2. 青岛海洋科学与技术试点国家实验室海洋渔业科学与食物产出过程功能实验室 农业农村部海水养殖病害防治重点实验室 中国水产科学研究院黄海水产研究所山东 青岛 266071;
3. 中国海洋大学海洋生命学院 山东 青岛 266003
2. Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Key Laboratory of Maricultural Organism Disease Control, Ministry of Agriculture and Rural Affairs, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao, Shandong 266071, China;
3. College of Marine Life Sciences, Ocean University of China, Qingdao, Shandong 266003, China
绿鳍马面鲀(Thamnaconus septentrionalis)曾是一种重要的海洋捕捞鱼类,其营养丰富,出肉率高,肉味鲜美,具有较高的营养和经济价值(徐大凤等, 2018)。随着市场需求量的增加和野生资源的频繁捕捞,野生绿鳍马面鲀资源几近枯竭。资源的枯竭促进了人工养殖的发展,绿鳍马面鲀的人工繁育技术已获成功(关健等, 2011、2012; 薛美岩等, 2012)。工厂化养殖规模逐渐扩大,现已成为一种极具开发潜力且值得推广的优良养殖品种(刘琨等, 2017、2019; 张立宁等, 2020)。但国内外尚未有绿鳍马面鲀病害的报道,这可能与绿鳍马面鲀的生物特性有关。绿鳍马面鲀鱼皮坚韧,对环境中的潜在病原有一定的抵御能力(李平伦等, 2003)。此外,虽然有研究报道过甲藻(Cochlodinium polykrikoides)对绿鳍马面鲀的毒性作用,以及有研究者发现野生绿鳍马面鲀体内的2种粘孢子虫寄生(Zhao et al, 2001; Kim et al, 2000),但在养殖条件下绿鳍马面鲀的细菌、病毒和寄生虫等病害尚未见报道。
近年来,随着我国北方网箱养殖的快速发展,许氏平鲉(Sebastes schlegeli)的养殖量也在逐年增加,但在养殖过程中各种病害也随之出现(林春媛等, 2011),在我国许氏平鲉养殖中,已报道哈维氏弧菌(Vibrio harveyi) (孟鹏等, 2010)、鱼肠道弧菌(Vibrio ichthyoenteri) (王庚申, 2012)、鳗利斯顿氏菌(Listonella anguillarum) (王庚申等, 2012)、淋巴囊肿病毒(lymphocystis disease virus) (Zheng et al, 2016)、异尖线虫(Anisakis spp.) (张雯倩等, 2017)、轮虫弧菌(Vibrio rotiferianus) (王凯等, 2019)、美人鱼发光杆菌美人鱼亚种(Photobacterium damselae subsp. damselae) (Zhang et al, 2019)等多种病害,造成巨大的养殖损失。
2018年11月,山东省烟台市蓬莱市一养殖场工厂化养殖的绿鳍马面鲀出现发病死亡情况;2019年4月,该养殖场的许氏平鲉也出现发病死亡情况,典型症状均为嘴部发红。本实验室分别从发病绿鳍马面鲀和许氏平鲉的内脏中分离得到优势菌株2018TS-1和2019SS-1,对其致病性进行了人工感染确认,并通过生理生化鉴定、16S rRNA和vapA基因测序分析,对病原菌进行鉴定。研究内容充实了国内工厂化养殖绿鳍马面鲀和许氏平鲉的疾病研究,为这2种经济鱼类的疾病防控和健康养殖提供了数据支撑。
1 材料与方法 1.1 病鱼来源患病绿鳍马面鲀和许氏平鲉均采自山东省烟台市蓬莱市某养殖场,使用水源为浅井地下水,盐度为28~30,pH为7.6~7.8,溶解氧为8.0 mg/L左右。绿鳍马面鲀约5000尾,体重为200~300 g,养殖于8个25 m3水体的水泥池中,水温为14℃;许氏平鲉鱼苗20 000尾,体重为30~50 g,养殖于8个25 m3水体的水泥池中,水温为12℃。
1.2 病原菌分离随机选取具有典型症状的发病鱼各10尾,首先在显微镜下观察病鱼的鳃、鳍、体表及内脏等组织,检测是否存在寄生虫感染。随后在无菌条件下,分别取病鱼的肝脏、脾脏和肾脏组织,划线培养于胰蛋白胨大豆琼脂(tryptose soya agar, TSA)和2216E培养基,培养温度为20℃。72 h后,挑取菌落形态一致的优势菌进行纯化,并用甘油保存于–80℃超低温冰箱备用。
1.3 人工感染感染实验用绿鳍马面鲀和许氏平鲉购自山东省烟台市某养殖场,体重为30~50 g,实验前分别养殖于300 L循环海水养殖系统中,养殖水温为16℃。感染实验前,随机取5尾鱼解剖,取肝脏、脾脏和肾脏,匀浆,涂布于TSA培养基,确定实验鱼未携带病原。将实验鱼随机分为4组,每组10尾,养殖于50 L整理箱,水温为16~18℃。利用PBS缓冲液将培养好的2018TS-1和2019SS-1菌液浓度调整至1×105、1×106、1×107和1×108 CFU/mL。采用背部肌肉注射方法,每尾绿鳍马面鲀注射感染2018TS-1菌液0.1 mL,每尾许氏平鲉注射感染2019SS-1菌液0.1 mL,对照组注射0.1 mL的PBS缓冲液,每组2个平行,饲养于相同条件下。注射感染后观察21 d,记录各组鱼的发病症状和死亡情况,根据改进的寇氏法(杨茂成, 1990)计算菌株的半数致死量(lethal dose 50%, LD50)。取死亡或濒死鱼的肝脏、脾脏和肾脏,进行病原菌的再次分离和16S r RNA基因测序鉴定。
1.4 病原菌的鉴定利用BIOLOG微生物鉴定系统(BIOLOG, 美国),通过GenⅢ微孔板试剂条对菌株2018TS-1和2019SS-1进行生理生化特征鉴定。利用引物27F (5′-AGAGTTTGATCCTGGTCAGAACGAACGCT-3')和1492R (5′-TACGGCTACCTTGTTACGACTTCACC CC-3′) PCR扩增菌株2018TS-1和2019SS-1的16S rRNA基因(Lane, 1991),利用引物A-layer 1F (ACAGTGCA CCGAAGGTTGAT) and A-layer 6R (ACGGCAGAGC TTGTCTACCT) PCR扩增菌株的vapA基因(Gulla et al, 2016),对获得的扩增产物进行测序。将得到的序列上传至GeneBank,并进行BLAST同源性比对(https://blast.ncbi.nlm.nih.gov)。选取GeneBank中的代表性菌株,使用MEGA 7.0软件,采用邻位相连法(neighbor- joining)构建系统发育进化树(bootstrap=1000)。
2 结果 2.1 病原分离绿鳍马面鲀共有3个养殖池出现发病情况,发病鱼嘴部充血发红,鱼鳍溃烂,少数头部轻微溃疡,解剖可见肝脏有出血点(图 1),日死亡率为0.4%~1.0%,累积死亡率约为25%;许氏平鲉共有5个养殖池出现发病死亡情况,病鱼嘴部发红,鳍部、尾部溃烂,肠道充满淡黄色液体(图 2),日死亡率约为1%,累积死亡率约为40%。
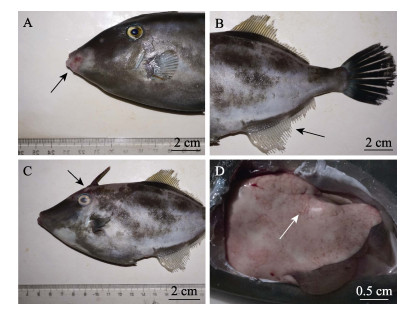
|
图 1 发病绿鳍马面鲀症状 Fig.1 The clinical signs of diseased T. septentrionalis A:嘴部发红;B:鱼鳍溃烂;C:体表溃疡;D:肝脏出血 A: Red mouth; B: Ulcer in the fin; C: Ulcer on the body; D: Liver congestion |
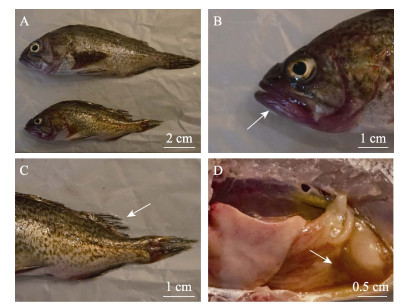
|
图 2 发病许氏平鲉症状 Fig.2 The clinical signs of diseased S. schlegeli A:发病鱼;B:嘴部发红;C:鱼鳍溃烂;D:肠道积液 A: Diseased fish; B: Red mouth; C: Ulcer in the fin; D: Intestinal hydrops |
肉眼和显微镜观察患病鱼体表和内脏,未发现寄生虫感染。利用TSA固体培养基,从所有发病鱼的肝脏、脾脏和肾脏可分离得到大量形态一致的淡黄色圆形不透明菌落。
2.2 人工感染实验通过背部肌肉注射感染的方式分别检测菌株2018TS-1对绿鳍马面鲀和2019SS-1对许氏平鲉的致病性。人工感染后死亡的鱼均表现出与自然感染一致的症状,包括典型的嘴部红肿、肝脏出血,另有注射部位红肿溃烂等症状。1×107 CFU/尾感染组的绿鳍马面鲀和许氏平鲉在感染后全部死亡,其他感染组部分死亡。2018TS-1对绿鳍马面鲀的半数致死量为1.78×105 CFU/尾,2019SS-1对许氏平鲉的半数致死量为0.89×105 CFU/尾,均具有较强的致病性(表 1)。从感染死亡的绿鳍马面鲀和许氏平鲉肝脏、脾脏和肾脏中可重新分离到大量形态一致的优势菌,分离菌落的16S rRNA测序结果与2018TS-1和2019SS-1一致,表明发病鱼死于该分离株的感染。对照组实验鱼均未发生死亡或出现异常症状。
|
|
表 1 绿鳍马面鲀与许氏平鲉人工感染实验 Tab.1 Experimental infection of T. septentrionalis and S. schlegeli |
BIOLOG GenⅢ试剂条鉴定结果见表 2,由表 2可知,2株菌对糊精、D-麦芽糖、蔗糖、D-水杨苷、D-甘露糖、D-果糖、D-甘露醇、明胶、甘油、L-精氨酸和L-谷氨酸等呈阳性反应。对龙胆二糖、D-松二糖、水苏糖、蜜二糖、D-半乳糖、L-岩藻糖、L-鼠李糖、肌苷、L-丙氨酸、奎宁酸和L-焦谷氨酸等呈阴性反应。经BIOLOG鉴定系统分析,2株菌生理生化特征与杀鲑气单胞菌最为接近,可信度为0.999。
|
|
表 2 菌株2018TS-1和2019SS-1生理生化特征 Tab.2 Physiological and biochemical analysis of 2018TS-1 and 2019SS-1 strain by Biolog |
利用PCR分别扩增菌株2018TS-1和2019SS-1的16S rRNA基因序列,Gene Bank登录号分别为OK258319和OK258320。测序结果经过BLAST比对分析显示,菌株2018TS-1和2019SS-1 16S rRNA基因在Gene Bank中同源性最高的均为杀鲑气单胞菌。进一步扩增得到了2株菌的vapA基因序列,2018TS-1和2019SS-1的GenBank登录号分别为OK300094和OK300095。从GenBank中选取代表性菌株,利用MEGA 7.0软件分别构建了16S rRNA基因和vapA基因的系统进化树。16S rRNA系统进化树显示,2018TS-1和2019SS-1均与杀鲑气单胞菌聚为一枝(图 3),与生理生化鉴定结果吻合。vapA系统进化树结果显示,2019TS-1和2018TS-1均与杀鲑气单胞菌杀日本鲑亚种聚为一枝(图 4)。综合以上结果,将这2株病原菌鉴定为杀鲑气单胞菌杀日本鲑亚种(Aeromonas salmonicida subsp. masoucida)。
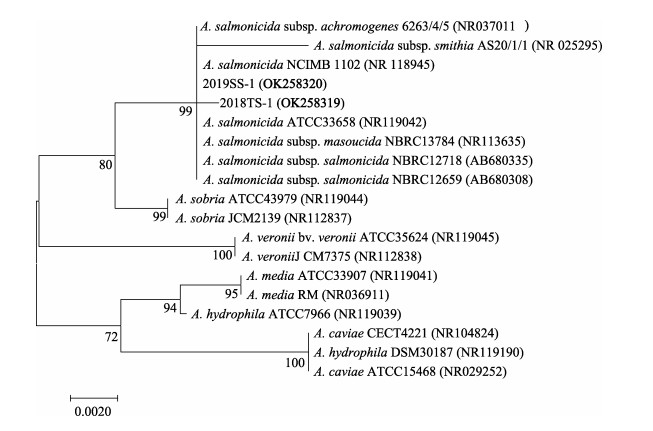
|
图 3 基于16S rRNA基因序列构建的系统发育进化树 Fig.3 Phylogenetic tree constructed by neighbor joining method based on sequences of 16S rRNA |
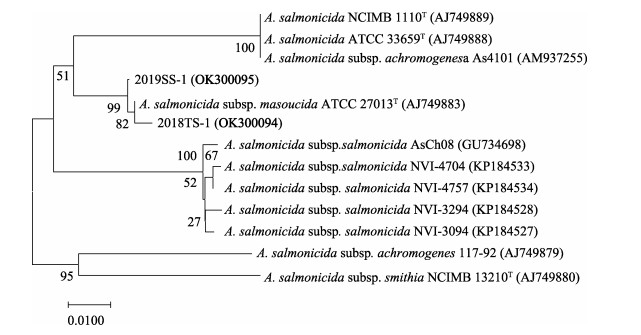
|
图 4 基于vapA基因序列构建的系统发育进化树 Fig.4 Phylogenetic tree constructed by neighbor joining method based on sequences of vapA |
杀鲑气单胞菌是一种重要的鱼类病原菌,在世界范围内广泛分布。根据菌株形态学和生理生化特征,如褐色色素产生、溶血性、蔗糖发酵、菌落大小等,分为5个亚种,分别为杀鲑亚种(A. salmonicida subsp. salmonicida)、杀日本鲑亚种(A. salmonicida subsp. masoucida)、无色亚种(A. salmonicida subsp. achromogenes)、史氏亚种(A. salmonicida subsp. smithia)和溶果胶亚种(A. salmonicida subsp. pectinolytica) (Holt et al, 1994; Pavan et al, 2000; Nash et al, 2006)。其中杀鲑亚种称为典型株,其他菌株均归为非典型株(Wiklund et al, 1998)。A层蛋白(A-layer)是杀鲑气单胞菌的毒力蛋白之一,由毒力阵列蛋白基因vapA编码(Lago et al, 2012),vapA基因是杀鲑气单胞菌分型和亚种鉴定的一个重要标准(Gulla et al, 2016),根据vapA基因的可变区将杀鲑气单胞菌分为14个亚型和一些单生菌株,以及无vapA基因的溶果胶亚种(Gulla et al, 2019)。本研究也采用了vapA基因分型的方法,并将分离到的病原2018TS-1和2019SS-1确定为杀日本鲑亚种。
传统认为无致病性的杀鲑气单胞菌溶果胶亚种属于嗜温性菌株,而其他致病性亚种均为嗜冷性菌株,通常感染冷水鱼类,如大西洋鲑(Salmo salar)、虹鳟(Oncorhynchus mykiss)、大西洋鳕鱼(Gadus morhua)、北极红点鲑(Salvelinus alpinus)、大西洋比目鱼(Hippoglossus hippoglossus)、大西洋狼鱼(Anarhichas lupus)等(Woo et al, 2017)。此次许氏平鲉和绿鳍马面鲀发病时的养殖水温分别为12℃和14℃,与先前研究相吻合。但近年来报道发现,杀鲑气单胞菌感染的宿主越来越广泛,在我国热带养殖品种中也有感染的病例报道,如鳜鱼(Siniperca chuatsi) (Lin et al, 2020)、石斑鱼(Epinephelus coioides) (Zhong et al, 2021)等,其中,感染石斑鱼的菌株SRW-OG1属于嗜温性的杀鲑亚种,可以在37℃下生长,改变了传统对杀鲑亚种的认识(Zhong et al, 2021)。
在国内,杀鲑气单胞菌杀日本鲑亚种已被报道可感染多种水产养殖动物,包括刺参(Apostichopus japonicus) (杨嘉龙等, 2007)、大西洋鲑(Du et al. 2015)、虹鳟(刁菁等, 2018)、中国大鲵(Andrias davidianus) (赵光伟等, 2019)等,本实验室也在山东省的养殖裸盖鱼(Anoplopoma fimbria) (王晓冉等, 2017)、半滑舌鳎(Cynoglossus semilaevis)和大菱鲆(Wang et al, 2020)中发现了杀鲑气单胞菌杀日本鲑亚种感染的病例。通过本研究进一步发现,杀鲑气单胞菌杀日本鲑亚种还可感染绿鳍马面鲀和许氏平鲉,这表明病原的宿主范围在不断扩大。杀鲑气单胞菌的基因组中含有大量的可移动元件,包括插入元件(insertion sequences, ISs)、基因组岛(genomic islands, GEIs)、转座子(transposons)和前噬菌体(prophages)等(Reith et al, 2008; Emond-Rheault et al, 2015),这些可移动原件增强了杀鲑气单胞菌的变异能力,也进一步增强了细菌对宿主、环境的适应能力(Juhas et al, 2009; Bellanger et al, 2014; Darmon et al, 2014),这可能是导致近年来杀鲑气单胞菌感染宿主种类和流行区域不断扩大的重要原因。
本次从患病绿鳍马面鲀和许氏平鲉中分离出来2株致病菌2018TS-1和2019SS-1,人工感染实验结果表明,菌株2018TS-1和2019SS-1能引发绿鳍马面鲀和许氏平鲉死亡,其症状与自然条件下发病症状一致,从人工感染发病鱼中可再次分离到优势菌2018TS-1和2019SS-1,表明这2株菌是此次发病的病原。通过生理生化鉴定、16S rRNA和vapA基因分型,确定病原为杀鲑气单胞菌杀日本鲑亚种。本研究是人工养殖绿鳍马面鲀病害的首例报道,为绿鳍马面鲀的病害防控提供了基础信息。尽管日本和韩国均发现过杀鲑气单胞菌感染许氏平鲉的病例(Izumikawa et al, 1997; Han et al, 2010),但本研究是国内首次报道许氏平鲉感染杀鲑气单胞菌。同时,本次在同一养殖场的不同养殖品种均发现杀鲑气单胞菌感染,表明场内可能存在水平传播,在养殖管理中,防疫措施仍需进一步加强。
BELLANGER X, PAYOT S, LEBLOND-BOURGET N, et al. Conjugative and mobilizable genomic islands in bacteria: Evolution and diversity. FEMS Microbiology Reviews, 2014, 38(4): 720-760 DOI:10.1111/1574-6976.12058 |
DARMON E, LEACH D R F. Bacterial genome instability. Microbiology and Molecular Biology Reviews, 2014, 78(1): 1-39 DOI:10.1128/MMBR.00035-13 |
DIAO J, LI L, WANG X L, et al. Isolation, identification and virulence factor detection of pathogenic Aeromonas salmonicida from rainbow trout (Oncorhynchus mykiss). Journal of Dalian Ocean University, 2018, 33(4): 435-443 [刁菁, 李乐, 王晓璐, 等. 虹鳟致病性杀鲑气单胞菌的分离鉴定及其毒力因子的检测. 大连海洋大学学报, 2018, 33(4): 435-443] |
DU Y S, YI M M, XIAO P, et al. The impact of Aeromonas salmonicida infection on innate immune parameters of Atlantic salmon (Salmo salar L). Fish and Shellfish Immunology, 2015, 44(1): 307-315 DOI:10.1016/j.fsi.2015.02.029 |
EMOND-RHEAULT J G, VINCENT A T, TRUDEL M V, et al. AsaGEI2b: A new variant of a genomic island identified in the Aeromonas salmonicida subsp. salmonicida JF3224 strain isolated from a wild fish in Switzerland. FEMS Microbiology Letters, 2015, 362(13): 1-18 |
GUAN J, CHEN Z X, ZHANG J N, et al. Post-embryonic development of filefish Thamnaconus modestus. Oceanologia et Limnologia Sinica, 2011, 42(4): 561-566 [关健, 陈志信, 张家男, 等. 绿鳍马面鲀(Thamnaconus modestus)仔、稚鱼生长发育特征研究. 海洋与湖沼, 2011, 42(4): 561-566] |
GUAN J, LIU H J, ZHENG Y Y, et al. Early developmental chharacteristics of external apparatus of bluefin leatherjacket Thamnaconus modestus. Progress in Fishery Sciences, 2012, 33(4): 26-33 [关健, 刘洪军, 郑永允, 等. 绿鳍马面鲀外部器官的早期发育. 渔业科学进展, 2012, 33(4): 26-33] |
GULLA S, BAYLISS S, BJÖRNSDÓTTIR B, et al. Biogeography of the fish pathogen Aeromonas salmonicida inferred by vapA genotyping. FEMS Microbiology Letters, 2019, 366(7): 1-7 |
GULLA S, LUND V, KRISTOFFERSEN A B, et al. vapA (A-layer) typing differentiates Aeromonas salmonicida subspecies and identifies a number of previously undescribed subtypes. Journal of Fish Diseases, 2016, 39(3): 329-342 DOI:10.1111/jfd.12367 |
HAN H J, KIM D Y, KIM W S, et al. Atypical Aeromonas salmonicida infection in the black rockfish, Sebastes schlegeli Hilgendorf, in Korea. Journal of Fish Diseases, 2010, 34(1): 47-55 |
HOLT J G, KRIEG N R, SNEATH P H A, et al. Bergey's manual of determinative bacteriology, Ninth Edition. Baltimore: Williams and Wilkins, 1994, 251–253
|
IZUMIKAWA K, UEKI N. Atypical Aeromonas salmonicida infection in cultured Schlegel's black rockfish. Fish Pathology, 1997, 32(1): 67-68 DOI:10.3147/jsfp.32.67 |
JUHAS M, VAN DER MEER J R, GAILLARD M, et al. Genomic islands: Tools of bacterial horizontal gene transfer and evolution. FEMS Microbiology Reviews, 2009, 33(2): 376-393 DOI:10.1111/j.1574-6976.2008.00136.x |
KIM C S, LEE S G, KIM H G. Biochemical responses of fish exposed to a harmful dinoflagellate Cochlodinium polykrikoides. Journal of Experimental Marine Biology and Ecology, 2000, 254(2): 131-141 DOI:10.1016/S0022-0981(00)00263-X |
LAGO E P, NIETO T P, FARTO R. Virulence factors of Aeromonas salmonicida subsp. salmonicida strains associated with infections in turbot Psetta maxima. Diseases of Aquatic Organisms, 2012, 99(2): 145-151 DOI:10.3354/dao02467 |
LANE D J. 16S/23S rRNA sequencing. In: Nucleic acid techniques in bacterial systematics. New York: John Wiley & Sons, 1991, 125–175
|
LI P L, LI D J, XU J B, et al. Key points of aquaculture technology of Thamnaconus septentrionalis in offshore netcage. Shandong Fisheries, 2003, 20(10): 10-11 [李平伦, 李德军, 徐金波, 等. 绿鳍马面鲀海上网箱养殖技术要点. 齐鲁渔业, 2003, 20(10): 10-11] |
LIN C Y, LIU Z W, WANG Y G, et al. Domestic and foreign research progress of cage culture Sebastes schlegeli disease. Hebei Fisheries, 2011(8): 50-54 [林春媛, 刘志伟, 王印庚, 等. 网箱养殖许氏平鲉疾病的国内外研究进展. 河北渔业, 2011(8): 50-54] |
LIN Q, LI J, FU X Z, et al. Hemorrhagic gill disease of Chinese perch caused by Aeromonas salmonicida subsp. salmonicida in China. Aquaculture, 2020, 519: 734775 DOI:10.1016/j.aquaculture.2019.734775 |
LIU K, LIU G, HUANG L, et al. Experiment on cage cultivation of large-size Thamnaconus septentrionalis Günther. Fishery Modernization, 2019, 46(6): 54-60 [刘琨, 刘刚, 黄亮, 等. 绿鳍马面鲀大规格苗种网箱培育试验. 渔业现代化, 2019, 46(6): 54-60] |
LIU K, ZHANG L L, ZHANG Q W, et al. Study on Thamnaconus septentrionalis under industrial aquaculture condition. Fishery Modernization, 2017, 44(3): 35-40 [刘琨, 张乐乐, 张庆文, 等. 绿鳍马面鲀工厂化养殖研究. 渔业现代化, 2017, 44(3): 35-40] |
MENG P, ZHAN W B, SHENG X Z. Identification and phylogenetic analysis of pathogen HV0811 isolated from the diseased Sebastodes fuscescens. Marine Sciences, 2010, 34(5): 48-54 [孟鹏, 战文斌, 绳秀珍. 从患病黑鲪分离病原菌HV0811的鉴定及其系统发育分析. 海洋科学, 2010, 34(5): 48-54] |
NASH J H, FINDLAY W A, LUEBBERT C C, et al. Comparative genomics profiling of clinical isolates of Aeromonas salmonicida using DNA microarrays. BMC Genomics, 2006, 7(1): 43 DOI:10.1186/1471-2164-7-43 |
PAVAN M E, ABBOTT S L, ZORZOPULOS J, et al. Aeromonas salmonicida subsp. pectinolytica subsp. nov., a new pectinase-positive subspecies isolated from a heavily polluted river. International Journal of Systematic and Evolutionary Microbiology, 2000, 50(3): 1119-1124 DOI:10.1099/00207713-50-3-1119 |
REITH M E, SINGH R K, CURTIS B, et al. The genome of Aeromonas salmonicida subsp. salmonicida A449: Insights into the evolution of a fish pathogen. BMC Genomics, 2008, 9(1): 427 |
WANG G S, WANG Y G, ZHANG Z, et al. Isolation, identification and pathogenicity of Listonella anguillarum from diseased cultured Sebastes schlegelii. Journal of Fisheries of China, 2012, 36(8): 1290-1296 [王庚申, 王印庚, 张正, 等. 引起养殖许氏平鲉死亡的鳗利斯顿氏菌的分离鉴定及致病性. 水产学报, 2012, 36(8): 1290-1296] |
WANG G S. Isolation and identification of two pathogenic bacteria and associated histopathology in cultured black rockfish (Sebastes schlegelii). Masterxs Thesis of Shanghai Ocean University, 2012 [王庚申. 养殖许氏平鲉两种细菌性病原的分离、鉴定及组织病理学研究. 上海海洋大学硕士研究生学位论文, 2012]
|
WANG K, WANG Y G, JIANG Y, et al. Isolation, identification, and biological characteristics of a pathogenic bacterial strain from cage-cultured black rockfish (Sebastes schlegelii). Progress in Fishery Sciences, 2019, 40(1): 119-126 [王凯, 王印庚, 姜勇, 等. 一株感染深水网箱养殖许氏平鲉的病原菌分离与鉴定. 渔业科学进展, 2019, 40(1): 119-126] |
WANG P, LI J, HE T T, et al. Pathogenic characterization of Aeromonas salmonicida subsp. masoucida turbot isolate from China. Journal of Fish Diseases, 2020, 43(10): 1145-1154 |
WANG X R, CHEN S Q, MO Z L, et al. Isolation and identification of Aeromonas salmonicida associated with furunculosis in cultured sablefish (Anoplopoma fimbria). Progress in Fishery Sciences, 2017, 38(5): 25-31 [王晓冉, 陈四清, 莫照兰, 等. 养殖裸盖鱼(Anoplopoma fimbria)疥疮病病原菌的分离与鉴定. 渔业科学进展, 2017, 38(5): 25-31] |
WIKLUND T, DALSGAARD I. Occurrence and significance of atypical Aeromonas salmonicida in non-salmonid and salmonid fish species: A review. Diseases of Aquatic Organisms, 1998, 32(1): 49-69 |
WOO P T K, CIPRIANO R C. Fish viruses and bacteria: Pathobiology and protection. London: CABI, 2017, 173–189
|
XU D F, LIU K, WANG P F, et al. Analysis of nutritional composition in the muscle of Thamnaconus septentrionalis. Marine Sciences, 2018, 42(5): 122-129 [徐大凤, 刘琨, 王鹏飞, 等. 绿鳍马面鲀肌肉营养成分分析和营养评价. 海洋科学, 2018, 42(5): 122-129] |
XUE M Y, ZHANG J, DU R B, et al. Effects of temperature and salinity on survival and growth of larvae Navodon septentrionalis. Transactions of Oceanology and Limnology, 2012(1): 63-67 [薛美岩, 张静, 杜荣斌, 等. 温度、盐度对绿鳍马面鲀幼鱼存活及生长的影响. 海洋湖沼通报, 2012(1): 63-67] |
YANG J L, ZHOU L, XING J, et al. Identification of Aeromonas salmonicida associated with skin ulceration of cultured sea cucumber Apostichopus japonicus and characterization of the extracellular products. Journal of Fishery Sciences of China, 2007, 14(6): 981-989 [杨嘉龙, 周丽, 邢婧, 等. 养殖刺参溃疡病杀鲑气单胞菌的分离、致病性及胞外产物特性分析. 中国水产科学, 2007, 14(6): 981-989] |
YANG M C. Statistics for veterinary science. Beijing: China Prospect Publishing House, 1990: 232-234 [杨茂成. 兽医统计学. 北京: 中国展望出版社, 1990: 232-234]
|
ZHANG L N, SHAO X B, SHAN L Z, et al. Scaled artificial breeding of Thamnaconus septentrionalis. Fisheries Science and Technology Information, 2020, 47(5): 258-260 [张立宁, 邵鑫斌, 单乐州, 等. 绿鳍马面鲀规模化人工繁育技术. 水产科技情报, 2020, 47(5): 258-260] |
ZHANG W Q, REN X T, ZHAO Y Q, et al. Investigation of Anisakis spp. larva infection in marine fish for sale in Yantai City. Chinese Journal of Parasitology and Parasitic Diseases, 2017, 35(5): 472-477 [张雯倩, 任晓彤, 赵玉绮, 等. 烟台市市售海鱼异尖线虫幼虫感染现况调查. 中国寄生虫学与寄生虫病杂志, 2017, 35(5): 472-477] |
ZHANG Z, YU Y X, WANG K, et al. First report of skin ulceration caused by Photobacterium damselae subsp. damselae in net-cage cultured black rockfish (Sebastes schlegeli). Aquaculture, 2019, 503: 1-7 |
ZHAO G W, SHA S, LI X L, et al. Isolation, identification and drug sensitivity test of the pathogenic bacteria from the Chinese giant salamander with splenic tuberosity. Chinese Journal of Veterinary Medicine, 2019, 55(02): 122-125 [赵光伟, 沙莎, 黎学练, 等. 大鲵脾脏结节症病原菌的分离鉴定及药敏试验. 中国兽医杂志, 2019, 55(2): 122-125] |
ZHAO Y J, SONG W B. Myxoproteus cheni sp. n. and Sinuolinea mai sp. n. (Myxosporea: Sinuolineidae) parasitic in the urinary bladder of marine fish (Thamnaconus septentrionalis Gunther, 1877) from the Yellow Sea, off the Qingdao Coast of China. Acta Protozoologica, 2001, 40(2): 125-130 |
ZHENG F R, LIU H Z, GUO X Y, et al. Isolation and identification of a new isolate of lymphocystis disease virus isolated from black rockfish (Sebastes schlegelii) in China. Aquaculture, 2016, 451: 340-344 |
ZHONG Y, QI W, XU W, et al. Insights into mesophilic virulence, antibiotic resistant and human pathogenicity: A genomics study on the Aeromonas salmonicida SRW-OG1 newly isolated from the Asian fish Epinephelus coioides. Aquaculture, 2021, 539: 736630 |



