2. 湖南师范大学 省部共建淡水鱼类发育生物学国家重点实验室 湖南 长沙 410081
2. State Key Laboratory of Developmental Biology of Freshwater Fish, Hunan Normal University, Changsha, Hunan 410081, China
鱼类对食物的消化和吸收与消化道密切相关。研究鱼体消化系统发育过程的形态结构与组织特征,有助于深入了解鱼类对摄食饵料的选择、分解、消化和吸收的内在机制;同时,可作为检测鱼类生长发育过程中消化能力和营养需求变化的技术手段,对鱼苗培育和养殖条件的优化具有重要的意义(Lipscomb et al, 2020; 张哲等, 2021)。近年来,国内外学者已对蓝鳍金枪鱼(Thunnus thynnus)(Yúfera et al, 2014)、条石鲷(Oplegnathus fasciantus)(区又君等, 2015)、大西洋白姑鱼(Argyrosomus regius)(Solovyev et al, 2016)、四指马鲅(Eleutheronema tetradactylum)(谢木娇等, 2017)、美洲黑石斑鱼(Centropristis striata)(张廷廷等, 2017)、褐菖鲉(Sebastiscus marmoratus)(杨佳喆等, 2019)等多种肉食性鱼类消化系统的形态和组织学进行了研究,为这些品种的苗种培育、仔稚鱼的饵料开发提供了有益的数据。
大口黑鲈(Micropterus salmoides)为引进鱼类,现已成为中国重要的特色淡水养殖品种之一。近年来,大口黑鲈的养殖方式由传统的投喂冰鲜野杂鱼发展到全程投喂人工配合饲料。然而,大口黑鲈仔鱼在转食人工配合饲料阶段极易出现消化及病原性疾病等问题,造成鱼苗大量死亡。目前,对大口黑鲈消化系统结构和功能的相关研究,主要集中在养殖方式、替代性饲料等对大口黑鲈消化道结构、消化酶活性以及肠道微生物群落组成等方面的影响(Li et al, 2020; Ma et al, 2020; Lin et al, 2020; Yang et al, 2020; 欧红霞等, 2020; 谢苏明等, 2021; Yin et al, 2021),未见大口黑鲈仔鱼消化系统的发育及其与转食人工配合饲料相关性的研究。本研究通过连续组织切片和扫描电镜技术,对开口摄食和转食人工配合饲料过程中大口黑鲈仔鱼的消化系统进行了组织学观察,并对部分死亡仔鱼的肠胃组织结构进行分析,以期为大口黑鲈幼苗培育条件的优化提供参考。
1 材料与方法 1.1 实验鱼及培育大口黑鲈受精卵取自广东佛山新荣水产有限公司。亲鱼(2+龄)经强化培育后,自然产卵于棕片上,布满受精卵的棕片运回实验室,置于约10 m3的室内水泥池中,微流水充气孵化。孵化用水的溶解氧(DO) > 6.0 mg/L,水温为(23±1)℃,pH为6.5~7.5。大口黑鲈仔鱼孵化出膜4 d平游后,转入体积约为1 m3的蓝桶中继续培育,每个桶中放入仔鱼约10 000尾,一共12个桶。以丰年虫(Artemia salina)作为开口饵料,每日投喂4次,并随着鱼苗的生长,逐渐调整饵料投喂量。每日上午吸污,换掉1/4水,清除排泄物与残饵。
1.2 仔鱼开口和转食人工配合饲料过程中成活率的统计设置3个时间点(12、16和20日龄)对大口黑鲈仔鱼进行转食人工配合饲料(粉料),每个时间点设置4个重复(即4个桶)。在转食粉料过程中,逐渐减少丰年虫的投喂量,并增大饲料粉投喂量。每日投喂3次,转食4 d后,全程投喂人工配合饲料。转食过程中,每日换掉1/4水,并分别于上午和下午吸污,清除排泄物与残料。30日龄时,统计各组实验鱼的成活率(Survival rate, SR, %),同时,对部分实验鱼的全长和体重进行测量。SR计算公式:
| $ \text{SR}=最终存活仔鱼数目/起始仔鱼数目×100\% $ |
全长测量:每桶实验鱼随机选取200尾,使用千分尺逐条测量。体重测量:测量全长后的实验鱼每10尾为1组,共20组,称量后计算每尾仔鱼的平均体重。
1.3 样品固定及组织切片的制作大口黑鲈仔鱼孵化出膜后第2天开始取样,20日龄前连续取样,20日龄后隔天取样。每次取样20尾,持续30 d。部分出现“拖便”以及即将死亡或刚死亡的仔鱼个体则单独取样。样品先经Bouin's液固定24 h以上,再采用70%乙醇小心清洗样品后,置于常温条件下保存。所有样品经80%、90%、100%酒精脱水,二甲苯透明,石蜡包埋处理后,再使用RM2135型石蜡切片机(Leica,德国)进行连续切片(厚度为5 μm)。染色前,石蜡切片放入42℃恒温箱中烘片3 h,随后放入二甲苯中脱蜡2 h。脱蜡后,切片依次经过100%、90%、80%、70%酒精覆水(各5 min),苏木精染色10 min,流水冲洗30 s,伊红染色30 s,流水冲洗30 s,随后经90%、100%酒精及二甲苯脱水,最后采用中性树胶封片。组织切片使用Nikon显微镜(日本)拍照。
1.4 扫描电镜样本制作剪取30日龄仔鱼的胃、幽门盲囊和肠道组织,分别放入预冷的2.5%的戊二醛溶液中固定12 h。使用0.01 mol/L磷酸盐缓冲液(PBS)漂洗组织3次,每次10 min,随后在1%的锇酸溶液中固定2 h,PBS缓冲液冲洗3次。梯度酒精(70%~100%)脱水,叔丁醇浸泡2 h,冷冻干燥仪干燥,离子溅射镀金,最后采用HITACHIX- 650 (日本)扫描电镜拍照观察。
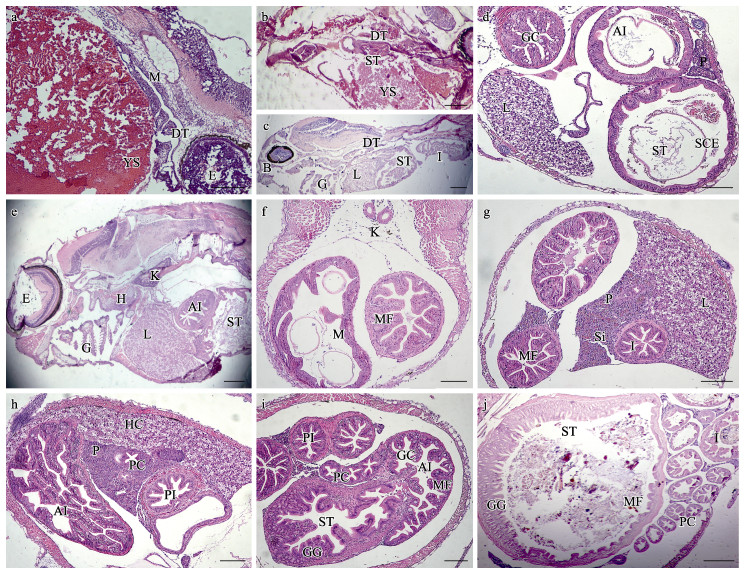
2 结果 2.1 大口黑鲈消化道系统发育过程的组织学特征大口黑鲈仔鱼孵出2 d后,消化道初步分化,消化腔和消化道上皮黏膜出现(图 1a)。4 d后,仔鱼可平游,卵黄囊和油球显著减小,口裂、食道扩大,胃组织出现,肛门与外界连通,此时,仔鱼具备了基本的摄食能力,开始进入混合营养期(图 1b)。6 d后,卵黄囊和油球基本消失,消化道逐渐分化,形成了口腔咽、胃、肠道、消化腺(图 1c);胃黏膜上皮细胞逐渐分化,分为黏膜层和外膜,出现黏膜褶,无杯状细胞;前肠上皮单层柱状细胞排列紧密,黏膜褶逐渐形成,后肠道黏膜褶排列紧密,分布密集杯状细胞;卵黄囊吸收后产生的空间主要被肝胰组织填充;肝脏细胞区域增大,胰脏出现,肝脏和胰脏独立,此时,仔鱼开始进入外源性营养期(图 1d)。
|
图 1 大口黑鲈仔鱼消化道系统发育的组织学观察(标尺=200 μm)
Fig.1 Histological observation of the development of digestive system of larval M. salmoides (Bar = 200 μm)
a:2 d仔鱼纵切;b:4 d仔鱼纵切;c:6 d仔鱼纵切;d:6 d仔鱼腹部横切;e:10 d仔鱼纵切;f:12 d仔鱼腹部横切;g:16 d仔鱼腹部横切;h:20 d仔鱼腹部横切;i:24 d仔鱼腹部横切;j:30 d仔鱼腹部横切 AI:前肠;B:口咽腔;DT:消化道;E:眼;H:心脏;G:鳃;GC:杯状细胞;GG:胃腺;HC:肝细胞;I:肠;IL:胰岛;K:肾;L:肝;M:黏膜;MF:黏膜褶;P:胰脏;PC:幽门盲囊;PI:后肠;SCE:单层柱状细胞;Si:血窦;ST:胃;YS:卵黄囊。 a: Longitudinal section of 2 day-post-hatching (dph) larval fish; b: Longitudinal section of 4 dph larval fish; c: Longitudinal section of 6 dph larval fish; d: Transverse section of 6 dph larval fish abdomen; e: Longitudinal section of 10 dph larval fish; f: Transverse section of 12 dph larval fish abdomen; g: Transverse section of 16 dph larval fish abdomen; h: Transverse section of 20 dph larval fish abdomen; i: Transverse section of 24 dph larval fish abdomen; j: Transverse section of 30 dph larval fish abdomen AI: Anterior intestine; B: Buccopharyngeal cavity; DT: Digestion tract; E: Eye; H: Heart; G: Gill; GC: Ggoblet cell; GGC: Gastric gland cell; HC: Hepatic cell; I: Intestine; IL: Islet; K: Kidney; L: Liver; M: Mucosa; MF: Mucosal fold; P: Pancreas; PC: Pyloric caecum; PI: Posterior intestine; SCE: Simple columnar epithelium; Si: Sinusoid; ST: Stomach; YS: Yolk sac. |
10日龄仔鱼的消化道已基本形成,肝脏、胰脏、胃和肠道紧密排列(图 1e)。12日龄仔鱼胃腔逐渐增大,胃和肠道黏膜褶皱逐渐增长,数量逐渐增多,仔鱼完全进入外源性营养期(图 1f)。16日龄仔鱼的肠道组织结构逐渐分明,自肠腔表面向里依次为黏膜层、黏膜下层、肌层与浆膜层,黏膜柱状上皮细胞体积增大、排列紧密,黏膜褶充满整个肠腔,杯状细胞明显增多;肝脏和胰脏细胞团显著增大,肝细胞团出现空泡结构;胰腺细胞排列紧密,血细胞与胰管分布于胰腺细胞之间,胰腺细胞逐渐向肠的方向延伸,可见酶原颗粒,仔鱼摄食能力进一步完善(图 1g)。
20日龄仔鱼肠道黏膜褶皱继续增加、伸长,卷曲程度趋于复杂,出现次级黏膜褶,黏膜褶充满整个肠腔,肠黏膜褶皱初具成体特性;幽门盲囊口径较细,与肠道组织结构非常类似,但黏膜褶较短,且数目稀少(多为4个黏膜褶);肝脏和胰脏细胞区域继续增大,肝脏细胞核规则,窦状隙减少,细胞间分布有大量脂肪小颗粒,肝脏组织结构近似成鱼,仔鱼完全具备了转食人工配合饲料的能力(图 1h)。
24日龄仔鱼胃部结构分明,胃壁增厚,胃腺数量增多,黏膜褶和黏膜下层结缔组织进一步分化,纵向黏膜褶皱增高、卷曲;前肠黏膜褶进一步复杂卷曲,前肠口径显著大于后肠,后肠黏膜褶紧密排列,肠黏膜褶柱状细胞清晰,分布大量杯状细胞,幽门盲囊紧贴胃部,纵向黏膜褶逐渐增大、增长,胃、肠道和幽门盲囊的结构趋于完整,近似成鱼(图 1i)。30日龄仔鱼胃腔继续增大,胃腺发达,固有层胃腺数量明显增多,黏膜褶不断分化增长;幽门盲囊形态结构与肠道相似,肠壁增厚,黏膜褶不断增多、增长,结构进一步发育完整(图 1j)。
对30日龄摄食正常的大口黑鲈仔鱼的胃、肠和幽门盲囊的内表皮进行扫描电镜观察,发现胃部内表皮呈相对规则的网状多边形状,有明显的孔洞间隙(图 2A),黏膜褶呈网状,黏膜表面多处凹陷,即胃小凹,胃小凹内分布密集的分泌孔,分泌孔径小而深(图 2B)。肠道内表面具有无固定形态的黏膜褶,黏膜褶长短不一,部分呈弯曲状,黏膜褶之间分布许多巨大的分泌孔,黏膜褶上分布大量的黏液细胞,黏液细胞周围可见分泌颗粒(图 2C和图 2D)。幽门盲囊内表面结构与肠道非常相似,内表面无成型的黏膜褶,黏膜褶之间充满了大小不一的分泌颗粒,部分黏膜褶上可见密集排列的黏液细胞(图 2E和图 2F)。
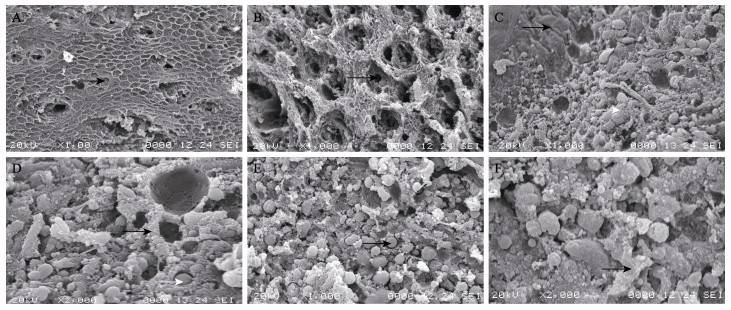
|
图 2 大口黑鲈30日仔鱼胃、中肠和幽门盲囊内表面扫描电镜观察 Fig.2 Scanning electron microscopy observation on the mucosal epithelium of the digestive tract in M. salmoides A:胃黏膜上皮及胃部黏膜褶(黑色箭头)×100;B:胃部胃小凹及分泌孔(黑色箭头)×1000;C:肠上表皮黏膜褶(黑色箭头)及分泌孔(白色箭头)×1000;D:肠上表皮黏液细胞(黑色箭头)及分泌颗粒(白色箭头)×2000;E: 幽门盲囊上皮细胞分泌孔和分泌颗粒(黑色箭头)×1000;F: 幽门盲囊上表皮黏液细胞(黑色箭头)×2000 A: Stomach mucosal epithelium and mucosal fold (black arrow) ×100; B: Gastric pit and secretory cell (black arrow) ×1000; C: Intestine mucosal fold (black arrow) and secretory cell (white arrow) ×1000; D: Intestine mucous cells (black arrow) and secretory granules (white arrow) ×2000; E: Pyloric caecum secretory cell and secretory granules (black arrow) ×1000; F: Pyloric caecum mucous cells (black arrow) ×2000 |
对大口黑鲈仔鱼开口摄食和转食饲料过程中,部分死亡个体的胃、肠道组织学进行了观察,结果见图 3。从图 3可以看出,与6日龄开口摄食丰年虫活饵的仔鱼相比(图 3C、D),死亡个体的胃部容积小,可见大量胃腺细胞和胃小凹,无成型的黏膜褶,胃内充满未消化的食物残渣,肠道黏膜褶短、数量少,杯状细胞数目稀少,胃和肠道发育不完善(图 3A)。与12~16日龄摄食丰年虫活饵的仔鱼相比(图 3F、G),死亡个体胃腔显著扩大,无成型的黏膜褶,胃腺细胞稀少,黏膜层脱落,胃腔中充满了大量未消化的食物残渣;肠道黏膜褶数目少,部分断裂脱落,可见少量食物残渣,摄食过多导致胃肿大损伤(图 3B、C)。
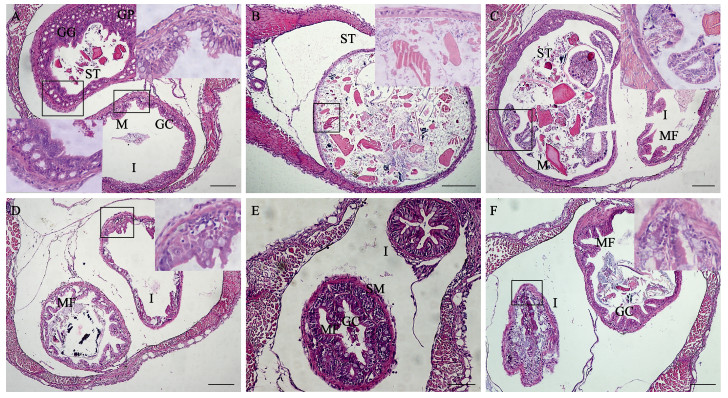
|
图 3 大口黑鲈仔鱼摄食过程中死亡个体的胃、肠组织学观察(标尺= 200 μm) Fig.3 Histological observation of stomach and intestine of the death larval M. salmoides (Bar = 200 μm) A:6日龄仔鱼腹部横切;B:10日龄仔鱼腹部横切;C:12日龄仔鱼腹部横切;D:16日龄仔鱼腹部横切;E:20日龄仔鱼腹部横切;F:24日龄仔鱼腹部横切。右上角图为黑色方框中的局部放大图。GC:杯状细胞;GG:胃腺;GP:胃小凹;I:肠;MF:黏膜褶;MS:肌肉层;ST:胃 A: Transverse section of 6 dph larval fish abdomen; B: Transverse section of 10 dph larval fish abdomen; C: Transverse section of 12 dph larval fish abdomen; D: Transverse section of 16 dph larval fish abdomen; E: Transverse section of 20 dph larval fish abdomen; F: Transverse section of 24 dph larval fish abdomen. The upper-right corner is a locally enlarged image of the black box; GC: Goblet cell; GGC: Gastric gland cell; GP: Gastric pit; I: Intestine; MF: Mucosal fold; MS: Muscle layer; ST: Stomach |
与20日龄摄食饲料粉料的仔鱼相比(图 1I),死亡个体胃腔正常,可见成型的胃部黏膜褶和胃小凹及少量食物颗粒,而前肠肠腔扩大,黏膜下层增厚,单层柱状上皮细胞排列稀疏,黏膜褶短,数量稀少;或肠道内表皮无成型的黏膜褶,无杯状细胞,黏膜褶断裂,黏膜层脱落,无食物残渣,肠道组织损伤(图 3D、E)。与24日龄摄食饲料粉料的仔鱼相比(图 1j),胃部内表皮可见部分明显的黏膜褶和胃小凹,但黏膜褶数量少,少量断裂脱落,肠道皱缩,无成型的黏膜褶和杯状细胞,肠腔中大量食物残渣未能排出体外,胃和肠道组织均有损伤(图 3F)。
2.3 大口黑鲈仔鱼不同时间点转食人工配合饲料后的存活率比较对大口黑鲈仔鱼在不同时间点转食人工配合饲料后30日龄的体长、体重以及SR进行了统计,结果见表 1。从表 1可以看出,在不同时间点转食人工配合饲料后,大口黑鲈仔鱼的体长、体重均无显着差异(P > 0.05),且12日龄仔鱼(45.59%)的SR低于16日龄(60.60%)和20日龄(69.83%)。此外,大口黑鲈仔鱼的体长、体重及SR均与转食人工配合饲料的时间成正相关,3组实验鱼的体长、体重及SR大小为12日龄 < 16日龄 < 20日龄。
|
|
表 1 大口黑鲈仔鱼不同时间点转食人工配合饲料后30日龄的生长及成活率比较 Tab.1 Comparison of growth level and survival rate of 30 dph larval M. salmoides after transformed artificial diet at different time points |
硬骨鱼类的消化系统早期发育通常可分为内源性营养期、内源和外源混合性营养期、外源性营养期3个阶段(Faccioli et al, 2014; Engrola et al, 2018)。大口黑鲈仔鱼消化系统的发育亦具有上述阶段性特征。0~4日龄仔鱼主要依靠吸收卵黄囊营养进行胚后发育,为内源性营养期;4~6日龄仔鱼的消化道初步分化,具备基本摄食能力,进入混合营养期。此时,应该及时并多次提供饵料诱导仔鱼开口。6日龄后,大口黑鲈仔鱼的卵黄囊消失,开始进入外源性营养期。这与草鱼(Ctenopharyngodon idellus)、蓝鳍金枪鱼、美洲黑石斑鱼等研究结果基本一致(阮国良等, 2012; Yúfera et al, 2014; 张廷廷等, 2017)。然而,不同鱼类早期发育阶段及其开口和摄食形成时间存在一定的差异。如大西洋白姑鱼(Solovyev et al, 2016)和褐菖鲉(杨佳喆等, 2019),在出膜后0~1 d为内源性营养期,2~5 d为混合营养期,6 d后为外源性营养期;哲罗鱼(Hucho taimen)出膜后0~10 d为内源性营养期,11~18 d为混合营养期,19 d后为外源性营养期(张永泉等, 2010)。这些差异与不同鱼类的遗传特性、卵质以及外界条件(水温)等因素密切相关。
胃内表皮的黏膜褶皱可以扩大消化道的贮存容积和消化面积,利于食物的充分消化和吸收,而胃腺的形成是仔鱼向成鱼过渡的标志(区又君等, 2015; Engrola et al, 2018)。6日龄大口黑鲈仔鱼胃腺出现,胃黏膜褶皱开始形成,出现胃小凹,表明此时仔鱼开始消化外源性营养物质。随着仔鱼的发育,胃部黏膜褶皱逐渐增加、延长,仔鱼消化与吸收外界营养物质的能力逐渐提升。20~24日龄仔鱼胃腺发达,黏膜纵向褶皱增高并复杂卷曲(图 1i和图 1h),表明20日龄后仔鱼胃腺消化能力完善。同时,本研究也观察到仔鱼在20日龄转食人工配合饲料后的SR较12、16日龄高(表 1)。30日龄仔鱼胃腺固有层增多,黏膜褶密集、增长,胃小凹间分布丰富的分泌孔,表明仔鱼在摄食人工配合饲料后可能促进了胃腺、黏膜褶的发育和完善。大口黑鲈仔鱼胃黏膜褶的出现及胃腺形成时间与鲈形目(Perciformes)黑鲷(Sparus macrocephalus)(23日龄)、大黄鱼(Larimichthys crocea) (21日龄)等大致相同,晚于鲇形目(Siluriformes)的鲇(Silurus asotus) (5日龄)、黄颡鱼(Pelteobagrus fulvidraco) (3日龄),早于鲽形目(Pleuronectiformes)大西洋牙鲆(Paralichthy sdentatus) (31日龄)、黄尾鲽(Limanda ferruginea) (36日龄)等鱼类,这可能与鱼类进化和分类地位相关(杨瑞斌等, 2009)。
鱼类肠道上皮的杯状细胞能分泌中性和酸性黏液,在乳化食物、润滑消化道、保护表面黏膜以及防御外界细菌等多个方面具有重要作用(Faccioli et al, 2016; Yúfera et al, 2018; 赵柳兰等, 2018)。6日龄大口黑鲈仔鱼肠道黏膜褶和杯状细胞出现,20日龄后,仔鱼肠道黏膜褶卷曲复杂,并出现了次级黏膜褶,杯状细胞数量显著增加,这有利于仔鱼开口摄食以及摄食人工配合饲料后食物的有效吞咽、消化产物的顺利运输以及排便(Hachero-Cruzado et al, 2009; Comabella et al, 2013)。Faccioli等(2014)和赵柳兰等(2018)研究表明,黏液细胞是一种多功能细胞,具有协调食物消化、润滑和排出的作用。大口黑鲈仔鱼幽门盲囊与肠道具有相似的结构,肠道和幽门盲囊黏膜褶具有丰富的黏液细胞和分泌孔(图 2)。表明20~30日龄大口黑鲈仔鱼已具备摄食人工配合饲料的能力。
鱼类的肝胰脏承担着体内代谢、排泄和解毒等重要生理功能。4~6日龄大口黑鲈仔鱼肝胰脏细胞团出现,并且肝脏和胰脏发育为独立器官,这与在褐菖鲉(杨佳喆等, 2019)、大鳞副泥鳅(Paramisgurnus dabryanus) (刘亚秋等, 2016)等研究结果相一致,而与具有弥散性肝胰腺的卵形鲹鲳(Trachinotus ovatus) (区又君等, 2011)和宽口裂腹鱼(Schizothorax eurystomus) (魏杰等, 2020)等不同。仔鱼外源性摄食的发生, 主要依赖于胰腺分泌的消化酶。6日龄仔鱼肝脏和胰脏细胞团独立出现,进一步表明仔鱼进入外源性营养阶段。16日龄后,仔鱼肝脏区域出现脂肪沉积区,胰脏周围可见酶原分泌颗粒,表明肝脏和胰脏结构与功能日益完善,这为大口黑鲈仔鱼对糖原和脂肪的高效吸收和存储提供了重要保证,表明16日龄后仔鱼基本具备摄食人工配合饲料的能力(Yúfera et al, 2018)。
3.2 大口黑鲈早期阶段营养转换期存活率低的原因分析在人工育苗和养殖条件下,早期仔鱼的口裂形成阶段、后期仔鱼的饵料转换期、稚鱼快速生长期以及高密度养殖过程中都会出现一定的死亡率,这与鱼体的消化吸收不良、肠道炎症以及疾病等密切相关(Dadzie et al, 2000; 陈猛猛等, 2015)。大口黑鲈仔鱼在开口摄食和转食人工配合饲料过程中死亡率较高,严重影响着大口黑鲈苗种的培育和供应。本研究观察到部分6日龄仔鱼的胃肠道发育仍不完善(图 3A),仔鱼死亡可能与消化道发育缓慢,未及时诱导开口或开口过早导致仔鱼摄取过多的食物而不能正常消化且堵塞消化道有关。大口黑鲈仔鱼在10~12日龄完全转入外源性营养期,随着生长发育的进行,营养需求逐渐增多,摄食量不断增大,部分仔鱼摄食过多,导致胃腔和肠腔显著扩大,黏膜脱落(图 3B、C),摄入的食物可能未及时有效地消化吸收,造成饥饿性或炎症性死亡。这也可能是12日龄仔鱼转食人工配合饲料后SR相对较低的原因之一(表 1)。随着仔鱼进一步生长发育,投喂饵料难以满足大口黑鲈仔鱼的生长需求。但鱼类只有消化系统发育完善,才能完全适应喂养饵料的改变(Yúfera et al, 2007; Lipscomb et al, 2020)。16~20日龄大口黑鲈仔鱼消化道和消化腺发育完善,此阶段仔鱼更易适应摄食人工配合饲料。养殖实验进一步观察到仔鱼在16日龄和20日龄转食人工配合饲料的SR较12日龄时高(表 1)。同时,本研究表明,16~24日龄死亡个体仔鱼的胃部和肠道腔体皱缩、黏膜褶断裂、黏膜层脱落(图 3D~图 3F),这可能与饲料粉料不能有效地消化吸收和排出体外进而引发营养缺陷和肠道阻滞、炎症等所导致的死亡有关(赵柳兰等, 2018)。此外,导致大口黑鲈早期阶段营养转换期SR低的原因可能还与肠道稳态、肠道益生菌、肠道炎症以及细菌、病毒性疾病等众多因素相关,后续还需进一步研究。
4 结论本研究通过组织切片和扫描电镜技术观察大口黑鲈早期消化系统的发育。结果显示,大口黑鲈4~ 6日龄仔鱼仍处于混合性营养期,需少量、多次投喂适口饵料诱导开口;6~16日龄仔鱼进入外源性营养期,此时,消化系统发育基本完成,应提供充足的饵料;16~24日龄仔鱼的消化系统发育完善,为最佳转食人工配合饲料时间,可选择适口且营养丰富的人工配合粉料,并适当延长转食饲料的时间,以提高大口黑鲈仔鱼的育苗成活率。
CHEN M M, LUO J, CHEN G H, et al. Embryonic development and morphologic observations of newly-hatched Cheilinus undulatus larvae. Progress in Fishery Sciences, 2015, 36(5): 38-44 [陈猛猛, 骆剑, 陈国华, 等. 波纹唇鱼(Cheilinus undulatus)的胚胎发育及初孵仔鱼的形态观察. 渔业科学进展, 2015, 36(5): 38-44] |
COMABELLA Y, FRANYUTTI AH, HURTADO A, et al. Ontogenetic development of the digestive tract in Cuban gar (Atractosteus tristoechus) larvae. Reviews in Fish Biology and Fisheries, 2013, 23(2): 245-260 DOI:10.1007/s11160-012-9289-z |
DADZIE S, ABOU-SEEDO F, AL-SHALLAL T. Reproductive biology of the silver pomfret, Pampus argenteus (Euphrasen), in Kuwait waters. Journal of Applied Ichthyology, 2000, 16(6): 247-253 DOI:10.1046/j.1439-0426.2000.00237.x |
ENGROLA S, ARAGÃO C, VALENTE L M P, et al. Nutritional modulation of marine fish larvae performance. Emerging issues in fish larvae research. Springer, Cham, 2018, 209-228 |
FACCIOLI C K, CHEDID R A, DO-AMARAL A C, et al. Morphology and histochemistry of the digestive tract in carnivorous freshwater Hemisorubim platyrhynchos (Siluriformes: Pimelodidae). Micron, 2014, 64: 10-19 DOI:10.1016/j.micron.2014.03.011 |
HACHERO-CRUZADO I, ORTIZ-DELGADO J B, BORREGA B, et al. Larval organogenesis of flatfish brill Scophthalmus rhombus L: Histological and histochemical aspects. Aquaculture, 2009, 286(1/2): 138-149 |
LI S, CHI S Y, CHENG X, et al. Effects of antimicrobial peptides on the growth performance, antioxidant and intestinal function in juvenile largemouth bass, Micropterus salmoides. Aquaculture Reports, 2020, 16: 100252 DOI:10.1016/j.aqrep.2019.100252 |
LIN S M, ZHOU X M, ZHOU Y L, et al. Intestinal morphology, immunity and microbiota response to dietary fibers in largemouth bass, Micropterus salmoide. Fish and Shellfish Immunology, 2020, 103: 135-142 DOI:10.1016/j.fsi.2020.04.070 |
LIPSCOMB T N, YANONG R P, RAMEE S W, et al. Histological, histochemical and biochemical characterization of larval digestive system ontogeny in black tetra Gymnocorymbus ternetzi to inform aquaculture weaning protocols. Aquaculture, 2020, 520: 734957 DOI:10.1016/j.aquaculture.2020.734957 |
LIU Y Q, GAO S T, HU Y, et al. Histological observation on the post-embryonic development of the digestive system in Paramisgurnus dabryanus. Journal of Chongqing Normal University (Natural Science Edition), 2016, 33(3): 31-37 [刘亚秋, 高胜涛, 胡雨, 等. 大鳞副泥鳅消化系统胚后发育组织学观察. 重庆师范大学学报(自然科学版), 2016, 33(3): 31-37] |
MA D M, FAN J J, ZHU H P, et al. Histologic examination and transcriptome analysis uncovered liver damage in largemouth bass from formulated diets. Aquaculture, 2020, 526: 735329 DOI:10.1016/j.aquaculture.2020.735329 |
OU H X, WANG G J, LI Z F, et al. Influence of different diets on intestinal histological morphologic structure of largemouth bass Micropterus salmoides. Fisheries Science, 2020, 39(6): 902-907 [欧红霞, 王广军, 李志斐, 等. 不同饲料对大口黑鲈肠道组织结构的影响. 水产科学, 2020, 39(6): 902-907] |
OU Y J, LI J E, AI L. A study on the histology of digestive system in early life stages of Oplegnathus fasciantus. Journal of South China Agricultural University, 2015, 36(1): 23-27 [区又君, 李加儿, 艾丽. 条石鲷早期发育阶段消化系统组织学研究. 华南农业大学学报, 2015, 36(1): 23-27] |
RUAN G L, YANG D Q, WANG W M. Ontogeny of the digestive tracts in Grass carp (Ctenopharyngodon idellus), Yellowcheck carp (Elopichthys bambusa) and Topmouthculter (Culter alburnus). Acta Hydrobiologica Sinic, 2012, 36(6): 1164-1170 [阮国良, 杨代勤, 王卫民. 草鱼、鳡和翘嘴鲌消化道组织的早期发育. 水生生物学报, 2012, 36(6): 1164-1170] |
SOLOVYEV M M, CAMPOVERDE C, ÖZTÜRK S, et al. Morphological and functional description of the development of the digestive system in meagre (Argyrosomus regius): An integrative approach. Aquaculture, 2016, 464: 381-391 DOI:10.1016/j.aquaculture.2016.07.008 |
WEI J, CAO X Q, REN Y L, et al. Anatomy and histological observation of digestive system in Schizothorax eurystomus. South China Fisheries Science, 2020, 16(1): 120-126 [魏杰, 曹希全, 任永丽, 等. 宽口裂腹鱼消化系统解剖和组织学观察. 南方水产科学, 2020, 16(1): 120-126] |
XIE M J, OU Y J, LI J E, et al. Histological observation of the post-embryonic development of digestive tract of Eleutheronema tetradactylum. Progress in Fishery Sciences, 2017, 38(2): 50-58 [谢木娇, 区又君, 李加儿, 等. 四指马鲅(Eleutheronema tetradactylum)消化系统胚后发育组织学观察. 渔业科学进展, 2017, 38(2): 50-58] |
XIE S M, XU G C, WANG Y Y, et al. Effects of feeding frequency on digestive enzymes, histomorphology, and gene expression of lipid metabolic enzymes of largemouth bass (Micropterus salmoides) reared in in-pond raceway culture systems. Journal of Fishery Sciences of China, 2021, 28(2): 157-166 [谢苏明, 徐钢春, 王裕玉, 等. 投喂频率对池塘循环水养殖大口黑鲈消化酶、组织结构及脂代谢酶基因表达的影响. 中国水产科学, 2021, 28(2): 157-166] |
YANG J Z, QI C, XU S L. Histological studies on development of the digestive system in larval and juvenile Sebastiscus marmoratus. Journal of Tropical Oceanography, 2019, 38(2): 58-66 [杨佳喆, 齐闯, 徐善良. 褐菖鲉仔、稚鱼消化系统发育的组织学观察. 热带海洋学报, 2019, 38(2): 58-66] |
YANG P, YANG W, HE M, et al. Dietary synbiotics improved the growth, feed utilization and intestinal structure of largemouth bass (Micropterus salmoides) juvenile. Aquaculture Nutrition, 2020, 26(2): 590-600 |
YANG R B, XIE C X, FAN Q X, et al. Histological and ultrastructural studies of the stomach and intestine in larvae of yellowcatfish pelteobagrus fulvidraco. Acta Hydrobiologica Sinic, 2009, 33(6): 1068-1077 [杨瑞斌, 谢从新, 樊启学, 等. 黄颡鱼仔稚鱼胃肠发育的显微和超微结构研究. 水生生物学报, 2009, 33(6): 1068-1077] |
YIN P, XIE S, ZHUANG Z, et al. Dietary supplementation of bile acid attenuate adverse effects of high-fat diet on growth performance, antioxidant ability, lipid accumulation and intestinal health in juvenile largemouth bass (Micropterus salmoides). Aquaculture, 2021, 531: 735864 |
YÚFERA M, DARIAS M J. The onset of exogenous feeding in marine fish larvae. Aquaculture, 2007, 268(1/2/3/4): 53-63 |
YÚFERA M, MOYANO F J, MARTÍNEZ R G. The digestive function in developing fish larvae and fry. From molecular gene expression to enzymatic activity. Emerging issues in fish larvae research. Springer, Cham, 2018: 51–86
|
YÚFERA M, ORTIZ-DELGADO J B, HOFFMAN T, et al. Organogenesis of digestive system, visual system and other structures in Atlantic bluefin tuna (Thunnus thynnus) larvae reared with copepods in mesocosm system. Aquaculture, 2014, 426/427(S): 126-137 |
ZHANG T T, CHEN C, SHAO Y X, et al. Histological observation on post-embryonic development of digestive system of Centropristis striata. Progress in Fishery Science, 2017, 38(3): 78-85 [张廷廷, 陈超, 邵彦翔, 等. 美洲黑石斑鱼(Centropristis striata)消化系统胚后发育的组织学观察. 渔业科学进展, 2017, 38(3): 78-85] |
ZHANG Y Q, LIU Y, YIN J S, et al. Morphology and histology of post-embryonic digestive system of Hucho taimen. Oceanologia et Limnologia Sinica, 2010, 41(3): 422-428 [张永泉, 刘奕, 尹家胜, 等. 哲罗鱼(Hucho tamen)消化系统胚后发育的形态与组织学的研究. 海洋与湖沼, 2010, 41(3): 422-428] |
ZHANG Z, JIANG L L, WANG Z, et al. Growth, development, and feeding characteristics of Thamnaconus modestus during early stages. Marine Science, 2021, 46(1): 1-13 [张哲, 姜良龙, 王臻, 等. 绿鳍马面鲀早期生长发育与摄食特性的研究. 海洋科学, 2021, 46(1): 1-13] |
ZHAO L L, CHEN J L, YANG S, et al. Analysis of the histological structure, the types and distribution of mucous cells of digestive tract in largemouth bass (Micropterus Samoides). Journal of Sichuan Agricultural University, 2018, 549-554 [赵柳兰, 陈侨兰, 杨淞, 等. 大口黑鲈消化道组织结构及黏液细胞的类型和分布. 四川农业大学学报, 2018, 549-554] |



