2. 青岛海洋科学与技术试点国家实验室海洋渔业科学与食物产出过程功能实验室 山东 青岛 266071;
3. 水产科学国家级实验教学示范中心 上海海洋大学 上海 201306;
4. 中国水产科学研究院长岛增殖实验站 山东 烟台 265800
2. Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, Shandong 266071, China;
3. National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai, 201306;
4. Long Island Breeding Experimental Station, Chinese Academy of Fishery Sciences, Yantai, Shandong 265800, China
糖原是牡蛎体内最直接有效的储能物质,也是影响牡蛎肉质肥美的主要呈味物质之一,其含量的高低直接影响到牡蛎的风味和营养品质,常作为牡蛎肉质性状重要的评判标准。与其他多数双壳贝类相比,牡蛎拥有较高的糖原成分(Beltrán-Lugo et al, 2006; Martínez-Pita et al, 2012; Chávez-Villalba et al, 2013; Anacleto et al, 2013; Vodáková et al, 2019),含量约占其干重的20%~40% (庾晋等, 2002)。2020年中华人民共和国农业农村部公告第324号公布的长牡蛎(Crassostrea gigas)“鲁益1号”和长牡蛎“海蛎1号”2个水产新品种均以高糖原为主要优势性状,高糖原性状成为牡蛎品质性状改良的重点(全国水产技术推广总站, 2020)。
高效、快速、高通量的糖原含量测定方法可为高糖原贝类新种质的培育提供技术支撑。目前,糖原含量的检测方法多以传统化学检测和试剂盒为主(杨柳等, 2018),虽然这些技术方法成熟,但耗时耗力,成本较高,会产生大量化学废液,不适于大批量糖原含量的快速、批量测定。近红外光谱(Near Infrared, NIR)技术作为现代常见的一种快速高效分析技术,可通过近红外光谱仪记录主要含氢基团的倍频和合频吸收(于征等, 2012),具有适用范围广、快速高效、便捷准确、对样品无破损等诸多优点(曲艺伟等, 2019),在水产科学研究领域应用广泛,尤其是在品质元素的检测研究上。例如,2008年建立的大西洋鲑(Salmo salar)脂肪和色素含量NIR分析模型,实现了脂肪和色素含量在鲑鱼肉质中的快速无损伤检测(Folkestad et al, 2008)。Fluckiger等(2011)利用NIR技术建立了活鲍(Abalone)、去壳新鲜鲍和冻干腹足肌样品糖原定量模型,完成了糖原含量绿色高效测定。此后,Miller等(2019)建立了新西兰绿唇贻贝(Perna canaliculus)以及大鳞大马哈鱼(Oncorhynchus tshawytscha)中蛋白质、水分、脂肪、灰分和碳水化合物等成分的NIR定量模型,进一步实现了多种成分的同步高效检测。在牡蛎研究中,已建立长牡蛎、葡萄牙牡蛎(C. angulata)、悉尼岩牡蛎(Saccostrea glomerata)和美洲牡蛎(C. virginica)水分、脂肪、糖原、总蛋白质、氨基酸、牛磺酸、Zn、Se和Ca等成分的NIR定量模型,且模型经验证均具有较高的准确度(王卫军等, 2015; 于颖等, 2016; 黄冠明等, 2020; Brown, 2011; Brown et al, 2012; Guévélou et al, 2016)。其中,NIR分析技术已成功应用到了新品种长牡蛎“鲁益1号”的选育中,大大提高了牡蛎的育种效率。可见NIR测定技术有效克服了化学检测方法的费时费力、成本高等问题,对高品质性状牡蛎的选育具有重要意义。
近江牡蛎(C. ariakensis),又称“赤蚝”,为广温、广盐性的巨牡蛎属贝类,是我国重要的经济养殖种和生态种(Wang et al, 2008)。目前,已有的研究主要集中在分子标记开发、群体遗传等方面(Huvet et al, 2008; Guo et al, 2012; Cong et al, 2013、2014; Zhong et al, 2014; She et al, 2015; 李春燕, 2017; 赵庆等, 2019; 周丽青等, 2020),糖原含量的快速检测方法尚未建立。本研究以近江牡蛎6种冻干组织为建模对象,结合其糖原含量和光谱数据建立各组织糖原含量的NIR检测模型,为开展近江牡蛎糖原含量的大规模测定提供方法和技术支撑。
1 材料与方法 1.1 实验仪器与设备傅里叶变换NIR光谱仪(Antaris MX, 美国),配备RESULTTM样本光谱采集的集成软件以及数据处理软件TQ analyst (Thermo Fisher, 美国);酶标仪(BIO- RAD, 美国);真空冷冻干燥机(CHRIST, Alpha 2-4LDplus, 德国)。
1.2 样品的采集与处理实验用近江牡蛎为性腺不同发育时期的成体,采自山东东营和滨州海区。新鲜近江牡蛎运回实验室后,分别取外套膜、鳃、闭壳肌、肝胰腺、唇瓣、性腺6个组织,放入1.5 mL离心管中,液氮速冻后置于–80℃冰箱中保存备用。测定前,利用真空冷冻干燥机干燥48 h后,研磨成细粉末分管保存,用于后续糖原含量的测定和NIR数据的采集。
1.3 糖原含量的化学测定近江牡蛎糖原含量的化学值测定采用微量蒽酮比色法(陈夕等, 2021)进行。主要步骤如下:称取20 mg粉末样品于10 mL试管中,加入30% KOH溶液(w/w) 2 mL,沸水浴消化60 min,期间每隔5~10 min摇晃试管,使其消化完全后,蒸馏水定容至5 mL,12 000 r/min离心8 min后,取上清液获得样品待测液。取稀释10倍的牡蛎待测液100 μL于EP管中,冰浴5 min,取0.2%的蒽酮硫酸溶液200 μL在冰浴条件下,贴管壁缓慢加至EP管中。所有样品统一摇匀之后在沸水浴中加热8 min,期间迅速颠倒混匀2次(每次不超过5 s),加热完成后用自来水冷却。室温静置30 min,移取200 μL于微孔板,利用酶标仪测定样品在620 nm的吸光值,每个样品设置3个重复。
使用浓度为1 mg/mL的葡萄糖标准液配置成不同浓度梯度的葡萄糖溶液,替代上述的待测液,按照上述方法进行实验,测定吸光值,绘制浓度与吸光值的标准曲线,建立回归方程。测得吸光值后,由标准曲线计算得到反应体系中葡萄糖的浓度。
1.4 近红外光谱的采集利用傅里叶变换NIR光谱仪(Antaris MX, 美国)获得光谱数据。采集样品光谱前,需使用RESULT集成软件编定样品光谱采集工作流,光谱扫描波数范围设定为10 000~4000 cm–1,扫描次数为128次,分辨率为8 cm–1,用log(1/R)漫反射方法表示吸收光谱。样品扫描前,先将光谱仪预热0.5 h,后将研磨好的样品加入光谱采集处的积分球石英杯中(杯直径为1 cm),样品高度控制在1.5 cm左右。每次扫描样品前,采集背景光谱来消除背景影响,样品扫描选用漫反射光谱。
1.5 模型的建立与验证利用TQ Analyst (version 9.1.17, 美国)软件处理采集到的6种组织的光谱数据,选用偏最小二乘法(Partial Least Squares, PLS)作为建立定量模型的化学计量方法,根据软件推荐的光谱范围,选用乘法散射矫正、一阶求导、Norris平滑等预处理方法,并对样本进行异常值的剔除,分别建立了7种近红外定量分析模型,其中,全软体部的光谱数据采用的是6种组织的光谱数据集。后对建立的定量模型进行外部验证和交叉验证,验证样本量占总样本的1/9,以确保模型的准确性和可信性。
2 结果 2.1 近江牡蛎6种冻干组织糖原化学含量测定结果利用微量蒽酮法测定了近江牡蛎外套膜156个、鳃155个、闭壳肌159个、肝胰腺144个、唇瓣148个、性腺147个,共909个样品的糖原含量。经过优化和验证处理,用于构建近江牡蛎7种样品糖原近红外定量模型的建模集和验证集所包含的样品数量,以及样品糖原含量范围值见表 1,其中,全软体部为6种组织的混合样品。全软体部、外套膜、鳃、闭壳肌、肝胰腺、唇瓣、性腺的建模集个数分别为643、76、83、90、69、55和85,验证集样本个数分别为46、12、13、17、9、7和13。建模集各组织糖原含量范围分别为全软体部7.11~602.20 mg/g,外套膜28.31~ 509.59 mg/g,鳃28.83~306.94 mg/g,闭壳肌7.12~ 103.09 mg/g,肝胰腺68.95~516.72 mg/g,唇瓣345.01~ 590.39 mg/g,性腺32.58~602.21 mg/g,各组织糖原含量的标准差均较大。同时,各组织糖原含量的标准差也均较大,其建模集糖原含量的标准差范围在21.02~186.97,验证集糖原含量的标准差范围在18.64~182.86。在近红外分析模型建立过程中,要求样品含量分布范围广。
|
|
表 1 近江牡蛎7个近红外光谱模型建模集和验证集样本数目和糖原含量范围 Tab.1 Number of samples and the glycogen content in the freeze-dried tissue modeling set and validation set of seven NIR models of C. ariakensis |
采用NIR光谱仪扫描近江牡蛎冻干样品909份,得到的NIR漫反射原始光谱如图 1所示,所有的光谱曲线走向基本一致。在全软体部、外套膜、鳃、闭壳肌、肝胰腺、唇瓣和性腺的糖原含量NIR分析模型的建立过程中,选择软件推荐的最佳光谱范围,光谱数据的最优预处理方法均为一阶求导(图 2)、乘法散射校正及Norris平滑,处理过程中的主要参数及最终主因子数见表 2。
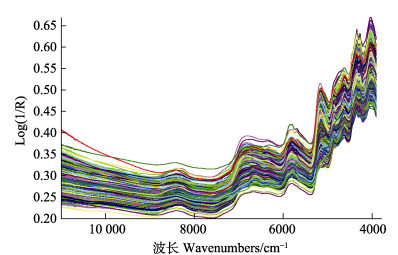
|
图 1 近江牡蛎冻干组织所有样本的NIR漫反射原始光谱 Fig.1 NIR diffuse reflectance original spectra of all samples of freeze-dried tissue of C. ariakensis |
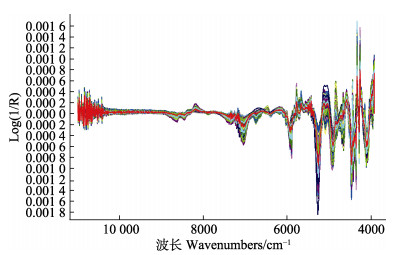
|
图 2 近江牡蛎冻干组织所有样本NIR光谱一阶求导处理 Fig.2 First-order derivation of NIR spectrum for all samples of freeze-dried tissue of C. ariakensis |
|
|
表 2 近江牡蛎糖原含量各样品建模集和验证集光谱数据处理参数 Tab.2 Spectral data processing parameters of the modeling set and verification set of NIR models for the glycogen content of C. ariakensis |
根据TQ Analyst软件推荐的异常值,进行样本剔除。异常值剔除后,建立了6种组织及全软体部的近红外定量模型。
建模过程中,相关系数越接近于1,相应的残差均方根越小,说明该模型越好。在各组织样本糖原含量建模的结果中,其RC均接近于1,其中,全软体部、外套膜、鳃、肝胰腺、唇瓣、性腺和闭壳肌糖原定量模型RC值范围是0.971 6~0.996 3,肝胰腺建模相关系数最高,为0.996 3;闭壳肌糖原定量模型RC值是所有模型中最低的,但也高达0.971 6,且7个模型的建模残差均方根(RMSEC)值在4.97~26.20之间,均相对较小,这些数据证明模型的预测值与化学真实值相关度较高。7个模型交叉验证相关系数(RCV)和外部验证的相关系数(REV)较高,均 > 0.94,最高为0.996 9,且对应的交叉验证残差均方根(RMSECV)值和外部验证残差均方根(RMSEP)值在4.59~30.30之间,也相对较小,进一步证明了该模型较高的可信度和准确性;模型验证的重要参数即验证样本真实值标准差与验证残差均方根的比值(RPD值)变化范围为4.06~11.60 (表 3)。研究结果表明,全软体部以及6种组织的糖原定量模型精确度高,可用于近江牡蛎冻干组织样品的糖原含量预测。
|
|
表 3 近江牡蛎各组织建模过程中的指标参数 Tab.3 Index parameters in the NIR modeling for each sample of C. ariakensis |
目前,糖原的检测方法主要有传统蒽酮比色法、基于蒽酮比色法基本原理的试剂盒法等(李春燕, 2017; 杨柳等, 2018)以及本实验室已建立的微量蒽酮比色法(陈夕等, 2021)。虽然传统的蒽酮比色法应用广泛,但样品预处理复杂,操作繁琐,不适于大批量样品的快速测定,同时,实验过程中浓硫酸的使用也存在较大的安全隐患。而试剂盒方法虽然在蒽酮硫酸使用量以及样品处理上作了改进等,但因其价格昂贵,极大地增加了实验成本。本实验室建立的微量蒽酮比色法虽降低了实验成本,简化了实验步骤,但在实验操作重仍旧费时费力,实验过程中会产生大量的化学废液,对环境污染严重。而NIR技术不仅可以进行大批量样品的测定,还具有快捷高效、省时省力等优点,且在实验过程中无化学废液产生,绿色环保。经过NIR技术检测后样品,其化学性质不会发生改变,不会造成样品的大量消耗,可用于后续的回收利用。本实验所用的近红外扫描样品,经短时间的近红外扫描获取光谱数据后,可迅速回收放于–80℃冰箱冷冻保存,不影响后续样品的使用。总的来说,NIR技术在测定糖原含量方面具有诸多优势,可以应用在牡蛎品质性状的改良上。
3.2 近江牡蛎NIR糖原定量模型的重要参数从图 3中可以看出,所建模型的RC值、RCV值以及REV值越接近1,模型的预测效果就越好。相较于前人在牡蛎上所建定量模型的数据,本研究建立的7个NIR糖原定量模型,以上3个指标值均在0.94以上,最高可达0.996 9,说明本实验所建模型的精确性更好。此外,RPD值也是评判模型准确度的重要指标,优质模型的RPD值需 > 2.5,且RPD值越高,模型就越好(Zhou et al, 2012)。本研究建立模型的RPD值远 > 2.5,最高可达11.6,这表明7个模型均具有较高的预测准确性。所建7个模型的建模集和验证集的SD值分别在21.02~186.97和18.64~182.86之间,可见建模样品糖原含量变化范围广,证明模型预测的精确度高和可信性强。
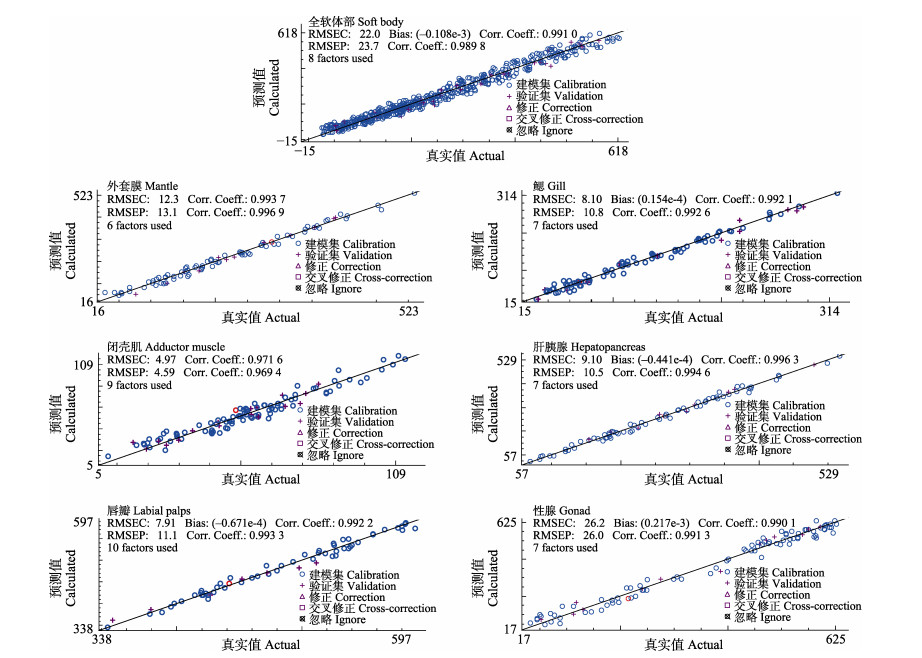
|
图 3 全软体部、外套膜、鳃、闭壳肌、肝胰腺、唇瓣、性腺糖原含量NIR模型主要参数 Fig.3 Main parameters of NIR model for glycogen content of the whole soft body, mantle, gill, adductor muscle, hepatopancreas, labial palps and gonad |
本研究样本采集于不同季节、2个不同地理群体,样本量多达900多份,样本代表性好。与已建立的长牡蛎、葡萄牙牡蛎等NIR品质成分定量模型相比,本研究采用的是分组织样品,与混组织样品相比,准确度会更高,可以满足不同样品糖原测定的需求。这是因为不同组织的糖原含量具有一定的差别,6种特定组织的NIR分析模型可以更准确的检测那些个体大、易于区分组织的样品的糖原含量。而全软体部模型由于其建立是依据6种组织的糖原和光谱数据,因此该模型适用于那些个体小、不易于区分组织的样品。本研究分别建立了包括全软体部、外套膜、鳃、闭壳肌、肝胰腺、唇瓣、性腺组织糖原的NIR检测模型,可以针对不同现场需求,用于样品糖原含量的检测,具有针对性高、适用性强的优势。
水分对光谱数据质量具有一定的影响。水分吸收区域在3500~3100 cm–1和800~750 cm–1,是光谱中具有非常高吸收值的区域,在该吸收区域会使得仪器设置失去灵敏度,此时产生的噪声会干扰模型的回归(Pedersen et al, 2003)。与鲜样模型相比,本研究采用的冻干组织样品,大大降低了水分对光谱质量的影响,使模型预测准确性更高。
3.3 NIR糖原定量模型在牡蛎品质性状测定中的应用随着生活水平的不断提高和牡蛎消费群体的不断扩大,人们更加追求高品质的牡蛎,尤其是鲜、甜等口感性状。目前,进入我国牡蛎高端市场的不同风味的牡蛎品种,几乎全部来自于进口,而且价格昂贵。2019年之前,国内获批的牡蛎新品种中,多数品种的主要优势性状都体现在生长、壳色等性状,牡蛎养殖业也主要靠“以量取胜”,规模、品质之间的矛盾日益突出。可以预见,随着贝类产业的发展,市场对贝类品质的需求更将向高质化和多元化发展,除了对生长速度等传统性状追求的基础上,对高糖原等高品质牡蛎的需求将更为迫切。针对不同牡蛎,国内外已建立了牡蛎多个品质性状成分含量测定的近红外模型,国外有多倍体长牡蛎、悉尼岩牡蛎和美洲牡蛎的水分、脂肪、糖原、总蛋白质NIR定量模型(Brown, 2011; Brown et al, 2012; Guévélou et al, 2016);国内主要有长牡蛎鲜样组织中水分、糖原、总蛋白质、总脂肪、Zn、Se、牛磺酸和灰分8种成分含量的NIR分析模型(王卫军等, 2015);葡萄牙牡蛎蛋白质、糖原、牛磺酸、Zn、Se和Ca 6种成分含量的NIR分析模型以及最早建立的牡蛎中氨基酸含量的NIR分析模型(于颖等, 2016; 黄冠明等, 2020),这些模型均具有较高的预测准确度,说明近红外光谱模型可以较好地应用在牡蛎营养元素的测定中。本研究建立的近江牡蛎糖原含量NIR分析模型,丰富了牡蛎NIR技术检测品质性状的方法,为今后实现快速近江牡蛎糖原成分的大规模测定提供了技术支撑。
4 结论本研究建立的7个糖原含量NIR分析模型,可以实现近江牡蛎外套膜、鳃、闭壳肌、肝胰腺、唇瓣、性腺及全软体部冻干组织样品中糖原含量的快速准确、大批量的测定,丰富了近江牡蛎品质性状的检测方法,为今后牡蛎品质性状改良等方面提供技术支撑。
ANACLETO P, MAULVAULT A L, BARRENTO S, et al. Physiological responses to depuration and transport of native and exotic clams at different temperatures. Aquaculture, 2013, 408-409: 136-146 DOI:10.1016/j.aquaculture.2013.05.035 |
BELTRÁN-LUGO A I, MAEDA-MARTINEZ A N, PACHECO- AGUILAR R, et al. Seasonal variations in chemical, physical, textural, and microstructural properties of adductor muscles of Pacific lions-paw scallop (Nodipecten subnodosus). Aquaculture, 2006, 258(1/2/3/4): 619-632 |
BROWN M R, KUBE P D, O'Connor S, et al. Application of near-infrared reflectance spectroscopy for the rapid chemical analysis of Sydney rock oyster (Saccostrea glomerata) and Pacific oyster (Crassostrea gigas). Journal of Shellfish Research, 2012, 31(4): 1051-1060 DOI:10.2983/035.031.0417 |
BROWN M R. Rapid compositional analysis of oysters using visible-near infrared Reflectance spectroscopy. Aquaculture, 2011, 317(4): 233-239 |
CHÁVEZ-VILLALBA J, SOYEZ C, AURENTZ H, et al. Physiological responses of female and male black-lip pearl oysters (Pinctada margaritifera) to different temperatures and concentrations of food. Aquatic Living Resources, 2013, 26(3): 263-271 DOI:10.1051/alr/2013059 |
CHEN X, WU B, WANG Y, et al. Establishment and optimization of micro-reaction system for determination of oyster glycogen content. South China Fisheries Science, 2021, 17(4): 126-132 [陈夕, 吴彪, 王岩, 等. 测定牡蛎糖原含量的微量反应体系的建立与优化. 南方水产科学, 2021, 17(4): 126-132] |
CONG R, KONG L, YU H, et al. Association between polymorphism in the insulin receptor-related receptor gene and growth traits in the Pacific oyster Crassostrea gigas. Biochemical Systematics and Ecology, 2014, 54: 144-149 DOI:10.1016/j.bse.2014.02.003 |
CONG R, LI Q, KONG L. Polymorphism in the insulin-related peptide gene and its association with growth traits in the Pacific oyster Crassostrea gigas. Biochemical Systematics and Ecology, 2013, 46: 36-43 DOI:10.1016/j.bse.2012.09.008 |
FLUCKIGER M, BROWN M R, WARD L R, et al. Predicting glycogen concentration in the foot muscle of abalone using near infrared reflectance spectroscopy (NIRS). Food Chemistry, 2011, 126(4): 1817-1820 DOI:10.1016/j.foodchem.2010.12.078 |
FOLKESTAD A, WOLD J P, RORVIK K, et al. Rapid and non- invasive measurements of fat and pigment concentrations in live and slaughtered Atlantic salmon (Salmo salar). Aquaculture, 2008, 280(1/2/3/4): 129-135 |
GUÉVÉLOU E, ALLEN S K. Use of Near Infrared Reflectance Spectroscopy (NIRS) for the rapid compositional analysis of di-, tri-, and tetraploid eastern oysters (Crassostrea virginica). Aquaculture, 2016, 459: 203-209 DOI:10.1016/j.aquaculture.2016.03.022 |
GUO X, LI Q, WANG Q Z, et al. Genetic mapping and QTL analysis of growth-related traits in the Pacific oyster. Marine Biotechnology, 2012, 14(2): 218-226 DOI:10.1007/s10126-011-9405-4 |
HUANG G M, GUO X, QI J F, et al. Establishment of quantitative model for six chemical compositions in Crassostrea angulata by near infrared spectroscopy. Spectroscopy and Spectral Analysis, 2020, 40(5): 1509-1513 [黄冠明, 郭香, 祁剑飞, 等. 葡萄牙牡蛎(Crassostrea angulata)六种化学成分近红外定量模型的建立. 光谱学与光谱分析, 2020, 40(5): 1509-1513] |
HUVET A, JEFFROY F, FABIOUX C, et al. Association among growth, food consumption -related traits and amylase gene polymorphism in the Pacific oyster Crassostrea gigas. Animal Genetics, 2008, 39(6): 662-665 DOI:10.1111/j.1365-2052.2008.01776.x |
LI C Y. Genetic mapping and gene dissection of nutritional and other important economic traits of the oyster. Doctoral Dissertation of University of Chinese Academy of Sciences, 2017 [李春燕. 牡蛎营养品质等重要经济性状的遗传定位与基因解析. 中国科学院大学博士研究生学位论文博士研究生学位论文, 2017]
|
MARTÍNEZ-PITA I, SANCHEZ-LAZO C, RUIZ-JARABO I, et al. Biochemical composition, lipid classes, fatty acids and sexual hormones in the mussel Mytilus galloprovincialis from cultivated populations in south Spain. Aquaculture, 2012, 358-359: 274-283 DOI:10.1016/j.aquaculture.2012.06.003 |
MILLER M R, PUDDICK J, SYMONDS J E, et al. Application of a Fourier transform—Near infrared reflectance spectroscopy method for the rapid proximate analysis of the greenshell mussel (Perna canaliculus) and king (Chinook) salmon (Oncorhynchus tshawytscha). Aquaculture Research, 2019, 50(6): 1668-1677 DOI:10.1111/are.14049 |
National Aquatic Technology Promotion Station. 2020 Guidelines for the promotion of new aquatic products. Beijing: China Agriculture Press, 2020: 68-86 [全国水产技术推广总站. 2020水产新品种推广指南. 北京: 中国农业出版社, 2020: 68-86]
|
PEDERSEN D K, MOREL S, ANDERSEN H J, et al. Early prediction of water-holding capacity in meat by multivariate vibrational spectroscopy. Meat Science, 2003, 65(1): 581-592 DOI:10.1016/S0309-1740(02)00251-6 |
QU Y W, ZHANG H, HAN X, et al. Establishment of near- infrared model of peanut fatty acids. Molecular Plant Breeding, 2019, 17(2): 568-578 [曲艺伟, 张鹤, 韩笑, 等. 花生脂肪酸近红外模型的建立. 分子植物育种, 2019, 17(2): 568-578] |
SHE Z, LI L, QI H, et al. Candidate gene polymorphisms and their association with glycogen content in the Pacific oyster Crassostrea gigas. Plos One, 2015, 10(5): 1-13 |
Vodáková B, Douda K. Variation in glycogen distribution among freshwater bivalve tissues: Simplified protocol and implications. Journal of Aquatic Animal Health, 2019, 31(1): 107-111 DOI:10.1002/aah.10057 |
WANG H Y, ZHANG G F, LIU X, et al. Classification of common oysters from North China. Journal of Shellfish Research, 2008, 27(3): 495-503 DOI:10.2983/0730-8000(2008)27[495:COCOFN]2.0.CO;2 |
WANG W J, YANG J M, DONG Y H, et al. Establishment of Near infrared models of eight components on fresh tissue of Pacific oyster Crassostrea gigas. Oceanologia et Limnologia Sinica, 2015, 46(4): 845-852 [王卫军, 杨建敏, 董迎辉, 等. 长牡蛎(Crassostrea gigas)鲜样组织八种成分含量近红外(NIR)模型的建立. 海洋与湖沼, 2015, 46(4): 845-852] |
YANG L, GUO H Y, SHAO C J, et al. Research progress of glycogen detection methods. Progress in Modern Biomedicine, 2018, 18(1): 175-179 [杨柳, 郭海云, 邵长健, 等. 糖原检测方法研究进展. 现代生物医学进展, 2018, 18(1): 175-179] |
YU J, ZHOU J. Delicacy of the sea: Oyster. Beijing Aquaculture, 2002(6): 48-49 [庾晋, 周洁. 海中珍馐——牡蛎. 北京水产, 2002(6): 48-49] |
YU Y, SONG J M, YU L D. Quantitative analysis of amino acids in oyster by FT-NIR spectroscopy. Marine Sciences, 2016, 40(12): 1-7 [于颖, 宋金明, 俞立东, 等. 牡蛎中氨基酸的傅里叶变换近红外光谱法快速定量测定. 海洋科学, 2016, 40(12): 1-7] |
YU Z, FANG F, PENG Z D, et al. New technologies for detecting seed vigor. Seed, 2012, 31(8): 52-55 [于征, 方芳, 彭祚登, 等. 基于新兴技术的种子活力检测方法研究. 种子, 2012, 31(8): 52-55] |
ZHAO Q, WU B, LIU Z H, et al. Cloning and expression analysis of hemoglobin geneⅠof Scapharca broughtonii. Progress in Fishery Sciences, 2019, 40(1): 84-91 [赵庆, 吴彪, 刘志鸿, 等. 魁蚶(Scapharca broughtonii)血红蛋白Ⅰ基因克隆及表达特征. 渔业科学进展, 2019, 40(1): 84-91] |
ZHONG X, LI Q, GUO X, et al. QTL mapping for glycogen content and shell pigmentation in the Pacific oyster Crassostrea gigas using microsatellites and SNPs. Aquaculture International, 2014, 22(6): 1877-1889 |
ZHOU L J, WU H, LI J T, et al. Determination of fatty acids in broiler breast meat by near-infrared reflectance spectroscopy. Meat Science, 2012, 90(3): 658-664 |
ZHOU L Q, ZHAO D, WU Z, et al. Review: Molecular mechanism of sex differentiation in major economic bivalves. Progress in Fishery Sciences, 2020, 41(5): 194-202 [周丽青, 赵丹, 吴宙, 等. 主要经济双壳贝类性别分化的分子机制概述. 渔业科学进展, 2020, 41(5): 194-202] |



