2. 中国水产科学研究院黄海水产研究所 农业农村部水产品质量安全检测与评价重点实验室山东 青岛 266071;
3. 青岛海洋科学与技术试点国家实验室 山东 青岛 266237;
4. 海洋食品精深加工关键技术省部共建协同创新中心 大连工业大学 辽宁 大连 116034
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory for Testing and Evaluation of Aquatic Product Quality and Safety, Ministry of Agriculture and Rural Affairs, Qingdao, Shandong 266071, China;
3. Pilot National Laboratory of Marine Science and Technology (Qingdao), Qingdao, Shandong 266237, China;
4. Key Technologies of Marine Food Intensive Processing Provincial and Ministerial Co-Construction of Collaborative Innovation Center, Dalian Polytechnic University, Dalian, Liaoning 116034, China
麻痹性贝类毒素(paralytic shellfish toxins, PSTs)是目前分布最广、危害最严重的一类藻毒素(贝类毒素),在全球范围内造成严重的生态和食品安全风险。据统计,全球由PSTs导致中毒事件年均约2000起(Wang et al, 2016),是国际社会的重点研究和管控目标(Visciano et al, 2016)。PSTs作为一类水溶性神经毒性生物碱小分子物质,由海洋甲藻和淡水蓝绿藻产生,海洋甲藻主要包括亚历山大藻(Alexandrium)、裸甲藻(Gymnodinium)、盾甲藻属(Pyrodinium) (Visciano et al, 2016),其中,亚历山大藻是PSTs的主要产毒藻。调查发现,我国近海广泛分布多种不同亚历山大藻,所产生的PSTs种类差异显著(Gu et al, 2013)。PSTs可经食物链传播蓄积于鱼类、贝类等物种体内,不仅影响物种正常生理状态,严重时危及人类生命安全(于仁成等, 2016)。我国自2016年发生“秦皇岛贻贝”中毒事件以来(张锦宏等, 2020),连续多次在河北和福建沿岸出现贻贝中PSTs超标事件(Huang et al, 2019; 张亚亚等, 2020)。其中,尤其是河北秦皇岛近海贻贝中2016—2021年均检出超出欧盟限量标准(800 μg STXeq/kg)的PSTs而引起了重大关注(呼晓群等, 2021),产生了十分严重的负面影响,不利于区域性贝类产业的发展和社会稳定。现已证实,秦皇岛贻贝中PSTs残留的主要肇事藻种为链状亚历山大藻(Alexandrium catenella) (Zhang et al, 2012)。
链状亚历山大藻广泛分布于我国黄渤海地区,并形成高丰度包囊分布于黄海北部的中心地区以及山东半岛东南部和南部苏北浅滩附近(Dai et al, 2020)。研究表明,自然条件下,链状亚历山大藻在形成包囊后会沉积在海底形成包囊床,造成目标海域连年暴发有害藻华(harmful algal blooms, HABs)(Kim et al, 2020; Condie et al, 2019; Gao et al, 2019),包囊床的存在也是秦皇岛海域贻贝中PSTs连年超标的主要潜在原因。自2016年中毒事件发生后,虽然秦皇岛政府逐步取消邻近海域贻贝养殖,但由于野生贻贝的大量存在,仍然对该地区消费者安全问题带来潜在风险(翟毓秀等, 2020)。因此,开展链状亚历山大藻在贻贝中的蓄积转化研究,明确贻贝中PSTs风险,具有重要的现实和科学意义。
海产贝类的滤食性特征导致其极易蓄积高浓度的PSTs,且经过一系列的代谢转化过程,降低PSTs对贝类自身的危害(马菲菲, 2012),但这种转化作用可能导致新PSTs组分的生成(汪娜等, 2013),且代谢产物的毒性当量因子(toxic equivalency factor, TEF)可能高于原型态物质,从而增加消费者安全风险。对贻贝来说,内脏团是PSTs蓄积的靶器官(邴晓菲等, 2017),可将PSTs累积到高水平,并进行代谢转化和消除(Freitas et al, 2019; Riisgard et al, 2012)。因此,以链状亚历山大藻(GY-H25株)这一优势PSTs产毒藻为研究对象,探究其所产生PSTs在紫贻贝(Mytilus galloprovincialis)中蓄积转化规律,并结合代谢产物形成和TEF考虑,评估紫贻贝中PSTs终端产物的风险程度,对于科学理解秦皇岛地区紫贻贝中PSTs风险并针对性构建区域性风险防控技术,具有积极的科学及现实意义。
1 材料与方法 1.1 实验材料 1.1.1 产毒藻、饵料藻及紫贻贝链状亚历山大藻(GY-H25株)购自上海光宇生物科技公司。实验室内以f/2培养液单种培养,温度为(20±1)℃,光照为54 μmol/(m2·s);光暗比12 h: 12 h。饵料藻选用小球藻(Chlorella vulgaris),同样条件下以f/2培养液培养。
紫贻贝购置于山东青岛码头,为周边海域野生贝,平均规格:壳长为(7±1) cm,壳宽为(3.5±0.5) cm,壳厚为(2.7±0.5) cm,总重(35±15) g,与秦皇岛海域所产紫贻贝为同一种,均起源于地中海(Borsa et al, 1999; 王晓琴等, 2019)。所用紫贻贝为当日采集,个头均一,置于保温箱中加入冰袋保存,2 h内运回,置于天然海水24 h曝气养殖容器环境下无投喂暂养2 d,以适应养殖环境,其死亡率稳定后即开始暴露实验。
1.1.2 仪器与设备光照培养箱(上海一恒科学仪器有限公司);AB-5500 QTRAP液相色谱–四极杆/离子阱复合质谱(AB SCIEX,美国),配有电喷雾离子源(ESI);Himac CR 22GⅡ高速离心机(Hitachi,日本);恒温水浴锅(上海蓝凯仪器仪表有限公司);XW-80A旋涡混合器(上海医大仪器厂);Milli-Q超纯水仪(Millipore,美国);固相萃取装置(Supelco,美国);GF/F玻璃纤维滤膜(Whatman,英国)。
1.1.3 化学试剂乙腈(质谱级)(Merck,美国);甲酸、甲酸铵(色谱级)(Fluka,瑞士);乙酸(色谱级) (Thermo,美国);超纯水(18.2 MΩ∙cm);其他未作特殊说明的试剂均为分析纯。
1.2 实验方法 1.2.1 链状亚历山大藻生长过程中产毒能力评估实验用藻初始接种密度为6.5×105 cells/L,培养用水为山东沿海天然海水,经0.45 μm纤维滤膜过滤、高温灭菌,添加f/2海水培养基(邵盛男等, 2011; 缪宇平等, 2009),接种链状亚历山大藻,置于智能光照培养箱中,温度为(20±1)℃,光照为54 μmol/(m2·s),光暗比12 h: 12 h。每天08:00取藻液样品,取样前充分摇匀,每瓶取样3次,每次取10 mL,加入150 μL鲁哥试液固定,充分摇匀(常孟阳等, 2019),从中取出100 μL,光学显微镜下进行藻细胞计数,取平均值用于评估产毒藻生长状况。
产毒藻密度达到2.5×107 cells/L时,取20 mL藻液于离心管中,5000 r/min离心15 min收集藻细胞,上清液8000 r/min离心10 min,2次离心的藻细胞合并(邵盛男等, 2011; 余新威等, 2018),加入5 mL 1%乙酸水溶液,冰浴超声破碎5 min,破碎8 s停2 s,4℃条件下8000 r/min离心10 min,取1 mL上清液过0.22 μm水系滤膜,存于进样小瓶中,进行PSTs分析。
1.2.2 紫贻贝暴露实验 1.2.2.1 暴露过程随机挑选540只紫贻贝平均分为3组,分别为对照组、低浓度组和高浓度组,放置在各盛有25 L海水的9个养殖容器中进行实验。实验共进行30 d,分为7 d暴露阶段和23 d代谢阶段。2个实验组产毒藻投喂均分早晚各8点2个时间段进行,控制早晚投喂量1∶2;对照组只投喂饵料藻。暴露阶段结束时,高浓度组180只紫贻贝共投喂产毒藻19.2×108 cells;低浓度组180只紫贻贝共投喂产毒藻8.0×108 cells。在暴露实验过程中各组均保持0.6%的稳定死亡率。
1.2.2.2 样品采集在暴露第0、0.5、1、2、4、6、7、8、10、12、14、18、22、26和30天采集毒素样品以测定毒素含量,随机取贝进行解剖,用清水将贝壳外表洗净,切断闭壳肌,仔细取出内脏和可食组织,在筛子上平铺沥水5 min,将所取组织分别均质、混匀,每3只贝匀浆为1个样品,每组取3个平行样品。
1.2.3 毒素检测 1.2.3.1 样品前处理参考Boundy等(2015)的前处理方法并改进:将所取组织称取5 g于50 mL离心管中,加入5 mL 1%的乙酸水溶液提取毒素,涡旋混合90 s,将离心管密封置于沸水中煮沸5 min (沸水没过试样液面),取出置于冷水下迅速冷却至室温,4500 r/min离心10 min,待净化。
移取上述提取液1 mL于2 mL离心管中,加入5 μL氨水,涡旋混匀,10 000 r/min离心5 min。依次用3 mL乙腈、3 mL 20%乙腈水溶液(含1%乙酸)、3 mL 0.1%氨水溶液活化Supelco ENVI-Carb固相萃取柱,加入500 μL提取液,再用700 μL超纯水淋洗,正压挤干,最后用1 mL 75%乙腈水溶液(含0.25%甲酸)洗脱混匀,过0.22 μm滤膜于进样小瓶中,4℃下保存,供液相色谱–串联质谱分析。
1.2.3.2 液相色谱及质谱条件样品分析在AB SCIEX 5500 QTRAP四极杆–线性离子阱复合质谱仪上进行。色谱柱为TSK-Amide-80 (3 μm, 2 mm× 15 cm),柱温为40℃,样品温度为4℃,进样体积为5 µL,流动相A为水(含2 mmol/L甲酸铵,50 mmol/L甲酸),B为95%乙腈水溶液(含2 mmol/L甲酸铵,50 mmol/L甲酸)。梯度洗脱条件:0~3.0 min,80% B;3.1~5.0 min,80%~40% B;5.1~10.0 min,40% B;10.1~11.0 min,40%~80% B;11.1~13.0 min,80% B;流速:0.4 mL/min。
采用喷雾电离子源(ESI)多反应监测(MRM)检测方式,离子源温度为550℃,喷雾电压为5000 V,–4500 V,气帘气压力为20 psi,雾化气压力为30 psi,辅助加热器压力为30 psi,碰撞气Medium。
1.2.4 数据处理 1.2.4.1 毒素结果计算试样中麻痹性贝类毒素含量按式(1)进行计算。
| $ X = \frac{{C \times V \times 1000}}{{m \times 1000}} \times n $ | (1) |
式中,X为试样中麻痹性贝类毒素的含量(μg/kg),C为样品溶液中麻痹性贝类毒素的浓度(ng/mL),V为试样定容体积(mL),m为试样质量(g),n为稀释倍数。
样品中贝类毒素的含量按照毒性因子,统一转换为STXeq来表示,计算公式见式(2):
| $ {\text{STXeq}} = \mathop \sum \limits_{i = 1}^n {X_i}{r_i} $ | (2) |
式中,Xi为各种亲水性贝类毒素含量,ri为毒性因子。
1.2.4.2 蓄积率计算蓄积率按照式(3)计算:
| $ A=({m}_{V}\times n\times \text{STXeq}/{10}^{3})/({V}_{藻}\times {\rho }_{藻}/{10}^{6}) $ | (3) |
式中,A为每时间点的蓄积率(%),mv为紫贻贝内脏质量(g),n为每一养殖容器紫贻贝数量,V藻为每筐投喂产毒藻体积(L),ρ藻为所投喂产毒藻密度(cells/L)。
1.2.4.3 摄食率测定投喂产毒藻后及1 h后充分混匀,各取水样5 mL,加入鲁哥试剂固定藻细胞,光学显微镜下进行藻细胞计数,计算水中饵料浓度。
实验中测得紫贻贝贝肉湿重,并由干重和湿重关系公式(程芬, 2018)计算得出紫贻贝干重,根据以下公式(梁伟锋, 2016)计算实验紫贻贝的摄食率(ingestion rate, IR, %):
| $ {\text{IR}} = V \times [\ln ({C_{e0}}/{C_{et}}) - \ln ({C_{c0}}/{C_{ct}})]/(w \times t) \times {C_{e0}} $ | (4) |
式中,IR为单位体重的摄食率(cells/g/h),Ce0和Cet为平行组实验开始和结束时水中的饵料浓度(×107 cells/L),Cc0和Cct为空白对照组实验开始和结束时水中的饵料浓度(×107 cells/L),V为实验水体体积(L),w为贝软体部干重(g),t为实验时间(h)。
实验数据采用SPSS 23和Excel 2018等软件整理分析,结果均采用单因素方差分析(one-way ANOVA),以平均值±标准差(Mean±SD)表示。采用Origin 8.5软件作图。
2 结果 2.1 紫贻贝中PSTs蓄积代谢动力学研究链状亚历山大藻以6.5×105 cells/L的初始密度接种,在培养18~24 d内即达到平台期,此阶段最高细胞密度为3.5×107 cells/L,单藻细胞产毒最高可达2.96 pg STXeq/cell。实验选取链状亚历山大藻实测单位体积毒素含量与藻细胞密度乘积最高点(22 d藻样)开展进一步的产毒能力研究,测得藻细胞密度为2.5×107 cells/L,单藻细胞产毒量为2 pg STXeq/cell。链状亚历山大藻可产9种毒素(图 1a),其中,最多的3种毒素分别为C2 (74.16%)、C1 (10.65%)和GTX5 (7.71%)。

|
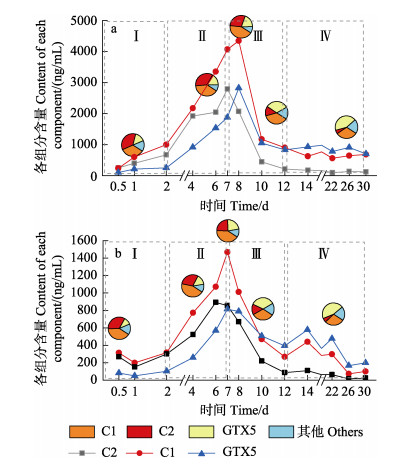
图 1 a:链状亚历山大藻产毒情况;b:紫贻贝内脏组织和可食组织PSTs蓄积含量对比;c:紫贻贝不同浓度暴露组内脏组织PSTs蓄积含量变化;d:紫贻贝不同浓度暴露组摄食率变化(n=6; Mean±SD) Fig.1 a: Toxicity of A. catenella; b: Comparison of PSTs accumulation in visceral tissue and edible tissue of M. edulis; c: Changes of PSTs accumulation in visceral tissue of M. edulis exposed to different concentrations; d: Changes of ingestion rate in M. edulis exposed to different concentrations (n=6; Mean±SD) |
在胁迫过程中,PSTs在紫贻贝体内的蓄积和代谢可划分为4个时期:0~2 d蓄积平缓,为初始暴露时期(时期Ⅰ);2~7 d内蓄积加快,以平均14.84%的蓄积速率持续蓄积至第7天达到峰值,为快速蓄积时期(时期Ⅱ);7~12 d内毒素含量以平均每天14.4%的排除速率快速代谢,为快速代谢时期(时期Ⅲ);12 d后趋于平缓,直到实验结束,为平稳期(时期Ⅳ)。如图 1c所示,高浓度和低浓度组呈现出一致趋势,高浓度组的最高蓄积含量是低浓度组的2.61倍,蓄积和代谢速率分别为1.37倍和0.96倍。在胁迫过程中,紫贻贝摄食率整体处于持续上升状态(图 1d),其对PSTs呈现出极强的蓄积能力,对其可食组织和内脏团的蓄积含量进行测定后比较发现,紫贻贝对PSTs的主要蓄积器官为内脏团,可高出可食组织35倍(图 1b),其中,高浓度组的蓄积浓度高达6 815.36 μg STXeq/kg,低浓度组也蓄积了超过2 614.78 μg STXeq/kg,超出国际限制安全标准(图 1c)。
2.2 紫贻贝体内毒素转化在高低2种浓度产毒藻的暴露下,紫贻贝的内脏中初始毒素组成相同,除dcNEO外,12种国标毒素组分均检出,其中,占比最高的3种毒素分别为C1、C2和GTX5,远超其他组分。随着暴露时间延长,各毒素百分比变化趋势也具有一致性,其中,C1、C2和GTX5毒素的占比变化最为明显。表现为C2呈稳定减少趋势,GTX5呈稳定增加趋势,C1则是先增加后减少。如图 2所示,GTX5占比虽然在前2个时期均为最低,占比在15%左右,但却呈持续升高趋势,在快速代谢时期其占比超过了C2,为33%左右,到平稳期时占比最高,达到了42.97%;C2占比则是持续下降,由初始的35.72%降至5.49%,在整个过程中从占比最高转变为占比最低;C1在实验过程中基本保持着高占比,35%左右,随着暴露进行也呈现了一定的增加趋势,后2个时期则呈现减少趋势。
|
图 2 紫贻贝不同浓度暴露组内脏组织中C1、C2、GTX5 3种毒素浓度变化及含量占比 Fig.2 Changes in concentrations and their content ratios of C1, C2 and GTX5 toxins in visceral tissues of M. edulis exposed to different concentrations a: 高浓度组; b: 低浓度组 a: High concentration group; b: Low concentration group |
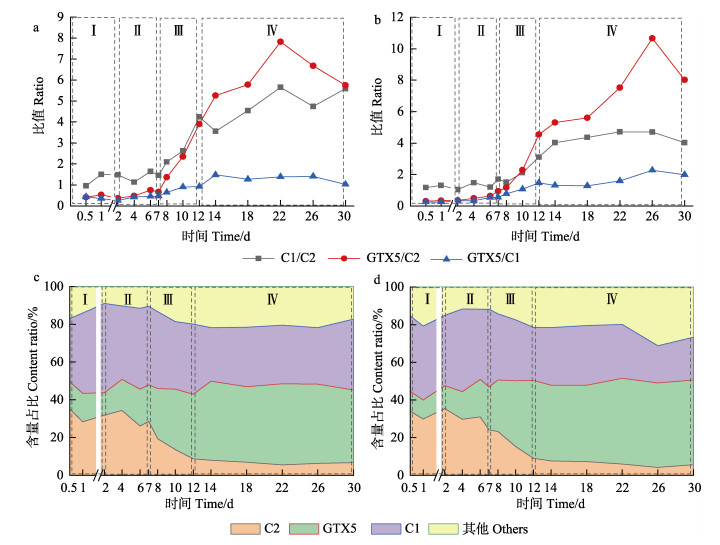
毒素的占比变化反映出其在紫贻贝体内必然存在一定的转化关系,以含量高且变化显著的C1、C2和GTX5毒素为切入点展开分析,通过面积反映3种主要毒素占比情况,结果如图 3c、图 3d所示。C1、C2和GTX5三者之间转化关系如图 3a、图 3b所示,采用比值计算法(朱明远等, 2003),3种毒素两两之间的转化程度可以通过折线图的斜率反映。代谢阶段能排除蓄积对毒素占比产生的影响,更直观地反映毒素之间的变化,因此,选取Ⅲ、Ⅳ时期进行分析:在Ⅲ时期,主要呈现为C1/C2 > GTX5/C2 > GTX5/C1的趋势,C2→C1的转化呈优势,其中,在低浓度组尤其显著;在Ⅳ时期,则呈现为GTX5/C2 > C1/C2 > GTX5/C1的趋势,C2→GTX5的转化则呈明显优势,而C2→C1的转化也较为显著。
|
图 3 紫贻贝不同浓度暴露组内脏组织中C1、C2、GTX5 3种毒素相互之间比值变化及含量占比变化 Fig.3 Changes of the ratios and content ratios of C1, C2 and GTX5 toxins in the visceral tissues of M. edulis exposed to different concentrations a和c:高浓度组;b和d:低浓度组 a and c: High concentration group; b and d: Low concentration group |
本研究中,C1 (TEF=0.01)、C2 (TEF=0.1)和GTX5 (TEF=0.1) 3种毒素间共存在3种转化关系,根据TEF毒性当量因子不同,C2→C1转化过程为高毒性转化为低毒性;C1→GTX5为低毒性转化为高毒性;C2→GTX5为同种毒性之间转化。高毒性毒素转化为低毒性毒素种类符合降低毒性的生物自我保护机制;低毒性转化为高毒性现象的一种可能的原因是贝类重新蓄积(田华, 2009),紫贻贝对自身毒素排除到环境中,由于没有及时将毒素从周围环境清除,造成紫贻贝对毒素的重新蓄积,进而转化为更高毒性毒素种类。
3 讨论本研究所用链状亚历山大藻GY-H25株购自上海光宇生物科技有限公司,主要PSTs组分为C2、C1和GTX5,单藻细胞产毒为2 pg STXeq/cell。在高、低2个浓度产毒藻的环境中蓄积7 d,紫贻贝的蓄积率分别为14.84%和19.92%;经过23 d的代谢,毒素排除率分别为74.39%和59.15%。影响贻贝蓄积量的因素包括藻的单细胞产毒、暴露浓度和持续时间,通过对已有研究结果(邱江兵, 2014; Sekiguchi et al, 2001; Tobke et al, 2021)对比并结合本实验发现,贻贝暴露于PSTs产毒藻的蓄积率在同属同种物种间无明显差异,且不随着藻种、单胞产毒、暴露浓度和持续时间变化。故暴露浓度与藻的单胞产毒联合作用决定蓄积量上限。与自然条件下相比,本实验由于产毒藻数量限制,远未达到有害藻华暴发时的暴露浓度,因此,蓄积总量远低于自然有害藻华暴露下秦皇岛贻贝蓄积总量。
紫贻贝快速代谢时期,内脏中PSTs含量急速下降,毒素排除率达72%,平均每天排除率为14.4%,与Bricelj等(1998)的研究结果一致。研究表明,麻痹性贝类毒素在内脏组织中的蓄积会造成贝类肠溶体膜破坏,对肠细胞产生毒害作用,以及造成谷胱甘肽巯基转移酶(glutathione S-transferase, GST)和谷胱甘肽还原酶(glutathione reductase, GR)等酶活性影响(Painefilu et al, 2020),包括肠细胞破碎、消化腺结缔组织空泡化等,因此,贝类个体正常生理机能受到严重影响(Álvarez et al, 2019)。并且该组织受损伤程度受暴露浓度影响,贝类内脏组织损伤情况随着暴露浓度的升高更加显著(Villalobos et al, 2020)。因此,高浓度组后期代谢速率减慢,更容易造成长期毒素滞留,威胁人类生命健康安全。
Choi等(2003)通过实验证明了PSTs在双壳贝类体内存在不同毒素间转化现象。Oshima(1995)研究证明,此种转化作用主要包括化学转化和酶促转化2种类型,具体表现为C1、C2向GTX5、GTX3转化,GTX2向GTX3、STX转化,NEO向STX的转化等。而紫贻贝内脏组织中毒素间相互转化以酶促转化中的天然还原剂催化的N1位羟基的还原反应为主导(如C1/2→GTX5),此外,还包括β型向α型差向异构体转化的化学转化过程(如C2→C1) (邱江兵, 2014)。研究表明,酸碱条件变化会影响紫贻贝体内C1和C2的差向异构化(田华, 2009);且C1、C2和GTX5同属于具有相同R4基团的N-磺酰氨甲酰基类毒素,而N-磺基转移酶可实现C1/2向GTX5的转化(邱江兵, 2014)。本研究中,链状亚历山大藻产毒主要为低毒性的C1/2,而紫贻贝内脏组织主要代谢转化产物为GTX5。由GTX5占比持续稳定上升推测,蓄积在贝类内脏中的毒素主要发生的是低毒性组分C1/2向高毒性组分GTX5的转化。结合C2占比的持续下降,GTX5/C2值持续升高,推测在快速代谢时期和平稳期,GTX5的转化来源主要是C2,C2在前2个时期保持高占比,后2个时期占比不断降低,进一步推测C2不断转化为C1和GTX5;C1占比在前2个时期升高而后2个时期降低,推测在快速代谢时期和平稳期,C1相关的转化中C1→GTX5占据主导,消耗C1使其含量占比减小。
自然环境中,紫贻贝对PSTs不敏感且具有滤食性特征,因此,可大量滤食有毒甲藻,从而蓄积高浓度的PSTs。而在紫贻贝PSTs蓄积靶器官—内脏组织中,蓄积的PSTs发生毒素组分间转化作用,使得低毒性毒素组分得以转化为高毒性组分,从而导致紫贻贝终端毒性与消费者摄食风险大大增加。本研究发现,紫贻贝经过23 d代谢消除,内脏组织中残留毒素仍远高出欧盟限值标准,且在12~30 d时间段的内脏组织中毒素含量趋于稳定,甚至由于毒素转化现象导致毒素含量呈现上升趋势,因此,难以预测需经过多少天代谢内脏中毒素含量可低于限制标准。Yu等(2021)首次发现一株渤海链状亚历山大藻产毒主要组分为GTX1-4类毒素,与此前发现的黄渤海域主产C1-2类毒素藻株相比具有更高毒性,高毒性藻株的出现增加了贝类暴露的风险。因此,需要加强重点养殖区紫贻贝中PSTs风险监控,及时发布预警信息,并提高消费者安全消费意识,以确保消费者健康安全,保护区域产业健康发展和社会稳定。
4 结论紫贻贝暴露于链状亚历山大藻后,表现出高蓄积和高残留的风险特征。内脏组织PSTs蓄积代谢在高、低浓度组暴露下具有一致规律,蓄积浓度高达6815.36 μg STXeq/kg,且代谢23 d后,毒素在内脏中残留含量仍超过限量标准。PSTs在紫贻贝内脏组织中存在以酶促转化中的还原反应为主导的低毒性向高毒性转化的趋势,主要表现为C1/C2→GTX5。其中,C2→GTX5为主要转化途径,其次是C1→GTX5。因此,紫贻贝摄食链状亚历山大藻后增加了PSTs的风险,开展针对性的强化监测和风险评估对保护消费者食用安全和渔业生产具有重要意义。本研究有助于深入了解紫贻贝中PSTs风险形成的机制,为系统研究PSTs风险性监测提供科学支撑。
ÁLVAREZ G, DIAZ P A, GODOY M, et al. Paralytic shellfish toxins in surf clams Mesodesma donacium during a large bloom of Alexandrium catenella dinoflagellates associated to an intense shellfish mass mortality. Toxins, 2019, 11(4): 188 DOI:10.3390/toxins11040188 |
BING X F, WU H Y, WANG Q, et al. Metabolic profile of paralytic shellfish toxin in scallop Chlamys farreri. Journal of Fishery Sciences of China, 2017, 24(3): 623-632 [邴晓菲, 吴海燕, 王群, 等. 麻痹性贝类毒素在栉孔扇贝体内的代谢轮廓. 中国水产科学, 2017, 24(3): 623-632] |
BORSA P, DAGUIN C, CAETANO S R, et al. Nuclear-DNA evidence that northeastern Atlantic Mytilus trossulus mussels carry M. edulis genes. Archives Ouvertes, 1999, 65: 504-507 |
BOUNDY M J, SELWOOD A I, HARWOOD D T, et al. Development of a sensitive and selective liquid chromatography- mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. Journal of Chromatography A, 2015, 1387: 1-12 DOI:10.1016/j.chroma.2015.01.086 |
BRICELJ V M, SHUMWAY S E. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Reviews in Fisheries Science, 1998, 6(4): 315-383 DOI:10.1080/10641269891314294 |
CHANG M Y, LI C L, DONG J, et al. Dynamic changes of phytoplankton composition during cyanobacteria blooms in aquaculture ponds. Progress in Fishery Sciences, 2019, 40(1): 36-45 [常孟阳, 李晨露, 董静, 等. 蓝藻水华暴发期间养殖池塘浮游藻类动态变化. 渔业科学进展, 2019, 40(1): 36-45 DOI:10.19663/j.issn2095-9869.20171227002] |
CHENG F. Effects of different microalgae and air exposure treatments on the intake and metabolism of Pteria penguin. Master´s Thesis of Hainan University, 2018, 62 [程芬. 不同单胞藻及干露处理对企鹅珍珠贝的摄食和代谢影响. 海南大学硕士研究生学位论文, 2018, 62]
|
CHOI M C, HSIEH D P H, LAM P K S, et al. Field depuration and biotransformation of paralytic shellfish toxins in scallop Chlamys nobilis and green-lipped mussel Perna viridis. Marine Biology, 2003, 143: 927-934 DOI:10.1007/s00227-003-1148-y |
CONDIE S A, OLIVER E C J, HALLEGRAEFF G M. Environmental drivers of unprecedented Alexandrium catenella dinoflagellate blooms off eastern Tasmania, 2012–2018. Harmful Algae, 2019, 87: 101628 DOI:10.1016/j.hal.2019.101628 |
DAI L, YU R C, GENG H X, et al. Resting cysts of Alexandrium catenella and A. pacificum (Dinophyceae) in the Bohai and Yellow Seas, China: Abundance, distribution and implications for toxic algal blooms. Harmful Algae, 2020, 93: 101794 DOI:10.1016/j.hal.2020.101794 |
FREITAS R, COPPOLA F, COSTA S, et al. The influence of temperature on the effects induced by triclosan and diclofenac in mussels. Science of the Total Environment, 2019, 663: 992-999 DOI:10.1016/j.scitotenv.2019.01.189 |
GAO Y, SASSENHAGEN I, RICHLEN M L, et al. Spatiotemporal genetic structure of regional-scale Alexandrium catenella dinoflagellate blooms explained by extensive dispersal and environmental selection. Harmful Algae, 2019, 86: 46-54 DOI:10.1016/j.hal.2019.03.013 |
GU H F, ZENG N, LIU T T. Morphology, toxicity, and phylogeny of Alexandrium (Dinophyceae) species along the coast of China. Harmful Algae, 2013, 27: 68-81 DOI:10.1016/j.hal.2013.05.008 |
HU X Q, XIE W C, LI M, et al. Review on accumulation and metabolism of paralytic shellfish toxins in toxins. Food and Chemistry, 2021, 37(3): 187-194 [呼晓群, 解万翠, 李敏, 等. 贝类中麻痹性贝类毒素的蓄积及代谢研究进展. 食品与机械, 2021, 37(3): 187-194 DOI:10.13652/j.issn.1003-5788.2021.03.034] |
HUANG H N, LU J L, LIN S E, et al. Simultaneous determination of twelve paralytic shellfish poisoning toxins in bivalve molluscs by UPLC-MS/MS and its applications to a food poisoning incident. Toxicon, 2019, 174: 1-7 |
KIM Y O, CHOI J, BAEK S H, et al. Tracking Alexandrium catenella from seed-bed to bloom on the southern coast of Korea. Harmful Algae, 2020, 99: 101922 DOI:10.1016/j.hal.2020.101922 |
LIANG W F. Effects of different factors on the feeding rate, oxygen consumption rate and ammonia excretion rate of rock scallop. Master´s Thesis of Dalian Ocean University, 2016, 55 [梁伟锋. 不同因子对岩扇贝摄食率、耗氧率和排氨率的影响. 大连海洋大学硕士研究生学位论文, 2016, 55]
|
MA F F. Biotransformation of paralytic shellfish toxins in shellfish and effect on its immune system. Master´s Thesis of Ocean University of China, 2012, 65 [马菲菲. 麻痹性贝毒在贝类中的生物转化及其对机体免疫系统的影响. 中国海洋大学硕士研究生学位论文, 2012, 65]
|
MIAO Y P, YUAN Q, ZHOU H N, et al. Effects of some ecological factors on the growth and toxin production of dinoflagellate Alexandrium minutum Amtk-9. Marine Fisheries, 2009, 31(3): 279-287 [缪宇平, 袁骐, 周宏农, 等. 环境因子对微小亚历山大藻Amtk-9生长与产毒的综合影响. 海洋渔业, 2009, 31(3): 279-287 DOI:10.3969/j.issn.1004-2490.2009.03.008] |
OSHIMA Y. Chemical and enzymatic transformation of paralytic shellfish toxins in marine organisms. Harmful Marine Algal Blooms. Paris, New York. Lavoisier Pub; Andover, England UK Intercept, 1995
|
PAINEFILU J C, BIANCHI V A, KROCK B, et al. Effects of paralytic shellfish toxins on the middle intestine of Oncorhynchus mykiss: Glutathione metabolism, oxidative status, lysosomal function and ATP-binding cassette class C (ABCC) proteins activity. Ecotoxicology and Environmental Safety, 2020, 204: 111069 DOI:10.1016/j.ecoenv.2020.111069 |
QIU J B. Metabolic transformation of paralytic shellfish toxins by bivalve molluscs and their physiological and biochemical responses. Master´s Thesis of Ocean University of China, 2014, 90 [邱江兵. 双壳贝类对麻痹性贝毒的代谢转化及其生理生化响应. 中国海洋大学硕士研究生学位论文, 2014, 90]
|
RIISGARD H U, BOTTIGER L, PLEISSNER D, et al. Effect of salinity on growth of mussels, Mytilus edulis, with special reference to Great Belt (Denmark). Open Journal of Marine Science, 2012, 2(4): 167-176 DOI:10.4236/ojms.2012.24020 |
SEKIGUCHI K, SATO S, KAGA S, et al. Accumulation of paralytic shellfish poisoning toxins in bivalves and an ascidian fed on Alexandrium tamarense cells. Fisheries Science, 2001, 67(2): 301-305 DOI:10.1046/j.1444-2906.2001.00228.x |
SHAO S N, MIAO Y P, ZHOU H N, et al. Effects of ecological factors on the growth of dinoflagellate Alexandrium catenella. Marine Fisheries, 2011, 33(1): 66-73 [邵盛男, 缪宇平, 周宏农, 等. 环境因子对链状亚历山大藻生长的影响. 海洋渔业, 2011, 33(1): 66-73] |
TIAN H. Study on the accumulation, transformation, depuration and diagnoses target of paralytic shellfish poisoning toxins. Doctoral Dissertation of Ocean University of China, 2009, 105 [田华. 麻痹性贝毒的累积、转化、排出过程及预警诊断指标研究. 中国海洋大学博士研究生学位论文, 2009, 105]
|
TOBKE J, GIARRATANO E, ORTIZ A, et al. Chitosan performance during paralytic shellfish toxins (PST) depuration of Mytilus chilensis exposed to Alexandrium catenella. Toxicon, 2021, 195: 48-57 |
VILLALOBOS L L G, TOBKE J L, MONTOYA N G, et al. Experimental exposure of the mussel Mytilus platensis (d'Orbigny, 1842) to the dinoflagellate Alexandrium catenella from Argentine Patagonia. Ecotoxicology, 2020, 29(2): 226-235 |
VISCIANO P, SCHIRONE M, BERTI M, et al. Marine biotoxins: Occurrence, toxicity, regulatory limits and reference methods. Frontiers in Microbiology, 2016, 7: 1051 |
WANG D Z, ZHANG S F, ZHANG Y, et al. Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: A molecular overview. Journal of Proteomics, 2016, 135: 132-140 |
WANG N, LIANG Y B, SONG T, et al. Accumulation, transformation and depuration of paralytic shellfish toxins by the scallop Patinopecten yessoensis. Marine Environmental Science, 2013, 32(4): 565-569 [汪娜, 梁玉波, 宋涛, 等. 麻痹性贝毒在虾夷扇贝体内累积、转化和排出过程研究. 海洋环境科学, 2013, 32(4): 565-569] |
WANG X Q, SAMUEL P S R, WU Y L, et al. Impact of seawater acidification on the energy budget of Mytilus galloprovincials. Progress in Fishery Sciences, 2019, 40(3): 21-30 [王晓琴, Samuel P S Rastrick, 吴亚林, 等. 海水酸化胁迫对紫贻贝能量分配的影响. 渔业科学进展, 2019, 40(3): 21-30] |
YU R C, LUO X. Status and research perspectives on toxic algae and phycotoxins in the coastal waters of China. Studia Marina Sinica, 2016, 51: 155-166 [于仁成, 罗璇. 我国近海有毒藻和藻毒素的研究现状与展望. 海洋科学集刊, 2016, 51: 155-166] |
YU R C, ZHANG Q C, LIU Y, et al. The dinoflagellate Alexandrium catenella producing only carbamate toxins may account for the seafood poisonings in Qinhuangdao, China. Harmful Algae, 2021, 103: 101980 |
YU X W, CHEN Y C, WANG Y J, et al. Effects of temperature and light intensity on growth and toxin productivity of Alexandrium minutum Z1. Transactions of Oceanology and Limnology, 2018, 9(5): 66-71 [余新威, 陈雨涔, 王亚军, 等. 温度、光照对微小亚历山大藻Z1产毒含量和组成的影响. 海洋湖沼通报, 2018, 9(5): 66-71] |
ZHAI Y X, GUO M M, JIANG Y H, et al. Analysis on the quality and safety risks of shellfish products. China Fishery Quality and Standards, 2020, 10(4): 1-25 [翟毓秀, 郭萌萌, 江艳华, 等. 贝类产品质量安全风险分析. 中国渔业质量与标准, 2020, 10(4): 1-25] |
ZHANG J H, LI H J, CAI M R, et al. Analysis on toxin monitoring result of shellfish samples in coastal waters in Zhangzhou City, 2018–2019. Strait Journal of Preventive Medicine, 2020, 26(3): 12-14 [张锦宏, 李海杰, 蔡茂荣, 等. 漳州市2018—2019年沿海海域贝类样品毒素监测结果分析. 海峡预防医学杂志, 2020, 26(3): 12-14] |
ZHANG Q C, QIU L M, YU R C, et al. Emergence of brown tides caused by Aureococcus anophagefferens Hargraves et Sieburth in China. Harmful Algae, 2012, 19: 117-124 |
ZHANG Y Y, YAN G W, WU H Y, et al. Establishment and application of detection methods to paralytic shellfish poisoning in water based on SPE and SPATT methods. Oceanologia et Limnologia Sinica, 2020, 51(2): 298-306 [张亚亚, 闫国旺, 吴海燕, 等. 基于SPE与SPATT的水体中麻痹性贝类毒素检测方法构建与应用. 海洋与湖沼, 2020, 51(2): 298-306] |
ZHU M Y, ZOU Y L, WU R J, et al. Accumulation and depuration of paralytic shellfish poisons (PSP) in Chinese scallop Chlamys farreri. Acta Oceanologica Sinica, 2003, 25(2): 75-83 [朱明远, 邹迎麟, 吴荣军, 等. 栉孔扇贝体内麻痹性贝毒的累积与排出过程研究. 海洋学报, 2003, 25(2): 75-83] |



