2. 中国水产科学研究院黄海水产研究所 农业农村部极地渔业可持续利用重点实验室 山东 青岛 266071;
3. 青岛海洋科学与技术试点国家实验室海洋药物与生物制品功能实验室 山东 青岛 266200
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory of Sustainable Development of Polar Fisheries, Ministry of Agriculture and Rural Affairs, Qingdao, Shandong 266071, China;
3. Laboratory for Marine Drugs and Bioproducts, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, Shandong 266200, China
南极磷虾(Euphausia superba)是地球上最大的单种生物资源,生物量约为6.5~10亿t,具有打造成为我国第二个远洋渔业的巨大潜力(赵宪勇等, 2016; 曹荣等, 2018; 潘晓炀等, 2019)。南极磷虾蛋白含量丰富,达到干重的65% (Tou et al, 2007; Wang et al, 2011),是全球最大的海洋动物蛋白资源宝库(刘志东等, 2017; 刘柯欣等, 2022)。近年来,相关研究已证明,南极磷虾多肽具有良好的生理活性,包括改善骨质疏松(Wang et al, 2015; Han et al, 2018)、调节血糖(Ji et al, 2017)、降血压(Zhao et al, 2019)、抗氧化(刘小芳等, 2020; 郑景如等, 2020)、缓解疲劳(徐恺, 2012)和改善皮肤光老化(于建伟等, 2021)等。鉴于丰富的资源量和良好的营养功能,南极磷虾蛋白肽的生产开发得到行业关注。目前,关于南极磷虾蛋白肽制备的研究集中在酶解工艺优化方面,主要涉及最适酶的筛选、酶解条件优化等(刘云娇等, 2019; 张华丹等, 2019; 孙如男等, 2020)。然而,由于南极磷虾自身矿物质含量较高,且在酶解处理时为调节最适反应条件需引入酸或碱,极易导致酶解后获得的蛋白肽盐分含量较高。较高的盐分含量,不仅会影响蛋白肽的口感和功能,也会对消费者健康带来一定的潜在风险,极大的限制产品的应用(Ras et al, 2000)。因此,针对酶解后的南极磷虾蛋白肽进行脱盐处理十分必要,但目前尚未见相关研究报道。
针对生物活性物质的脱盐方法主要包括透析、超滤、纳滤、电渗析和大孔树脂吸附等(夏光华等, 2013)。纳滤是一种由压差驱动的膜分离技术,作为目前膜分离研究领域的热点,其具有基于物理分离有利于保持物质活性和风味、不发生相变节能因而运行成本低、滤膜通量高对单价离子截留率低等优点(于国才等, 2011),近年来,在食品行业中越来越多地被应用于蛋白肽的分离、浓缩和脱盐过程(刘亮等, 2013; Benedetti et al, 2016; 王长伟等, 2019)。综上所述,本研究采用纳滤技术对南极磷虾蛋白肽进行脱盐处理,通过单因素实验和正交实验对蛋白肽浓度、纳滤压力、循环次数等工艺条件进行优化,以期为高品质南极磷虾蛋白肽的工业化生产应用提供技术支持。
1 材料与方法 1.1 实验材料脱脂南极磷虾粉:山东青岛南极维康生物科技有限公司;碱性蛋白酶(Alcalase 2.4 L, 200 000 U/mL):丹麦诺维信生物技术有限公司;Lowry法蛋白浓度测定试剂盒:北京索莱宝科技有限公司;磷酸二氢钠、磷酸氢二钠、NaOH、HCl等试剂:分析纯,国药集团化学试剂有限公司。
1.2 仪器设备XS-Y-MINI-2型有机膜多功能实验设备:南京诺润机械科技有限公司;TFN-18-200 200D型纳滤膜:山东博纳生物科技集团有限公司;SRJX-8-13型马弗炉:天津泰斯特仪器有限公司;DK-98-Ⅱ型电炉:天津泰斯特仪器有限公司;UV1-102II型紫外/可见分光光度计:上海天美科学仪器有限公司;BILON-6000Y型喷雾干燥机:上海比朗仪器制造有限公司;ST3100型pH计:奥豪斯仪器(常州)有限公司;BSA224S-CW型电子分析天平:赛多利斯科学仪器有限公司;SHA-B型恒温振荡器:常州智博瑞仪器制造有限公司;HH-2型数显恒温水浴锅:国华电器有限公司;LXJ-IIB型离心机:上海安亭科学仪器厂。
1.3 实验方法 1.3.1 南极磷虾蛋白肽的制备参考张华丹等(2019)的实验方法制备南极磷虾蛋白肽:称取适量脱脂南极磷虾粉,按照料液比1∶6 (g/mL)添加pH为7.5的磷酸盐缓冲液,混匀,根据脱脂南极磷虾粉质量加入2% (v/m)的碱性蛋白酶,55℃酶解4 h后于95℃加热灭酶20 min,冷却至室温,5000 r/min离心20 min,收集上清液,在进风温度为200℃、出风温度为85℃条件下,经喷雾干燥即得南极磷虾蛋白肽。
1.3.2 纳滤脱盐处理工艺南极磷虾蛋白肽经蒸馏水稀释配制成实验浓度,在纳滤设备料液罐中投料2 L,控制过膜压力稳定在实验压力进行脱盐处理,待压力降至0.3 MPa以下,使用蒸馏水将罐内溶液补至2 L后继续处理相应实验循环次数。待最后1次循环处理压力降至0.3 MPa后,将处理液经管路放出,测定相应评价指标。
1.3.3 单因素实验设计进行蛋白肽浓度、纳滤压力、循环次数的单因素优化:蛋白肽浓度为3%,循环次数为1次,纳滤压力分别为0.6、0.8、1.0、1.2和1.4 MPa进行纳滤脱盐;压力为1.0 MPa,循环次数为1次,蛋白肽浓度分别为1%、2%、3%、4%和5%进行纳滤脱盐;蛋白肽浓度为3%,压力为1.0 MPa,循环次数分别为1、2、3、4、5进行纳滤脱盐。以南极磷虾蛋白肽的脱盐率和蛋白损失率为评价指标,确定各单因素的最佳条件。
1.3.4 正交实验设计根据单因素实验结果进行蛋白肽浓度、纳滤压力、循环次数等三因素的正交实验优化,实验水平设置见表 1。根据正交表L9(33)进行实验,根据南极磷虾蛋白肽的脱盐率和蛋白损失率结果,确定最佳脱盐工艺条件。
|
|
表 1 正交实验因素水平表 Tab.1 Factors and levels used in the orthogonal tests |
脱盐处理前后料液中的盐分含量按照SC/T 3011-2001《水产品中盐分的测定》的规定执行。脱盐率按照以下公式计算:
脱盐率/%=(脱盐前料液中盐分含量–脱盐后料液中盐分含量)/脱盐前料液中盐分含量×100
1.3.6 蛋白损失率的测定按照试剂盒说明书的规定测定脱盐处理前后料液中的蛋白含量。蛋白损失率按照以下公式计算:
蛋白损失率/%=(脱盐前料液中蛋白含量–脱盐后料液中蛋白含量)/脱盐前料液中蛋白含量×100
1.3.7 脱盐处理后南极磷虾蛋白肽的组成分析按照正交实验确定的最优纳滤工艺条件对南极磷虾蛋白肽进行脱盐处理,处理后的蛋白肽溶液经喷雾干燥后进行组成分析:水分含量按照GB 5009.3-2016《食品安全国家标准食品中水分的测定》的规定执行;蛋白质含量按照GB 5009.5-2016《食品安全国家标准食品中蛋白质的测定》的规定执行;灰分含量按照GB 5009.4-2016《食品安全国家标准食品中灰分的测定》的规定执行;盐分含量按照SC/T 3011-2001《水产品中盐分的测定》的规定执行;分子量分布按照GB 31645-2018《食品安全国家标准胶原蛋白肽》中附录A的规定执行;氨基酸组成按照GB 5009.124-2016《食品安全国家标准食品中氨基酸的测定》的规定执行。
1.4 数据处理实验数据采用平均值±标准差(Mean±SD)的形式表示,采用Excel 2016、IBM SPSS 20.0和Origin 2018等软件进行数据处理分析和图表绘制。采用单因素方差分析(one-way ANOVA)进行组间比较,P < 0.05为差异显著。
2 结果与讨论 2.1 单因素实验蛋白肽浓度对南极磷虾蛋白肽的脱盐率和蛋白损失率的影响结果见图 1。当蛋白肽浓度为1%~3%时,脱盐率随着蛋白肽浓度的增加而逐渐升高;当蛋白肽浓度为3%时,脱盐率最高,达到(69.07±2.25)%,显著高于其他实验组(P < 0.05);而后随着蛋白肽浓度的增加,脱盐率有所下降。蛋白肽浓度较低时,纳滤时间较短,导致脱盐效果不佳;而当蛋白浓度较高时,纳滤膜表面被截留的溶质质量浓度不断增加,浓差极化不断加强,膜的透过通量下降,进而影响脱盐效果(岳三峰, 2017)。蛋白肽浓度在1%~5%范围内进行脱盐处理,各实验组的蛋白损失率在(7.63±0.22)%~(8.75± 0.31)%之间;处理过程中,逃水现象和浓差极化现象的发生引起蛋白损失(刘亮等, 2013),但各实验组的蛋白损失率均可控制在较低水平,达到良好的蛋白回收效果。因此,确定蛋白肽浓度3%为最佳实验条件。

|
图 1 蛋白肽浓度对南极磷虾蛋白肽脱盐率和蛋白损失率的影响 Fig.1 Effect of peptides concentration on the desalination rate and protein loss rate during nanofiltration of Antarctic krill peptides 不同字母表示不同实验组间具有显著性差异(P < 0.05)。下同。 Different letters represent significant differences between different experimental groups (P < 0.05). The same as below. |
纳滤压力对南极磷虾蛋白肽的脱盐率和蛋白损失率的影响结果见图 2。在0.6~1.0 MPa的纳滤压力范围内,随着压力增加,蛋白肽的脱盐率不断增加;当纳滤压力为1.0 MPa时,脱盐率为(77.22±2.65)%,显著高于0.6 MPa和0.8 MPa实验组(P < 0.05);而后随着纳滤压力的增加,脱盐率有所下降。在纳滤压力为0.6~1.4 MPa范围内进行脱盐处理,各实验组的蛋白损失率在(7.87±0.13)%~(9.01±0.33)%之间,蛋白损失均较少。在实际生产中,压力过高会使滤膜发生极限压降现象,形成水锤作用而导致滤膜衰减加剧,损坏膜组件(杨砾等, 2012)。因此,确定纳滤压力1.0 MPa为最佳实验条件。

|
图 2 纳滤压力对南极磷虾蛋白肽脱盐率和蛋白损失率的影响 Fig.2 Effect of pressure on desalination rate and protein loss rate during nanofiltration of Antarctic krill peptides |
循环次数对南极磷虾蛋白肽的脱盐率和蛋白损失率的影响结果见图 3。在循环次数为1~3次时,随着循环次数的增多,南极磷虾蛋白肽的脱盐率逐渐升高,而随着循环次数的增加,脱盐率趋于平稳;在循环次数在1~5次范围内进行脱盐处理,各实验组的蛋白损失率在(7.35±0.12)%~(8.36±0.19)%之间,蛋白损失均较少。补水循环会降低浓差极化现象,提高渗透速率,提升脱盐处理效果(孔凡丕等, 2010)。考虑到循环处理2次,脱盐效果已较好,达到(82.88±2.32)%,与循环处理3次无明显差异(P > 0.05),且循环次数增多会增加实际生产操作难度和成本,因此,确定循环次数2次为最佳实验条件。

|
图 3 循环次数对南极磷虾蛋白肽脱盐率和蛋白损失率的影响 Fig.3 Effect of cycle times on desalination rate and protein loss rate during nanofiltration of Antarctic krill peptides |
在单因素实验基础上,采用L9 (33)正交实验设计研究蛋白肽浓度、纳滤压力和循环次数等三因素对脱盐效果的影响,结果见表 2。由表 2可知,极差R的波动幅度代表了实验因素对脱盐效果的影响程度,各实验因素中,蛋白肽浓度对脱盐效果的影响最大,其次为循环次数,影响最小的为纳滤压力。经k值分析得到南极磷虾蛋白肽脱盐处理最佳条件:蛋白肽浓度k2、循环次数k3、纳滤压力k3,即蛋白肽浓度3%,压力1.2 MPa,循环次数3次。经验证,在推荐的最佳工艺条件下,南极磷虾蛋白肽的脱盐率可达到(86.35±2.11)%,蛋白损失率为(9.10±0.35)%,证明该工艺可行。
|
|
表 2 正交实验结果 Tab.2 The results of the orthogonal tests |
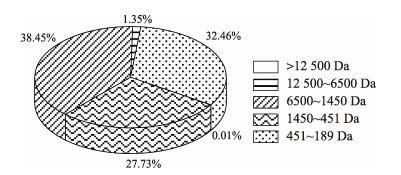
经脱盐处理后获得的南极磷虾蛋白肽的组成分析结果见表 3、图 4。由表 3可知,采用优化工艺获得的南极磷虾蛋白肽,盐分含量为(1.14±0.12)%,蛋白质含量为(92.73±2.29)%;蛋白肽共检出16种氨基酸,含有7种必需氨基酸,其中,谷氨酸含量最高,天冬氨酸含量次之,必需氨基酸含量占总氨基酸含量的(40.06±0.10)%,必需氨基酸与非必需氨基酸的比值为(66.82±0.28)%,符合联合国粮农组织/世界卫生组织规定的优质蛋白标准(必需氨基酸占氨基酸总量的40%,必需氨基酸与非必需氨基酸的比值为60%)。由图 4可知,蛋白肽的分子量主要在189~6500 Da,其中,451~1450 Da占比最高(38.45±1.37)%,而分子量在3000 Da以下的占比达到(88.91±2.19)%,符合生物活性肽分子量分布范围(谢博等, 2021)。综上可知,采用纳滤脱盐后获得的南极磷虾蛋白肽品质良好,营养价值较高。
|
|
表 3 脱盐后南极磷虾蛋白肽的组成 Tab.3 The compositions of the desalinated Antarctic krill peptides |
|
图 4 脱盐后南极磷虾蛋白肽的分子量分布 Fig.4 Molecular weight distribution of the desalinated Antarctic krill peptides |
本研究通过单因素实验和正交实验,确定了影响南极磷虾蛋白肽纳滤脱盐效果的因素顺序:蛋白肽浓度 > 循环次数 > 纳滤压力;最佳工艺条件:蛋白肽浓度3%,纳滤压力1.2 MPa,循环次数3次;在该条件下,南极磷虾蛋白肽的脱盐率可达到(86.35±2.11)%。经优化工艺脱盐处理后获得的南极磷虾蛋白肽盐分含量低、氨基酸组成和分子量分布理想,品质良好。本研究对于南极磷虾蛋白的高效利用和高质产品开发具有重要参考价值。
BENEDETTI S, PRUDENCIO E S, MüLLER C M O, et al. Utilization of tofu whey concentrate by nanofiltration process aimed at obtaining a functional fermented lactic beverage. Journal of Food Engineering, 2016, 171: 222-229 DOI:10.1016/j.jfoodeng.2015.10.034 |
CAO R, YU Y K, ZHAO L, et al. Study on the protein autolysis process of Antarctic krill (Euphausia superba) and its influencing factors. Progress in Fishery Sciences, 2018, 39(6): 114-118 [曹荣, 余奕珂, 赵玲, 等. 南极磷虾(Euphausia superba)起捕后蛋白自溶进程及其影响因素. 渔业科学进展, 2018, 39(6): 114-118] |
HAN L H, MAO X Z, WANG K, et al. Phosphorylated peptides from Antarctic krill (Euphausia superba) ameliorated osteoporosis by activation of osteogenesis-related MAPKs and PI3K/AKT/GSK-3β pathways in dexamethasone-treated mice. Journal of Functional Foods, 2018, 47: 447-456 DOI:10.1016/j.jff.2018.06.004 |
JI W, ZHANG C H, JI H W. Purification, identification and molecular mechanism of two dipeptidyl peptidase Ⅳ (DPP- Ⅳ) inhibitory peptides from Antarctic krill (Euphausia superba) protein hydrolysate. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 1064, 56-61 |
KONG F P, LIU L, SUN Z, et al. Desalination of dairy whey by nanofiltration. Transactions of the CSAE, 2010, 26(12): 363-366 [孔凡丕, 刘鹭, 孙卓, 等. 纳滤技术对干酪乳清脱盐性能的影响. 农业工程学报, 2010, 26(12): 363-366] |
LIU K X, LIN S Y, HU S J, et al. Advances in nutritional and functional properties and food safety of Antarctic krill protein. Food Science, 2022, 43(7): 263-272 [刘柯欣, 林松毅, 胡胜杰, 等. 南极磷虾蛋白营养与功能特性及食用安全性研究进展. 食品科学, 2022, 43(7): 263-272] |
LIU L, LIU L Z, WANG Y, et al. Application of refining in sturgeon skin collagen peptide by ultrafiltration and nanofiltration. Journal of Wuhan Polytechnic University, 2013, 32(2): 6-10 [刘亮, 刘亮忠, 王燕, 等. 超滤纳滤在鲟鱼皮胶原蛋白肽精制中的应用. 武汉工业学院学报, 2013, 32(2): 6-10] |
LIU X F, YAN Z, LENG K L, et al. Composition analysis and evaluation of the antioxidative and ACE inhibitory activities of polypeptides from Antarctic krill. Food Research and Development, 2020, 41(23): 7-13 [刘小芳, 颜征, 冷凯良, 等. 南极磷虾多肽的组成及其抗氧化与ACE抑制活性. 食品研究与开发, 2020, 41(23): 7-13] |
LIU Y J, ZHANG H Y, LIU S H, et al. Response surface optimization of proteolytic process and protein peptide composition analysis of Antarctic krill. Modern Food Science and Technology, 2019, 35(1): 144–151, 280 [刘云娇, 张海燕, 刘淑晗, 等. 响应面优化南极磷虾蛋白酶解工艺及蛋白肽组分分析. 现代食品科技, 2019, 35(1): 144–151, 280] |
LIU Z D, WANG L M, CHEN X Z, et al. Research progress on the protein derived from Antarctic krill (Euphausia superba Dana). Food and Fermentation Industries, 2017, 43(7): 242-251 [刘志东, 王鲁民, 陈雪忠, 等. 南极磷虾蛋白的研究进展. 食品与发酵工业, 2017, 43(7): 242-251] |
PAN X Y, YANG L X, WANG X Y, et al. Effects of freezing and thawing cycles on taste components of minced Antarctic krill. Progress in Fishery Sciences, 2019, 40(2): 155-160 [潘晓炀, 杨林莘, 王晓燕, 等. 冻融循环对南极磷虾虾肉糜滋味成分的影响. 渔业科学进展, 2019, 40(2): 155-160] |
RAS E T, POMANTOC J J, TUMULAK E P, et al. ETRAS thermal desalination system. Desalination, 2000, 132: 353-356 |
SUN R N, LENG K, GAO H, et al. Optimization of enzymatic hydrolysis preparation of the protein-based material for the production of Antarctic krill metal-chelating peptide. Food Science and Technology, 2020, 45(7): 159-165 [孙如男, 冷凯良, 高华, 等. 南极磷虾金属螯合肽蛋白基料的酶解制备工艺优化. 食品科技, 2020, 45(7): 159-165] |
TOU J C, JACZYNSKI J, CHEN Y C. Krill for human consumption: Nutritional value and potential health benefits. Nutrition Reviews, 2007, 65(2): 63-77 |
WANG C W, LI B F, SONG W S, et al. Preparation of oyster protein peptide and its function in promoting growth and development. Journal of Anhui Agricultural Sciences, 2019, 47(17): 161-164 [王长伟, 李八方, 宋文山, 等. 牡蛎蛋白肽的制备及促进生长发育功能. 安徽农业科学, 2019, 47(17): 161-164] |
WANG L Z, XUE C H, WANG Y M, et al. Extraction of proteins with low fluoride level from Antarctic krill (Euphausia superba) and their composition analysis. Journal of Agricultural and Food Chemistry, 2011, 59(11): 6108-6112 |
WANG Y C, WANG S S, WANG J F, et al. Preparation and anti-osteoporotic activities in vivo of phosphorylated peptides from Antarctic krill (Euphausia superba). Peptides, 2015, 68: 239245 |
XIA G H, SHEN X R, JIU Z Q, et al. Desalination of microporous absorption resin on antioxidant peptides from tilapia skin collagen. Modern Food Science and Technology, 2013, 29(5): 1052-1056 [夏光华, 申铉日, 酒志强, 等. 大孔树脂对罗非鱼皮胶原蛋白抗氧化肽脱盐作用的研究. 现代食品科技, 2013, 29(5): 1052-1056] |
XIE B, FU H, YANG F. Research progress on preparation, purification, identification and structure-activity relationship of bioactive peptides. Science and Technology of Food Industry, 2021, 42(5): 383-391 [谢博, 傅红, 杨方. 生物活性肽的制备、分离纯化、鉴定以及构效关系研究进展. 食品工业科技, 2021, 42(5): 383-391] |
XU K. Experimental study of the functions of Antarctic krill peptide on fatigue resistance, anti-hypoxia, anti-aging and immunity improvement. Master′s Thesis of Ocean University of China, 2012 [徐恺. 南极磷虾肽抗疲劳、耐缺氧以及抗衰老、提高免疫力实验研究. 中国海洋大学硕士研究生学位论文, 2012]
|
YANG L, DU H B, RAN X J, et al. Analysis of nanofiltration membrane pollution cause and operation management. Membrane Science and Technology, 2012, 32(2): 92-95 [杨砾, 杜海波, 冉祥军, 等. 纳滤膜污染原因分析及运行管理. 膜科学与技术, 2012, 32(2): 92-95] |
YU G C, HE H, JIN Z, et al. Nanofiltration for concentration and purification of corn peptides and their effects on antialcoholism activity. Food Science, 2011, 32(8): 10-14 [于国才, 何慧, 靳桢, 等. 纳滤技术浓缩纯化玉米肽及对其醒酒活性的影响. 食品科学, 2011, 32(8): 10-14] |
YU J W, DU F, TAO Y, et al. Study on anti-aging activity of peptide from Antarctic krill. Science and Technology of Food Industry, 2021, 42(20): 372-376 [于建伟, 杜芬, 陶宇, 等. 南极磷虾肽抗皮肤光老化作用研究. 食品工业科技, 2021, 42(20): 372-376] |
YUE S F. CFD simulation for nanofiltration concentration polarization and its influential factors. Master′s Thesis of Harbin Institute of Technology, 2017 [岳三峰. 纳滤过程浓差极化行为的CFD模拟与影响因素研究. 哈尔滨工业大学硕士研究生学位论文, 2017]
|
ZHANG H D, ZHANG L Y, ZHANG G Y, et al. Optimization of proteolytic conditions of Antarctic krill by response surface methodology. The Food Industry, 2019, 40(7): 94-98 [张华丹, 张玲云, 张国玉, 等. 响应面法优化南极磷虾蛋白酶解工艺条件. 食品工业, 2019, 40(7): 94-98] |
ZHAO X Y, ZUO T, LENG K L, et al. Engineering science and technology challenges in the Antarctic krill fishery. Strategic Study of CAE, 2016, 18(2): 85-90 [赵宪勇, 左涛, 冷凯良, 等. 南极磷虾渔业发展的工程科技需求. 中国工程科学, 2016, 18(2): 85-90] |
ZHAO Y Q, ZHANG L, TAO J, et al. Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (Euphausia superba): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs). Food Research International, 2019, 121: 197-204 |
ZHENG J R, SUN X P, CAI Z Y, et al. Optimizing the antioxidant components of Antarctic krill protein peptides. Journal of Food Safety and Quality, 2020, 11(1): 189-195 [郑景如, 孙馨娉, 蔡紫仪, 等. 优选南极磷虾蛋白肽抗氧化活性组分. 食品安全质量检测学报, 2020, 11(1): 189-195] |



