 230: 627-636 |
WANG H L, LI T J, LIN S N, et al. Study on variation of volatile flavor components and sensory quality of enzymatic hydrolysate of shrimp leftovers under different enzymatic hydrolysis processes. Cereal and Feed Industry, 2018, 1: 53-59 [王红丽, 李天骄, 林圣楠, 等. 不同酶解工艺下对虾下脚料酶解液挥发性风味成分变化及感官品质研究. 粮食与饲料工业, 2018, 1: 53-59] |
WANG K, SUN N, LI D, et al. Enzyme-controlled hygroscopicity and proton dynamics in sea cucumber (Stichopus japonicus) ovum peptide powders. Food Research International, 2018, 112(10): 241-249 |
WANG Y H. Food analysis. Beijing: China Light Industry Press, 2010, 75 [王永华. 食品分析. 北京: 中国轻工业出版社, 2010, 75] |
WANG Y, GAO Y, LIANG W, et al. Identification and analysis of the flavor characteristics of unfermented stinky tofu brine during fermentation using SPME-GC-MS, e-nose, and sensory evaluation. Journal of Food Measurement and Characterization, 2020, 14(1): 597-612 |
WU N, GU S, TAO N, et al. Characterization of important odorants in steamed male Chinese mitten crab (Eriocheir sinensis) using gas chromatography-mass spectrometry- olfactometry. Journal of Food Science, 2014, 79(7): 1250-1259 |
XIE J, SUN B, ZHENG F, et al. Volatile flavor constituents in roasted pork of mini-pig. Food Chemistry, 2008, 109(3): 506-514 |
XU Y X, QU S Y, LI T, et al. Effects of different proteases on the flavor characteristics of blue clam hydrolysate. Food Science, 2021, 42(4): 190-196 [徐永霞, 曲诗瑶, 李涛, 等. 不同蛋白酶对蓝蛤酶解液风味特性的影响. 食品科学, 2021, 42(4): 190-196] |
YANG D, LIN F, HUANG Y, et al. Separation, purification, structural analysis and immune-enhancing activity of sulfated polysaccharide isolated from sea cucumber viscera. International Journal of Biological Macromolecules, 2020, 155: 1003-1018 |
YUAN W P, LIU C H, WANG X J, et al. Analysis and comprehensive evaluation of nutritional components in different parts of Apostichopus japonicus. Science and Technology of Food Industry, 2010, 31(5): 348-350 [袁文鹏, 刘昌衡, 王小军, 等. 仿刺参不同部位营养成分的分析及综合评价. 食品工业科技, 2010, 31(5): 348-350] |
ZHANG J, WANG M J, MA J J, et al. Analysis of nutritional components in gonad of Apostichopus japonicus. Food Science, 2013, 34(14): 232-236 [张健, 王茂剑, 马晶晶, 等. 仿刺参生殖腺营养成分分析. 食品科学, 2013, 34(14): 232-236] |
ZHANG Y Q, MA X T, DAI Z Y. Comparison of nonvolatile and volatile compounds in raw, cooked, and canned yellowfin tuna (Thunnus albacores). Journal of Food Processing and Preservation, 2019, 43(10): 1-11 |
ZHAO J, DING Q, SUN Y, et al. Comparative analysis of flavor components and flavor characteristics in mushroom soup and enzymatic hydrolysate. Food Science, 2016, 37(24): 99-104 [赵静, 丁奇, 孙颖, 等. 香菇菌汤及酶解液中滋味成分及呈味特性的对比分析. 食品科学, 2016, 37(24): 99-104] |
ZHAO J, WANG M, XIE J, et al. Volatile flavor constituents in the pork broth of black-pig. Food Chemistry, 2017, 226: 51-60 |
ZHAO L, CAO R, WANG L Z, et al. Study on the characteristic flavor change of Porphyra yezoensis before and after roasting. Progress in Fishery Sciences, 2021, 42(4): 199-207 [赵玲, 曹荣, 王联珠, 等. 条斑紫菜烤制前后特征风味变化研究. 渔业科学进展, 2021, 42(4): 199-207] |
ZHAO M M, CUI C, LIU S. Preparation of different meat flavor thermal reactants from low value fish protease hydrolysates. Journal of Food and Biotechnology, 2006, 25(3): 1-6 [赵谋明, 崔春, 刘珊. 低值鱼蛋白酶解产物制备不同肉香型热反应物. 食品与生物技术学报, 2006, 25(3): 1-6] |
ZHAO Q, XUE Y, SHEN Q. Changes in the major aroma-active compounds and taste components of Jasmine rice during storage. Food Research International, 2020, 133: 109160 |
ZHAO Y, CHEN H H, WANG Y S, et al. The release law of flavoring substances and the structure of flavoring peptides during the proteolysis of blue mussel. Food and Machinery, 2015, 31(1): 18-24 [赵阳, 陈海华, 王雨生, 等. 紫贻贝蛋白酶解过程中呈味物质释放规律和呈味肽结构. 食品与机械, 2015, 31(1): 18-24] |
ZHOU Y Q, WANG Z J. Extraction and analysis on fishy odor- causing compounds in the different part of carp. Chinese Journal of Analytical Chemistry, 2006(S1): 165-167 [周益奇, 王子健. 鲤鱼体中鱼腥味物质的提取和鉴定. 分析化学, 2006(S1): 165-167] |
ZOU Y, KANG D, LIU R, et al. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrasonic Sonochemistry, 2018, 46: 36-45 |



