 鱼消化相关酶活性的比较分析
鱼消化相关酶活性的比较分析
 鱼的消化生理特性,测定并比较分析了3种
鱼的消化生理特性,测定并比较分析了3种 鱼[高体
鱼[高体 (Seriola dumerili)、黄条
(Seriola dumerili)、黄条 (Seriola lalandi)、五条
(Seriola lalandi)、五条 (Seriola quinqueradiata)]的消化系统(胃、幽门盲囊、前肠、中肠、后肠和肝脏)中5种消化相关酶(胰蛋白酶、脂肪酶、淀粉酶、碱性磷酸酶和酸性磷酸酶)活性与组织分布特点。结果显示,3种
(Seriola quinqueradiata)]的消化系统(胃、幽门盲囊、前肠、中肠、后肠和肝脏)中5种消化相关酶(胰蛋白酶、脂肪酶、淀粉酶、碱性磷酸酶和酸性磷酸酶)活性与组织分布特点。结果显示,3种 鱼中5种消化相关酶主要分布在幽门盲囊、肝脏和肠道中。3种
鱼中5种消化相关酶主要分布在幽门盲囊、肝脏和肠道中。3种 鱼胃组织中胃蛋白酶活性无差异。幽门盲囊中胰蛋白酶活性:黄条
鱼胃组织中胃蛋白酶活性无差异。幽门盲囊中胰蛋白酶活性:黄条 > 高体
> 高体 > 五条
> 五条 (P < 0.05),高体
(P < 0.05),高体 肝脏组织中胰蛋白酶活性显著高于其他2种
肝脏组织中胰蛋白酶活性显著高于其他2种 鱼(P < 0.05);胃、中肠、后肠组织中α-淀粉酶活性:五条
鱼(P < 0.05);胃、中肠、后肠组织中α-淀粉酶活性:五条 > 黄条
> 黄条 > 高体
> 高体 (P < 0.05),幽门盲囊、前肠组织中α-淀粉酶活性:黄条
(P < 0.05),幽门盲囊、前肠组织中α-淀粉酶活性:黄条 > 五条
> 五条 > 高体
> 高体 (P < 0.05);胃、幽门盲囊组织中脂肪酶活性:黄条
(P < 0.05);胃、幽门盲囊组织中脂肪酶活性:黄条 > 五条
> 五条 > 高体
> 高体 (P < 0.05),前肠、后肠、肝脏中脂肪酶活性:五条
(P < 0.05),前肠、后肠、肝脏中脂肪酶活性:五条 > 黄条
> 黄条 > 高体
> 高体 (P < 0.05);3种
(P < 0.05);3种 鱼的酸、碱性磷酸酶活性组织分布趋势基本一致,其中,黄条
鱼的酸、碱性磷酸酶活性组织分布趋势基本一致,其中,黄条 幽门盲囊组织中酸、碱性磷酸酶活性最高(P < 0.05)。研究表明,3种
幽门盲囊组织中酸、碱性磷酸酶活性最高(P < 0.05)。研究表明,3种 鱼消化相关酶活性的组织分布特点基本一致,幽门盲囊是5种酶作用的主要靶器官,除胰蛋白酶外,高体
鱼消化相关酶活性的组织分布特点基本一致,幽门盲囊是5种酶作用的主要靶器官,除胰蛋白酶外,高体 其他4种酶活性均显著低于其他2种
其他4种酶活性均显著低于其他2种 鱼,黄条
鱼,黄条 幽门盲囊和肠道的5种酶活性显著偏高。结果可为揭示
幽门盲囊和肠道的5种酶活性显著偏高。结果可为揭示 属鱼类的消化生理特性、研制适宜
属鱼类的消化生理特性、研制适宜 属鱼类消化特点和种特异性生长的高效专用配合饲料提供理论依据。
属鱼类消化特点和种特异性生长的高效专用配合饲料提供理论依据。 黄条
黄条 五条
五条 消化酶活力 分布特征
消化酶活力 分布特征 “十三五”以来,为拓展海水养殖新空间、养护近海渔业资源、保护近岸海域环境,我国大力发展深远海养殖,各种深远海大型养殖平台不断投入运行,但深远海养殖“养什么、怎么养”的问题日益凸显,成为深远海养殖持续健康发展的重要制约瓶颈。






本团队于2017年突破黄条













实验鱼于2020年12月取自福建东山富闽洋水产有限公司网箱养殖基地,网箱规格为10 m×10 m× 8 m,养殖密度为15 kg/m3。实验所取高体


采集3种



本实验蛋白浓度测定方法依照上海碧云天生物技术有限公司生产的Bradford蛋白浓度测定试剂盒(P0006)利用酶标仪(BIO-RAD iMARK,美国)进行操作;酶活性测定方法依照南京建成α-淀粉酶试剂盒(C016-1-1)、胰蛋白酶试剂盒(A080-2)利用分光光度计(普析TU-1810,中国)进行操作;胃蛋白酶试剂盒(A080-1-1)、脂肪酶试剂盒(A054-2-1)、碱性磷酸酶试剂盒(A059-2)和酸性磷酸酶试剂盒(A060-2)用酶标仪(BIO-RAD iMARK,美国)按照试剂盒说明书进行操作。
测定酶活性前将样品取出用0.9%的生理盐水冲洗,称取0.1 g组织使用灭菌解剖剪将其剪碎,加入900 µL样品匀浆介质,使用样品破碎仪(净信JXFSTPRP-24, 中国)使组织破碎匀浆,匀浆液于3500 r/min离心15 min后吸取上清液,于–20℃冰箱保存待用,15 d内测定完毕,测定脂肪酶、酸性磷酸酶、碱性磷酸酶活性时,再将10%浓度的上清液稀释至2%浓度待用。
胃蛋白酶活力单位定义:每毫克组织蛋白37℃每分钟分解蛋白生成1 μg酪氨酸相当于1个酶活力单位。胰蛋白酶活力单位定义:在pH 8.0、37℃条件下,每毫克蛋白中含有的胰蛋白酶每分钟使吸光度值(OD值)变化0.003即为1个酶活力单位。α-淀粉酶活力单位定义:组织中每毫克蛋白在37℃与底物作用30 min,水解10 mg淀粉定义为1个淀粉酶活力单位。脂肪酶活力单位定义:在37℃条件下,每毫升酶液在反应体系中与底物反应1 min,每消耗1 mmol底物为1个酶活力单位。碱性磷酸酶活力单位定义:每克组织蛋白在37℃与基质作用15 min产生1 mg酚为1金氏单位。酸性磷酸酶活力单位定义:每克组织蛋白在37℃与基质作用30 min产生1 mg酚为1金氏单位。
1.4 统计分析采用SPSS 22.0软件对实验数据进行分析,运用单因子方差分析(one-way ANOVA)和Duncan检验法对同一种鱼不同组织和同一组织不同种鱼酶活性数据进行显著性差异分析和多重比较分析,设定差异显著性水平P值为0.05,所有数值均采用平均值±标准差(Mean±SD)表示。
2 结果 2.1 消化系统的外部形态比较




3种







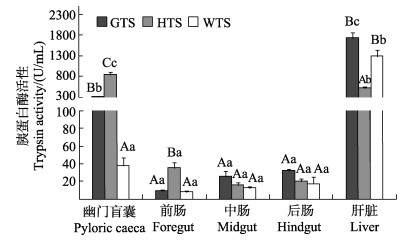
|
图 1 3种  ;HTS:黄条 ;HTS:黄条 ;WTS:五条 ;WTS:五条 。不同大写字母表示同一组织不同种鱼间消化酶活性变化差异显著(P < 0.05),不同小写字母表示同一种鱼不同组织间酶活性变化差异显著(P < 0.05)。下同。
GTS: S. dumerili; HTS: S. lalandi; WTS: S. quinqueradiata; different capital letters indicate significant difference of digestive enzymes activities in same tissue from different species (P < 0.05); different lowercase letters indicate significant difference of digestive enzymes activities in different tissues from the same species (P < 0.05). 。不同大写字母表示同一组织不同种鱼间消化酶活性变化差异显著(P < 0.05),不同小写字母表示同一种鱼不同组织间酶活性变化差异显著(P < 0.05)。下同。
GTS: S. dumerili; HTS: S. lalandi; WTS: S. quinqueradiata; different capital letters indicate significant difference of digestive enzymes activities in same tissue from different species (P < 0.05); different lowercase letters indicate significant difference of digestive enzymes activities in different tissues from the same species (P < 0.05). The same as below. |
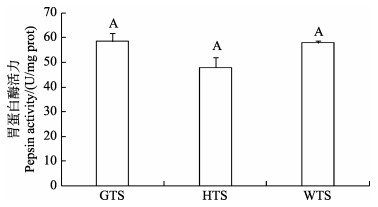
|
图 2 3种 |
3种










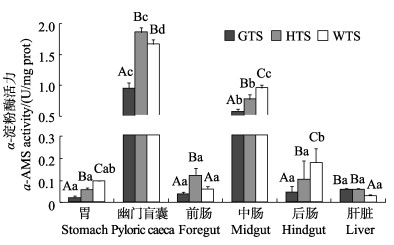
|
图 3 3种 |
3种











|
图 4 3种 |
3种














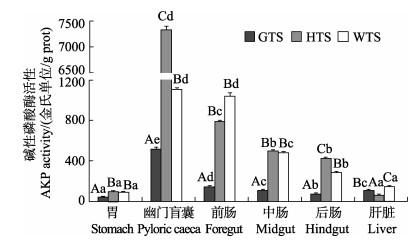
|
图 5 3种 |
如图 6所示,高体












|
图 6 3种 |
本研究比较了3种

已有研究表明,同一种鱼类不同生长阶段其消化酶活性存在一定差异,养殖环境及饵料与消化酶活性也存在一定关系,一定范围内盐度、温度的变化也是消化酶活力变化的影响因素(陈慕雁等, 2004; 白晓慧等, 2007; 庄平等, 2008; Miegel et al, 2010),同时消化酶活性与其食性关系密切,但即使食性相同的鱼类其酶活性也存在一定的差异,这也是生物本身对环境的一种适应机制(谭北平, 1995)。本研究发现,同一养殖条件同一季节和相同饵料投喂条件下,高体

















食物进入胃后刺激胃酸分泌,胃是胃蛋白酶分泌的最大靶器官(周景祥等, 2000; 章龙珍等; 2016)。通常肉食性鱼类具有较高的蛋白质消化能力,本研究发现,3种
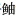








消化道中的淀粉酶主要是消化吸收食物中的碳水化合物和糖类。张正荣等(2020)研究了黄条





脂肪酶主要是由鱼类的肝胰脏分泌,在鱼类的消化生理中占据很重要的地位。3种



酸性磷酸酶(ACP)是溶酶体的一种标志酶,参与吞噬细胞的胞内消化、核酸和蛋白质、磷酸酯的代谢、免疫调节等重要生命活动(Li et al, 2009)。本研究发现,幽门盲囊是酸性磷酸酶作用的主要靶器官,3种

碱性磷酸酶作为一种吸收酶在鱼类的肠道均有分布,参与脂类、葡萄糖、钙和无机磷等营养物质的吸收和转运(Tengjaroenkul et al, 2000; Cara et al, 2003)。3种






本研究比较分析了3种



BAI X H, XIONG C X. Different feed including the type and feed ingredients, different protein source and protein content, feed additives and feed processing had significant effect on the digestive enzymes of fish. China Feed, 2007(10): 20 [白晓慧, 熊传喜. 不同饲料对鱼类消化酶活性的影响. 中国饲料, 2007(10): 20 DOI:10.3969/j.issn.1004-3314.2007.10.009] |
CARA J B, MOYANO F J, CARDENAS S, et al. Assessment of digestive enzyme activities during larval development of white bream. Journal of Fish Biology, 2003, 63: 48-58 DOI:10.1046/j.1095-8649.2003.00120.x |
CHEN M Y, ZHANG X M. Recent advances in digestive physiology of marine fish larvae-juvenile. Progress in Fishery Sciences, 2004, 25(3): 81-88 [陈慕雁, 张秀梅. 海水鱼类仔稚鱼消化生理学研究进展. 渔业科学进展, 2004, 25(3): 81-88 DOI:10.3969/j.issn.1000-7075.2004.03.014] |
DENSTADLI V, VEGUSDAL A, KROGDAHL A, et al. Lipid absorption in different segments of the gastrointestinal tract of Atlantic salmon (Salmo salar L.). Aquaculture, 2004, 240: 385-398 DOI:10.1016/j.aquaculture.2004.06.030 |
DENTON J, YOUSEF I, KUKSIS A. Bile acid composition of rainbow trout, Salmo gairdneri. Lipids, 1974, 9: 945-951 DOI:10.1007/BF02533816 |
FOUNTOULAKI E, ALEXIS M N, NENGAS I, et al. Effect of diet composition on nutrient digestibility and digestive enzyme levels of gilthead sea bream (Sparus aurata L.). Aquaculture Research, 2005, 36: 1243-1251 DOI:10.1111/j.1365-2109.2005.01232.x |
FU X H, SUN M, SUN S C. Activity of digestive enzymes in Scophthalmus maximus. Journal of Fishery Sciences of China, 2005, 12(1): 26-32 [付新华, 孙谧, 孙世春. 大菱鲆消化酶的活力. 中国水产科学, 2005, 12(1): 26-32 DOI:10.3321/j.issn:1005-8737.2005.01.006] |
HUANG J, XIONG B X, CHEN J, et al. Activity and distribution of digestigestive enzymes for paddlefish (Polyodon spathula). Journal of Huazhong Agricultural University, 2013, 32(1): 110-115 [黄瑾, 熊邦喜, 陈洁, 等. 匙吻鲟的消化酶分布及其活性. 华中农业大学学报, 2013, 32(1): 110-115 DOI:10.3969/j.issn.1000-2421.2013.01.021] |
IIJIMA N, TANAKA S, OTA Y. Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiology and Biochemistry, 1998, 18: 59-69 DOI:10.1023/A:1007725513389 |
KAPOOR B G, SMITH H, VERIGHINA I A. The alimentary canal and digestion in teleosts. Advances in Marine Biology, 1975, 13: 109-239 |
KOFUJI P Y M, AKIMOTO A, HOSOKAWA H, et al. Seasonal changes in proteolytic enzymes of yellowtail Seriola quinqueradiata (Temminck & Schlegel; Carangidae) fed extruded diets containing different protein and energy levels. Aquaculture and Fisheries Management, 2005, 36(7): 696-703 |
LI J Y, SUN X Q, ZHENG F R, et al. Histochemical localization and characterization of AKP, ACP, NSE, and POD from cultured Apostichopus japonicus. Chinese Journal of Oceanology and Limnology, 2009, 27(3): 550-554 DOI:10.1007/s00343-009-9125-z |
LI J, HE R G, WANG X D. Comparative studies on digestive enzymatic activities of the four alimentary organs in Acipenser sinensis. Fisheries Science Technology Information, 2001(3): 99-102 [李瑾, 何瑞国, 王学东. 中华鲟消化酶活性分布的研究. 水产科技情报, 2001(3): 99-102 DOI:10.3969/j.issn.1001-1994.2001.03.010] |
LI R, XU Y J, LIU X Z, et al. Morphometric analysis and internal anatomy of yellowtail kingfish (Seriola aureovittata). Progress in Fishery Sciences, 2017, 38(1): 142-149 [李荣, 徐永江, 柳学周, 等. 黄条  (Seriola aureovittata)形态度量与内部结构特征. 渔业科学进展, 2017, 38(1): 142-149] (Seriola aureovittata)形态度量与内部结构特征. 渔业科学进展, 2017, 38(1): 142-149] |
LIU H M. Study on the histology and enzyme in the digestive tract of Ophicephalus argus. Master′s Thesis of Ocean University of China, 2006, 28-29 [刘红梅. 乌鳢消化系统组织学及消化酶的研究. 中国海洋大学硕士研究生学位论文, 2006, 28-29] |
LIU W, CHEN Q H, TANG F J, et al. Comparison of activities of digestive enzymes among four culter species in Xingkai Lake. Chinese Journal of Fisheries, 2017, 30(5): 23-27 [刘伟, 陈清华, 唐富江, 等. 兴凯湖四种野生鲌消化酶活性比较研究. 水产学杂志, 2017, 30(5): 23-27 DOI:10.3969/j.issn.1005-3832.2017.05.005] |
LIU X Z, XU Y J, LI R, et al. Analysis and evaluation of nutritional composition of the muscle of yellowtail kingfish (Seriola aureovittata). Progress in Fishery Sciences, 2017, 38(1): 128-135 [柳学周, 徐永江, 李荣, 等. 黄条  (Seriola aureovittata)肌肉营养组成分析与评价. 渔业科学进展, 2017, 38(1): 128-135] (Seriola aureovittata)肌肉营养组成分析与评价. 渔业科学进展, 2017, 38(1): 128-135] |
MANKURA M, KAYAMA M, SAITO S. Wax ester hydrolysis by lipolytic enzymes in pyloric ceca of various fishes. Bulletin of the Japanese Society of Scientific Fisheries, 1984, 50(12): 2127-2131 DOI:10.2331/suisan.50.2127 |
MIEGEL R P, PAIN S J, VAN WETTERE W H E J, et al. Effect of water temperature on gut transit time, digestive enzyme activity and nutrient digestibility in yellowtail kingfish (Seriola lalandi). Aquaculture, 2010, 308(3/4): 145-151 |
NI S W, GUI M Y, LIU H L. Investigation on the comparison of protease activities in grass carp, common carp, silver carp, big head carp and tilapla nilotica. Acta Zoologica Sinica, 1993, 39(2): 160-168 [倪寿文, 桂明远, 刘焕亮. 草鱼、鲤、鲢、鳙和尼罗非鱼肝胰脏和肠道蛋白酶活性的初步探讨. 动物学报, 1993, 39(2): 160-168 DOI:10.3321/j.issn:0001-7302.1993.02.015] |
NORTY M, LEARN W S. Digestive enzymes study. Journal of Japan´ s Fisheries Society, 1960, 26(7): 685-690 [北御门, 学野新光. 消化酶素研究. 日本水产学会志, 1960, 26(7): 685-690] |
OU Y J, LUO Q, LI J E. Distribution and cryopreservation of alkaline phosphatase (AKP) and acid phosphatase (ACP) in Trachinotus ovatus. South China Fisheries Science, 2011, 7(2): 49-54 [区又君, 罗奇, 李加儿. 卵形鲳鲹碱性磷酸酶和酸性磷酸酶的分布及其低温保存. 南方水产科学, 2011, 7(2): 49-54 DOI:10.3969/j.issn.2095-0780.2011.02.008] |
SONG B L, CHEN G, YE F L, et al. Relation between lipase activities and environmental factors in the young fish of Rachycentron canadum. Journal of Jinan University (Natural Science), 2007, 8(5): 531-536 [宋波澜, 陈刚, 叶富良, 等. 军曹鱼幼鱼脂肪酶的活力与环境因子的关系. 暨南大学学报(自然科学版), 2007, 8(5): 531-536] |
TAN B P. Studies on protease activity of some predatory fishes in coastal area of Taihu Lake. Journal of Hubei Agricultural College, 1995, 15(2): 96-98 [谭北平. 太湖沿岸区几种肉食性鱼类蛋白酶活性的研究. 湖北农学院学报, 1995, 15(2): 96-98] |
TENGJAROENKUL B, SMITH B J, CACECI T, et al. Distribution of intestinal enzyme activities along the intestinal tract of cultured Nile tilapia, Oreochromis niloticus L. Aquaculture, 2000, 182: 317-327 DOI:10.1016/S0044-8486(99)00270-7 |
WANG B, SUN P X, DONG Z F. Biological characteristics and aquaculture of yellowtail kingfish (Seriola lalandi). Fishery Modernization, 2005(3): 18-20 [王波, 孙丕喜, 董振芳. 黄尾  的生物学特性与养殖. 渔业现代化, 2005(3): 18-20 DOI:10.3969/j.issn.1007-9580.2005.03.007] 的生物学特性与养殖. 渔业现代化, 2005(3): 18-20 DOI:10.3969/j.issn.1007-9580.2005.03.007] |
WANG H T. The research on immune system and disease resistance of flounder, Paralichthys olivaceus. Doctoral Dissertation of Institution Oceanology, Chinese Academy of Sciences, 2000, 70-80 [王宏田. 牙鲆免疫系统和抗病力的研究. 中国科学院海洋研究所博士研究生学位论文, 2000, 70-80] |
WANG R, ZHANG S C. Distribution of alkline phosphatase in amphioxus Branchiostoma belcheri tsingtaunese. Journal of Ocean University of Qingdao, 2001, 31(1): 81-85 [王锐, 张士璀. 碱性磷酸酶在文昌鱼体内的分布. 青岛海洋大学学报, 2001, 31(1): 81-85] |
WEI W, ZHANG H Y, SHI A J. The study of relationship between acid phosphatase activity and oyster defence. Acta Hydrobiologica Sinica, 2001, 25(4): 413-415 [魏炜, 张洪渊, 石安静. 育珠蚌酸性磷酸酶活力与免疫反应关系的研究. 水生生物学报, 2001, 25(4): 413-415] |
WU R X, GE W, HONG W S, et al. Digestive enzymes of adult mudskipper Boleophthalmus pectinirostris. Journal of Fishery Sciences of China, 2007, 14(1): 99-105 [吴仁协, 戈薇, 洪万树, 等. 大弹涂鱼成鱼消化酶活性的研究. 中国水产科学, 2007, 14(1): 99-105] |
YANG G Q, ZHANG Y, WANG N M, et al. Comparison on activities of phosphatase and alexin in the serum and tissue of three kinds of sturgeons. Journal of Fisheries Science, 2008, 21(1): 53-58 [杨贵强, 张颖, 王念民, 等. 三种鲟鱼血清及组织中磷酸酶和补体活性的比较. 水产学杂志, 2008, 21(1): 53-58] |
YU T, SHI L C, QU D F, et al. Activities of digestive enzyme in Pseudobagrus fulvidraco. Journal of Jilin Agricultural University, 2002, 24(5): 92-94 [余涛, 施立才, 曲东风, 等. 黄颡鱼消化酶活性的初步研究. 吉林农业大学学报, 2002, 24(5): 92-94] |
ZHAI Z H. Cell biology. Beijing: Higher Education Press, 1995: 141-142 [翟中和. 细胞生物学. 北京: 高等教育出版社, 1995: 141-142]
|
ZHANG L Z, CHEN N N, ZHANG T, et al. The activity and distribution of digestive enzymes in 2-year-old Acipenser baerii. Marine Fisheries, 2016, 38(2): 166-173 [章龙珍, 陈宁宁, 张涛, 等. 2龄西伯利亚鲟消化酶活性分布. 海洋渔业, 2016, 38(2): 166-173] |
ZHANG Z R, LIU X Z, YU Y, et al. The variations of digestive enzymes in larval and juvenile Seriola aureovittata. Progress in Fishery Sciences, 2020, 41(2): 61-68 [张正荣, 柳学周, 于毅, 等. 黄条  仔稚幼鱼消化酶活性变化研究. 渔业科学进展, 2020, 41(2): 61-68] 仔稚幼鱼消化酶活性变化研究. 渔业科学进展, 2020, 41(2): 61-68] |
ZHENG J S, FENG X Y. Distribution of intestinal enzymes in digestive tract of tract of Sebastes schlegeli. Journal of Fishery Sciences of China, 2002, 9(4): 309-314 [郑家声, 冯晓燕. 许氏平  . 中国水产科学, 2002, 9(4): 309-314] . 中国水产科学, 2002, 9(4): 309-314] |
ZHOU J X, ZHANG D M, HUANG Q, et al. Studies on the activities of amylase of walleye (Stizosedion vitreum). Journal of Jilin Agricultural University, 2000, 22(3): 107-110 [周景祥, 张东鸣, 黄权, 等. 大眼  鲈蛋白酶活性的研究. 吉林农业大学学报, 2000, 22(3): 107-110] 鲈蛋白酶活性的研究. 吉林农业大学学报, 2000, 22(3): 107-110] |
ZHUANG P, ZHANG L Z, TIAN H J, et al. Effects of salinity on digestive enzyme activities of juvenile Acipenser schrenckii. Journal of Fishery Sciences of China, 2008, 2: 198-203 [庄平, 章龙珍, 田宏杰, 等. 盐度对施氏鲟幼鱼消化酶活力的影响. 中国水产科学, 2008, 2: 198-203] |



