2. 中国水产科学研究院淡水研究中心淡水渔业与种质资源利用重点实验室 江苏 无锡 214000
2. Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi, Jiangsu 214000, China
细胞色素c (cytochrome c,Cytc)是一种可溶性氧化还原活性血红素蛋白。作为线粒体呼吸链(mitochondrial respiratory chain, MRC)中传递电子的载体,影响ATP的产生。Cytc缺失或功能失调会引起线粒体呼吸链功能异常,ATP缺乏,导致细胞死亡。正常状态下Cytc位于线粒体内膜,参与电子传递,当细胞受凋亡信号刺激后便从线粒体释放至细胞质(Welchen et al, 2016)。Cytc在被释放到细胞质之后,与凋亡酶激活因子1 (Apaf-1)形成凋亡小体,促进caspases-9前体的自身活化,并触发caspase级联反应导致细胞凋亡(Chereau et al, 2005)。Cytc还可以通过干扰电子传递,阻断能量合成,促进自由基产生等方式参与细胞凋亡过程(Huettemann et al, 2011、2012)。
细胞凋亡是由一系列相关基因严格调控的细胞程序性死亡,在抵御病原入侵、维持机体内环境稳态等方面有着重要意义(Elmore, 2007)。在对哺乳动物细胞凋亡的研究中,普遍认为Cytc从线粒体释放到细胞质中是凋亡开始的关键一步(Adrain et al, 2000)。对鳞翅目昆虫Cytc与细胞凋亡的研究结果与哺乳动物类似,都认为Cytc在调节细胞凋亡中起着关键作用(Liu et al, 2012)。随着对Cytc在细胞凋亡和免疫方面的不断深入研究,越来越多证据表明Cytc能够参与病毒感染诱导的细胞凋亡。白斑综合征病毒(white spot sydrome virus, WSSV)刺激可诱导凡纳滨对虾(Litopenaeus vannamei)肝胰腺和血细胞中Cytc基因的表达(Hu et al, 2016)。赤点石斑鱼(Epinephelus akaara)神经坏死病毒(RGNNV)诱导的石斑鱼肝细胞凋亡与Cytc释放相关(Chen et al, 2007)。在无脊椎动物中,He等(2015)研究发现,家蚕(Bombyx mori)微孢子虫感染宿主BMC细胞后通过下调Apaf-1和Cytc的表达抑制细胞凋亡,为自身生存创造最佳环境。家蚕Cytc基因能通过介导线粒体通路诱导细胞凋亡参与家蚕核型多角体病毒(BmNPV)应答(Wang et al, 2019)。克氏原螯虾(Procambarus clarkii)是我国主要的水产经济物种,病害的发生尤其是WSSV引起的暴发性疾病制约着克氏原螯虾养殖业健康发展(郝晨光, 2020; 徐日俊等, 2020)。然而,尽管Cytc在细胞凋亡和能量代谢中发挥重要作用,但对Cytc介导的细胞凋亡在克氏原螯虾WSSV病毒感染中的作用目前还未见报道。
鉴于细胞凋亡在机体免疫方面的重要作用,本研究克隆克氏原螯虾凋亡细胞色素c基因(PcCytc)全长,分析PcCytc在克氏原螯虾各组织中的表达情况,并通过RNA干扰技术探究PcCytc在WSSV感染过程中参与细胞凋亡的机制。以期更深入地了解凋亡相关因素在克氏原螯虾免疫反应中的潜在作用。
1 材料与方法 1.1 实验材料本研究所用克氏原螯虾取自中国水产科学研究院淡水渔业研究中心扬中实验基地。选取体重为20 g左右的克氏原螯虾,在实验室水族箱暂养1周,养殖温度为24~27℃。1周后,随机选取健康完整的雌雄对虾各3尾,分别取其肝胰腺、鳃、肠、胃、淋巴、心脏、肌肉、眼柄神经组织,并将3尾虾的同种组织样品混合于1管,立即置于–80℃保存。
1.2 引物设计根据实验室转录组数据得到的部分Cytc基因保守序列,通过Primer-BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi)设计PcCytc的上下游引物(表 1),并委托苏州金唯智公司合成。
|
|
表 1 实验中所用引物序列 Tab.1 Sequences of primers used in this study |
用Trizol提取总RNA,提取的RNA浓度和纯度用微型紫外分光光度计(Q5000)测定,RNA产物通过1.2%琼脂糖凝胶电泳确定完整性。使用RevertAid First Strand cDNA Synthesis Kit (索莱宝)合成用于RACE (rapid amplification of cDNA ends)的模板cDNA。
RACE降落PCR技术和半巢式PCR技术分别用于扩增PcCytc的3′端和5ʹ端。实验所用引物见表 1。第1次扩增反应以3ʹ-、5ʹ-cDNA为模板进行,第2次扩增反应以对应的第1次扩增反应产物稀释10倍为模板。用SanPrep Column DNA Gel Extraction Kit (生工, 上海)纯化扩增的DNA片段。纯化后的DNA与pMD18T连接,之后转入冰上融化的DH5α感受态细胞过夜培养,筛选阳性单克隆并进行菌落PCR鉴定,将含有目的基因的菌液测序(生工, 上海)。成功获得5ʹ和3ʹ端序列,然后,通过DNAstar软件进行拼接,最终获得PcCytc的cDNA全长序列。
1.4 生物信息学分析根据基因测序结果,使用NCBI中的ORF finder (http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi)寻找基因序列的开放阅读框(ORF),并翻译其编码的氨基酸序列;氨基酸同源性通过DNAMAN V.9软件进行比较;然后通过ExPASy (http://www.expasy.org)和Signal P3.0程序(http://www.cbs.dtu.dk/services/SignalP)分析蛋白质的理化性质和信号肽的存在。跨膜结构域由TMHMM (http://www.cbs.dtu.dk/services/TMHMM)预测。此外,使用在线工具SWISS-MODEL (https://swissmodel.expasy.org)通过同源建模方法模拟蛋白质的三维结构;使用Mafft和MEGA 10.0软件基于NJ方法构建系统发育树。
1.5 PcCytc的表达分析为了分析基因在不同组织中的分布和表达模式,使用HiScript 1st Strand cDNA Synthesis Kit (诺唯赞, 南京)将总RNA反转录为第一链cDNA。以上述所制备的cDNA为样品,浓度稀释到(55±2) ng/μL,使用ChamQ Universal SYBR qPCR Master Mix试剂盒进行荧光定量PCR。每个样品均设3个重复,以β-actin为内参基因。实验所用引物见表 1。反应体系为20 μL:1 μL模板cDNA,0.8 μL上游引物,0.8 μL下游引物,10 μL ChamQ Universal SYBR qPCR Master Mix,7.4 μL ddH2O。反应程序:预变性95℃ 30 s;95℃ 10 s,60℃ 30 s,40个循环;95℃ 15 s,60℃ 60 s,95℃ 15 s。计算方法采用2‒ΔΔCt法。
1.6 WSSV感染实验实验开始前,在200尾克氏原螯虾中随机选取20尾,经检测均为WSSV阴性。之后,选取100尾平均体重为20 g的健康克氏原螯虾,用于攻毒实验。将实验虾随机分为2组,每组50尾,分别于第2腹节注射PBS和WSSV。对照组注射100 μL无菌磷酸盐(PBS, pH7.4),实验组注射100 μL WSSV (1.0×108拷贝数/mL)。在注射后0、6、12、24、48和96 h,分别随机选取3尾完整、健康的克氏原螯虾,取其肝胰腺、肠道和肌肉组织,立即置于–80℃保存。用qRT-RCR检测各组织中PcCytc在不同时间的表达量,具体步骤同1.5所述。该实验同时设置3个平行。
1.7 RNA干扰(RNA interfere, RNAi)实验以普通cDNA为模板,经EX-Taq酶扩增获得含有T7启动子的DNA模板,并用1.2%的琼脂糖凝胶进行电泳检测。按照T7 RNAi Transcription Kit (诺唯赞, 南京)试剂盒说明书合成dsRNA,并用同样的方法合成dsGFP。按照5~8 μg/g虾的比例注射dsRNA。注射实验分为3组,分别为注射PBS组、注射dsGFP组和注射dsCytc组,每组3个平行,各50尾克氏原螯虾。分别于注射后24、48、72、96和120 h取肌肉,提取总RNA,用实时荧光定量PCR (qRT-PCR)法检测干扰效率,并在干扰效率最强时间段内注射WSSV。以β-actin为内参测定PcCytc的相对mRNA水平。
为分析PcCytc对WSSV感染过程的调控作用,将克氏原螯虾分为4组:dsCytc+WSSV组、dsGFP+ WSSV组、WSSV组和PBS组,每组3个平行,每个平行50尾克氏原螯虾。dsCytc+WSSV组和dsGFP+ WSSV组在分别注射上述剂量dsRNA 24 h后,注射100 μL WSSV (1.0×108拷贝数/mL),并同时注射WSSV组。分别于注射WSSV后的0、24、48和72 h采集各组3只克氏原螯虾的肌肉,提取DNA,并将24 h时采集的肌肉提取RNA。将提取的DNA进行荧光定量PCR检测,并根据WSSV标准曲线的线性方程(Y= –3.715x+41.983,R2=0.996 6)和Ct值计算出每1 mg组织WSSV病毒的拷贝数。提取的RNA通过qRT-PCR检测凋亡相关基因的表达水平。RNA的提取、cDNA的制备以及定量实验同1.3和1.5所述。
1.8 统计分析所有数据均以平均值±标准差(Mean±SD, n=3)表示,并使用SPSS 25.0进行显著性分析,显著水平设为P < 0.05和P < 0.01。使用GraphPad Prism 8.0.1和Origin 2018作图。
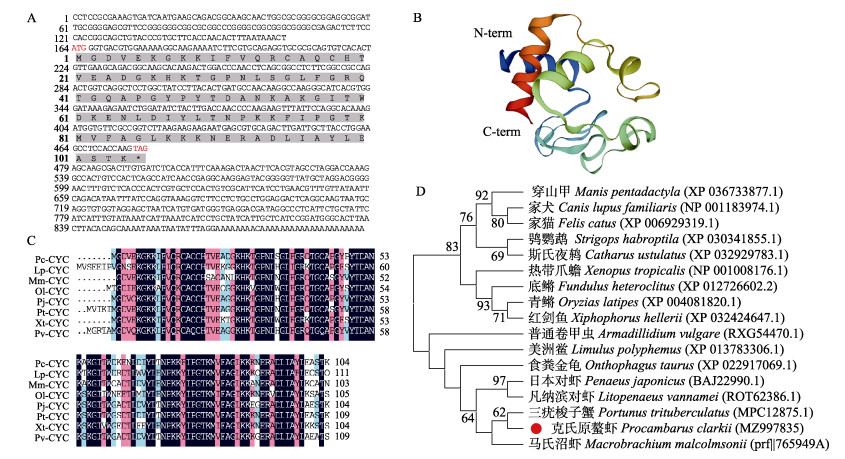
2 结果与分析 2.1 PcCytc的序列分析PcCytc基因全长为897 bp,包括163 bp的5′-UTR、419 bp的3′-UTR和315 bp开放阅读框,编码104个氨基酸(图 1A)。ExPASy Prot-Param预测其分子量为11.49 kD,理论等电点为9.49。SMART程序对蛋白质结构域的预测表明,PcCytc无信号肽,无跨膜结构;共有5个磷酸化位点,其中丝氨酸位点1个,苏氨酸位点1个,酪氨酸位点3个。PcCytc在4~103位置包含1个典型的Cytochrom_C结构域。三级结构图展示了PcCytc的N端和C端(图 1B)。
|
图 1 PcCytc基因生物信息学分析 Fig.1 Bioinformatics analysis of PcCytc A:PcCytc基因核苷酸及推导氨基酸序列,红色标出的为起始密码子(ATG)和终止密码子(TAG),灰色部分序列为Cytochrom-C结构域对应的氨基酸序列;B:PcCytc蛋白三级结构;C:PcCytc与其他物种Cytc的氨基酸序列多重比对;D:PcCytc系统发育进化树 A: Nucleotide sequence and deduced amino acid sequence of gene PcCytc, the red marks the start codon (ATG) and the stop codon (TAG), the gray part of the sequence is the Cytochrom-C domain corresponding to the cDNA sequence; B: The tertiary structure of PcCytc; C: Multiple alignment of the amino acid sequences of PcCytc and Cytc of other species; D: Phylogenetic tree of PcCytc |
利用NCBI BLASTP对PcCytc基因进行同源性比较,结果显示,PcCytc基因与三疣梭子蟹(Portunus trituberculatus)、日本对虾(Penaeus japonicus)、凡纳滨对虾等物种具有较高的氨基酸序列同源性,分别为89.42%、84.62%和84.47% (图 1C)。系统进化树中,PcCytc基因与三疣梭子蟹聚为一支,所有甲壳类聚为一大支,其他物种聚为一大支(图 1D)。
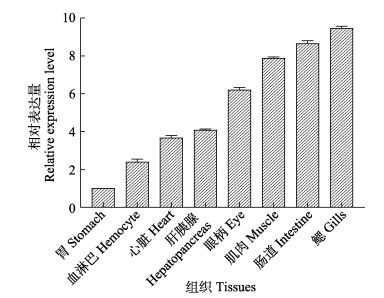
2.2 PcCytc在不同组织中的表达应用qRT-PCR技术,探讨PcCytc在克氏原螯虾肝胰腺、鳃、心、肠道、胃、眼柄、肌肉和血淋巴等组织中的表达差异。如图 2所示,PcCytc基因在所有组织中均有表达,而且主要在鳃和肠中表达,在胃中表达最低。鳃中表达量为胃中表达量的9.46倍。其次在肠道和肌肉中表达也较高,分别为胃表达量的8.65和7.88倍。
|
图 2 PcCytc在各组织的相对表达量 Fig.2 Relative expression level of PcCytc in different tissues |
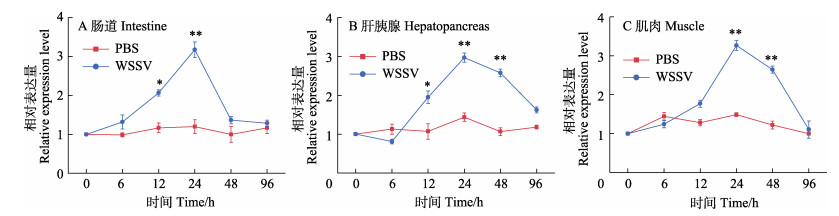
在肠道、肝胰腺和肌肉中(图 3),相比于PBS组,注射WSSV组PcCytc的表达水平在前24 h呈不断上调趋势,并在24 h达最高值,分别约为此时PBS组表达量的2.65、2.07和2.20倍,均存在极显著性差异(P < 0.01),之后表达水平开始下调,直到96 h恢复至正常水平,整体表现为诱导表达模式。在48 h时,尽管注射WSSV组肝胰腺和肌肉中PcCytc的表达水平有所下调,但仍分别为此时PBS组表达量的2.39和2.17倍,存在显著差异(P < 0.05)。
|
图 3 克氏原螯虾肠道(A)、肝胰腺(B)和肌肉(C)中PcCytc基因的WSSV刺激表达谱 Fig.3 WSSV stimulation expression profile of PcCytc gene in the intestine (A), hepatopancreas (B) and muscle (C) of P. clarkii *表示差异显著(P < 0.05),**表示差异极显著(P < 0.01),下同。 * denotes significant difference (P < 0.05), ** denotes highly significant difference (P < 0.01). The same as below. |
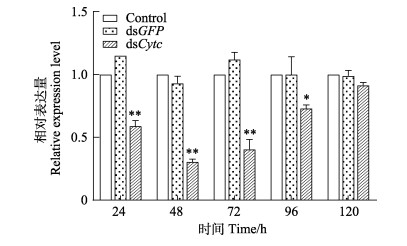
考虑到实验中dsRNA和WSSV需要肌肉注射(注射部位会影响RNA干扰效率),且肌肉中PcCytc表达量较高(史晓丽等, 2018; 范红弟等, 2020),基因干扰后选择肌肉继续后续的定量研究。在RNA干扰后24、48、72、96和120 h进行PcCytc基因的qRT-PCR检测,验证干扰效率。以未注射组为对照,如图 4所示,在注射后24、48、72、96和120 h dsCytc组克氏原螯虾体内PcCytc的表达量分别为未注射组的59.9%、30.5%、40.5%、73.0%和91.5%。说明PcCytc的干扰效率明显,而且在注射后48 h和72 h内干扰效率最强。
|
图 4 克氏原螯虾PcCytc在dsCytc干扰后的表达 Fig.4 PcCytc mRNA expression profiles after silencing by dsCytc |
注射dsCytc后,在WSSV感染下,克氏原螯虾肌肉中WSSV的病毒拷贝数见图 5。如图 5所示,注射dsCytc组的病毒拷贝数始终高于其他3组,在注射WSSV的24和48 h dsCytc组克氏原螯虾WSSV的病毒拷贝数与未注射组相比极显著增加(P < 0.01),在注射WSSV的72 h表现为显著增加(P < 0.05)。其中,在注射WSSV的24 h,WSSV组、dsGFP+WSSV组和dsCytc组克氏原螯虾WSSV的病毒拷贝数分别约为103.18、103.21和104.82拷贝数/mg;在注射WSSV的48 h,WSSV组、dsGFP+WSSV组和dsCytc组克氏原螯虾WSSV的病毒拷贝数分别约为104.97、104.98和106.31拷贝数/mg;在72 h分别上升为105.59、105.61和106.76拷贝数/mg。
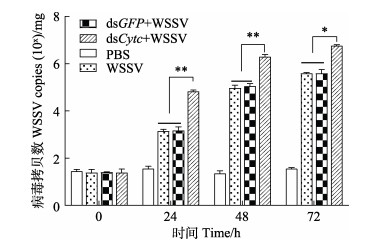
|
图 5 RNAi和WSSV注射后克氏原螯虾体内病毒拷贝数变化 Fig.5 Changes in virus copies in P. clarkii after RNAi and WSSV injection |
注射dsCytc后,在WSSV感染条件下,克氏原螯虾肌肉中凋亡相关基因的表达量如图 6A所示。与PBS组相比,其他各组在注射WSSV 24 h后克氏原螯虾bcl-2、bax和caspase-3基因均表现为不同程度的表达上调。其中,注射dsCytc组的bcl-2和caspase-3基因表达量与未注射组相比差异极显著(P < 0.01),分别表现为表达上调和下调。各组间的克氏原螯虾bax基因表达量差异显著(P < 0.05)。图 6B展示了bcl-2/bax值的变化,其中注射dsCytc组bcl-2/bax值与dsGFP组和WSSV组相比显著升高(P < 0.01);注射dsGFP组和WSSV组与PBS组相比显著降低(P < 0.05)。
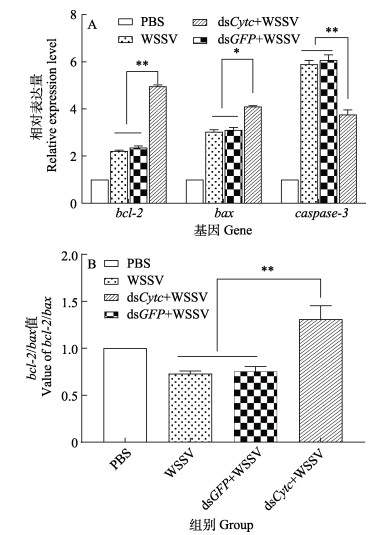
|
图 6 RNAi和WSSV注射后克氏原螯虾凋亡相关基因表达变化 Fig.6 Changes in expression of apoptosis-related genes in P. clarkii after RNAi and WSSV injection |
本研究克隆获得克氏原螯虾PcCytc基因全长序列,PcCytc基因全长为897 bp,编码282个氨基酸。结构预测表明,PcCytc含有1个保守的Cytochrom_C结构域,证明其与能量产生有关(Gnaiger et al, 2002)。蛋白同源比对分析表明,该蛋白氨基酸序列与其他物种的Cytc氨基酸序列具有很高的同源性,说明PcCytc在进化上趋于保守,系统发生进化树的结果也证明了该结果。
PcCytc在克氏原螯虾几乎所有主要的组织中均有表达,推测它能够参与线粒体能量代谢(赵丹等, 2012)。PcCytc在肠道和肌肉等高能量消耗组织中表现出相对较高的表达,这与先前在凡纳滨对虾中的研究一致(胡文燕, 2016)。在克氏原螯虾的主要免疫和呼吸组织——鳃中观察到最高表达水平,表明PcCytc可能参与呼吸和免疫等生物过程。根据以上结果,推测PcCytc在不同组织中可能发挥不同的功能。
病毒感染过程与细胞凋亡发生密切相关(于学武, 2010),一方面,细胞通过凋亡过程可以清除病毒,抵抗病毒感染(Nainu et al, 2015);另一方面,病毒在与宿主细胞博弈过程中会进化出一些拮抗或劫持策略,以利于病毒的复制与传播(Miao et al, 2016)。Hu等(2016)研究表明,Cytc能够参与病毒感染过程。在本研究中,当克氏原螯虾感染WSSV后,肠道、肝胰腺和肌肉中PcCytc的表达均显著上调(P < 0.05),并在24 h达最高值(P < 0.01)。说明PcCytc参与了WSSV病毒感染过程。此外,考虑到PcCytc能够做为线粒体呼吸链中的关键要素参与ATP产生(向飞等, 2020),PcCytc的低表达导致能量缺乏。推测,一旦病毒破坏了宿主细胞的能量代谢,宿主可能通过上调PcCytc的表达来弥补损失。
RNAi实验揭示了克氏原螯虾PcCytc在WSSV感染过程中的作用。在WSSV感染后的24、48 h,PcCytc基因RNAi组病毒的拷贝数与未干扰组相比呈极显著增加(P < 0.01),在72 h表现为显著增加(P < 0.05)。该结果表明,PcCytc在抑制WSSV在克氏原螯虾体内复制、延迟感染过程中承担了重要角色。为进一步确认PcCytc是否主要通过细胞凋亡途径抑制WSSV感染,检测部分凋亡相关的重要基因的表达变化。caspase介导的细胞凋亡是甲壳动物抗病毒免疫的重要机制(王磊等, 2020),其中caspase-3又被称为半胱氨酸蛋白32 (cysteine protease 32, CPP32),作为一种效应蛋白调控细胞凋亡,在凋亡信号传导途径中发挥主导功能,其表达量直接反映细胞凋亡程度(Lamkanfi et al, 2010)。而且Rijiravanich等(2008)研究表明,通过RNAi敲除caspase-3可降低受到低剂量WSSV攻击的凡纳滨对虾的死亡率。bcl-2家族蛋白是线粒体凋亡反应的重要守门人,这些蛋白质包括促凋亡(例如bax、bid和bad)和抗凋亡(例如bcl2、mcl-1和bcl-XL)成员(Levine et al, 2008)。它们通过控制线粒体外膜通透性(MOMP) (Chipuk et al, 2008),诱导细胞凋亡因子的释放或阻止细胞色素c从线粒体中释放而抑制半胱天冬酶活性来调节细胞凋亡(Gottlieb, 2001; Scorrano et al, 2003)。其中,bcl-2可以通过阻止细胞色素c释放到细胞质中从而抑制caspase级联反应的激活来阻止细胞凋亡(Chen et al, 2019);bax是bcl-2家族蛋白中第1个鉴定的促凋亡成员,依赖于p53途径介导程序性细胞死亡过程(Knudson et al, 2001; Suzuki et al, 2003)。重要的是,bcl-2/bax的比值被认为是细胞凋亡进程的指标,比值升高表示凋亡被抑制,降低表示凋亡被促进(王乔雨等, 2021)。在本研究中,与PBS组相比,仅注射WSSV组克氏原螯虾的bcl-2、bax和caspase-3基因均表现为不同程度的表达上调,且存在极显著差异(P < 0.01)。说明WSSV能导致克氏原螯虾肌肉细胞凋亡的发生,这和在泥蟹(Scylla paramamosain) (Lin et al, 2020)、凡纳滨对虾(Kakoolaki et al, 2016)、中国明对虾(Fenneropenaeus chinensis) (宋光年, 2010)、三疣梭子蟹(王磊等, 2020; 题兴斌等, 2021)中的研究一致。此外,注射dsCytc组caspase-3的表达量显著下调(P < 0.01),说明在干扰PcCytc后细胞凋亡受到抑制,注射dsCytc组bcl-2/bax比值显著升高(P < 0.01),也证明了该结论。结合上述PcCytc能够显著抑制WSSV在克氏原螯虾体内复制的结论,推测PcCytc能够通过调节血细胞的凋亡来抑制WSSV在克氏原螯虾体内的复制。
综上所述,本研究克隆获得了克氏原螯虾PcCytc基因序列,并发现在WSSV感染后其在克氏原螯虾中的表达上调。PcCytc被干扰后,WSSV的拷贝数显著升高,而且凋亡基因表达水平也发生显著变化。本研究表明,PcCytc可能通过调节细胞凋亡对病毒感染产生抑制作用,该结论为克氏原螯虾响应WSSV感染的免疫机制提供了一些新的见解。
ADRAIN C, MARTIN S J. The mitochondrial apoptosome: A killer unleashed by the cytochrome seas. Trends in Biochemical Sciences, 2000, 26(6): 390-397 |
CHEN J, GONG Y, ZHENG H, et al. SpBcl2 promotes WSSV infection by suppressing apoptotic activity of hemocytes in mud crab, Scylla paramamosain. Developmental and Comparative Immunology, 2019, 100: 103421 DOI:10.1016/j.dci.2019.103421 |
CHEN S P, WU J L, SU Y C, et al. Anti-Bcl-2 family members, zfBcl-xL and zfMcl-1a, prevent cytochrome c release from cells undergoing betanodavirus-induced secondary necrotic cell death. Apoptosis, 2007, 12: 1043-1060 DOI:10.1007/s10495-006-0032-x |
CHEREAU D, ZOU H, SPADA A P, et al. A nucleotide binding site in caspase-9 regulates apoptosome activation. Biochemistry, 2005, 44(13): 4971-4976 DOI:10.1021/bi047360+ |
CHIPUK J E, GREEN D R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization?. Trends in Cell Biology, 2008, 18(4): 157-164 DOI:10.1016/j.tcb.2008.01.007 |
ELMORE S. Apoptosis: A review of programmed cell death. Toxicologic Pathology, 2007, 35(4): 495-516 DOI:10.1080/01926230701320337 |
FAN H D, LI Y D, YANG Q B, et al. Characterization and expression analysis of MKK7 in black tiger shrimp (Penaeus monodon) under different stressors. Journal of Fishery Sciences of China, 2020, 27(7): 748-758 [范红弟, 李运东, 杨其彬, 等. 斑节对虾MKK7基因的克隆及在不同胁迫条件下的表达分析. 中国水产科学, 2020, 27(7): 748-758] |
GNAIGER E, KUZNETSOV A V. Mitochondrial respiration at low levels of oxygen and cytochrome c. Biochemical Society Transactions, 2002, 30(2): 252-258 DOI:10.1042/bst0300252 |
GOTTLIEB R A. Mitochondria and apoptosis. Biological Signals and Receptors, 2001, 10: 147-161 DOI:10.1159/000046884 |
HAO C G. Effects of three environmental factors on WSSV proliferation in Procambarus clarkii. Master´s Thesis of Hebei Agricultural University, 2020 [郝晨光. 三种环境因子对克氏原螯虾体内WSSV增殖的影响. 河北农业大学硕士研究生学位论文, 2020]
|
HE X, FU Z, LI M, et al. Nosema bombycis (Microsporidia) suppresses apoptosis in BmN cells (Bombyx mori). Acta Biochimica et Biophysica Sinica, 2015, 47(9): 696-702 DOI:10.1093/abbs/gmv062 |
HU W Y, YAO C L. Molecular and immune response characterizations of a novel AIF and cytochrome c in Litopenaeus vannamei defending against WSSV infection. Fish and Shellfish Immunology, 2016, 56: 84-95 DOI:10.1016/j.fsi.2016.06.050 |
HU W Y. Cloning and expression characterizations of some apoptotic genes in Litopenaeus vannamei immune response. Master´s Thesis of Jimei University, 2016 [胡文燕. 凡纳滨对虾部分凋亡相关基因的克隆及免疫反应特征. 集美大学硕士研究生学位论文, 2016]
|
HÜETTEMANN M, LEE I, GROSSMAN L I, et al. Phosphorylation of mammalian cytochrome c and cytochrome c oxidase in the regulation of cell destiny: Respiration, apoptosis, and human disease. In: KADENBACH B. Mitochondrial oxidative phosphorylation: Nuclear-encoded genes, enzyme regulation, and pathophysiology. Advances in Experimental Medicine and Biology, 2012, 748: 237–264
|
HÜETTEMANN M, PECINA P, RAINBOLT M, et al. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion, 2011, 11(3): 369-381 DOI:10.1016/j.mito.2011.01.010 |
KAKOOLAKI S, AFSHARNASAB M, SEDEH M F, et al. The effect of created hemolymph apoptosis on WSSV gama- vaccinated shrimp, Litopenaeus vannamei in WSSV disease control. Iranian Journal of Fisheries Sciences, 2016, 15(1): 301-310 |
KNUDSON C M, JOHNSON G M, LIN Y, et al. Bax accelerates tumorigenesis in p53-deficient mice. Cancer Research, 2001, 61(2): 659-665 |
LAMKANFI M, KANNEGANTI T D. Caspase-7: A protease involved in apoptosis and inflammation. International Journal of Biochemistry and Cell Biology, 2010, 42(1): 21-24 DOI:10.1016/j.biocel.2009.09.013 |
LEVINE B, SINHA S, KROEMER G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy, 2008, 4(5): 600-606 DOI:10.4161/auto.6260 |
LIN S, HE Y, GONG Y, et al. SpBOK inhibits WSSV infection by regulating the apoptotic pathway in mud crab (Scylla paramamosain). Developmental and Comparative Immunology, 2020, 106: 103603 DOI:10.1016/j.dci.2019.103603 |
LIU K Y, YANG H, PENG J X, et al. Cytochrome c and insect cell apoptosis. Insect Science, 2012, 19: 30-40 DOI:10.1111/j.1744-7917.2011.01431.x |
MIAO Y, LIANG A, FU Y. Baculovirus antiapoptotic protein P35 regulated the host apoptosis to enhance virus multiplication. Molecular and Cellular Biochemistry, 2016, 423: 67-73 DOI:10.1007/s11010-016-2825-8 |
NAINU F, TANAKA Y, SHIRATSUCHI A, et al. Protection of insects against viral infection by apoptosis-dependent phagocytosis. Journal of Immunology, 2015, 195(12): 5696-5706 DOI:10.4049/jimmunol.1500613 |
RIJIRAVANICH A, BROWDY C L, WITHYACHUMNARNKUL B. Knocking down caspase-3 by RNAi reduces mortality in Pacific white shrimp Penaeus (Litopenaeus) vannamei challenged with a low dose of white-spot syndrome virus. Fish and Shellfish Immunology, 2008, 24(3): 308-313 DOI:10.1016/j.fsi.2007.11.017 |
SCORRANO L, KORSMEYER S J. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochemical and Biophysical Research Communications, 2003, 304(3): 437-444 DOI:10.1016/S0006-291X(03)00615-6 |
SHI X L, MENG X H, KONG J, et al. cDNA cloning of the FBA gene in Fenneropenaeus chinensis and its expression and functional analysis after WSSV infection. Progress in Fishery Sciences, 2018, 39(2): 112-119 [史晓丽, 孟宪红, 孔杰, 等. 中国明对虾FBA基因克隆及其在白斑综合征病毒感染中的表达及功能分析. 渔业科学进展, 2018, 39(2): 112-119] |
SONG G N. Cloning, expression and function analysis of a caspase gene in Chinese shrimp Fenneropenaeus chinensis. Institute of Oceanology, Master´s Thesis of Chinese Academy of Sciences, 2010 [宋光年. 中国明对虾凋亡基因caspase的克隆、表达及功能的初步分析. 中国科学院研究生院(海洋研究所)硕士研究生学位论文, 2010]
|
SUZUKI H, KURITA M, MIZUMOTO K, et al. p19ARF-induced p53-independent apoptosis largely occurs through BAX. Biochemical and Biophysical Research Communications, 2003, 312(4): 1273-1277 DOI:10.1016/j.bbrc.2003.11.071 |
TI X B, LÜ J J, SONG L, et al. Cloning and expression analysis of 14-3-3 gene in Portunus trituberculatus after exposure to low salt and pathogenic stress. Progress in Fishery Sciences, 2021, 42(1): 134-143 [题兴斌, 吕建建, 宋柳, 等. 三疣梭子蟹14-3-3基因的克隆及其在低盐和病原胁迫后的表达分析. 渔业科学进展, 2021, 42(1): 134-143] |
WANG L, REN X Y, SONG L, et al. Cloning and expression analysis of the Apaf-1 gene in Portunus trituberculatus after exposure to low salt and pathogenic stress. Progress in Fishery Sciences, 2020, 41(4): 85-93 [王磊, 任宪云, 宋柳, 等. 三疣梭子蟹Apaf-1基因的克隆及其在低盐和病原胁迫后的表达分析. 渔业科学进展, 2020, 41(4): 85-93] |
WANG Q Y, SUN H N, ZHOU Q, et al. Folic acid and vitamin B12 inhibit arsenic-induced apoptosis of SH-SY5Y cells via Bcl-2/Bax pathway. Chinese Jouranl of Endemiology, 2021, 40(1): 5-11 [王乔雨, 孙洪娜, 周齐, 等. 叶酸和维生素B12通过Bcl-2/Bax途径抑制砷诱导SH-SY5Y细胞凋亡. 中华地方病学杂志, 2021, 40(1): 5-11] |
WANG X Y, WU K H, PANG H L, et al. Study on the role of Cytc in response to BmNPV infection in silkworm, Bombyx mori (Lepidoptera). International Journal of Molecular Sciences, 2019, 20: 4325 DOI:10.3390/ijms20184325 |
WELCHEN E, GONZALEZ D H. Cytochrome c, a hub linking energy, redox, stress and signaling pathways in mitochondria and other cell compartments. Physiologia Plantarum, 2016, 157(3): 310-321 DOI:10.1111/ppl.12449 |
XIANG F, XUE D D, LUO J, et al. Effects and mechanism of mitochondrial transcription factor A and cytochrome c oxidase pathway in the energy production of hypoxic cardiomyocytes of rats regulated by tumor necrosis factor receptor associated protein. Chinese Journal of Burns, 2020, 36(8): 651-657 [向飞, 薛冬冬, 罗佳, 等. 线粒体转录因子A和细胞色素c氧化酶途径对肿瘤坏死因子受体相关蛋白1调节大鼠缺氧心肌细胞能量生成的作用及机制. 中华烧伤杂志, 2020, 36(8): 651-657] |
XU R J, GU Z M, LI L J, et al. A comparative study on the epidemiology of WSSV infection of crayfish in ponds and rice fields. Chinese Fisheries, 2020(6): 63-66 [徐日俊, 顾泽茂, 李莉娟, 等. 池塘与稻田养殖小龙虾感染WSSV的流行病学比较研究. 中国水产, 2020(6): 63-66] |
YU X W. Research progress on the molecular mechanism of virus infection-induced cell apoptosis. Modern Journal of Animal Husbandry and Veterinary Medicine, 2010(6): 79-82 [于学武. 病毒感染诱导细胞凋亡分子机制的研究进展. 现代畜牧兽医, 2010(6): 79-82] |
ZHAO D, HE L F, LIU H, et al. Mitochondria, cytochrome C, caspase and apoptosis. Journal of Medical Pest Control, 2012, 28(12): 1337-1340 [赵丹, 贺莉芳, 刘晖, 等. 线粒体、细胞色素C、Caspase与细胞凋亡. 医学动物防制, 2012, 28(12): 1337-1340] |



