2. 中国水产科学研究院黄海水产研究所 青岛海洋科学与技术试点国家实验室海洋渔业科学与食物产出过程功能实验室农业农村部海水养殖病害防治重点实验室 青岛市海水养殖流行病学与生物安保重点实验室 山东 青岛 266071;
3. 中国海洋大学水产动物病害与免疫学实验室 山东 青岛 266003
2. Yellow Sea Fisheries Research Institute; Chinese Academy of Fishery Sciences, Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao); Key Laboratory of Maricultural Organism Disease Control, Ministry of Agriculture and Rural Affairs; Qingdao Key Laboratory of Mariculture Epidemiology and Biosecurity, Qingdao, Shandong 266071, China;
3. Laboratory of Pathology and Immunology of Aquatic Animals, Ocean University of China, Qingdao, Shandong 266003, China
疱疹样病毒粒子感染贝类的案例首次报道于1972年,引起一批实验用美洲牡蛎(Crassostrea virginica)种贝死亡,这也是此类病毒感染无脊椎动物的首例报道(Farley et al, 1972)。牡蛎疱疹病毒(Ostreid herpesvirus 1, OsHV-1)感染案例最早发现于1991年,引起育苗场长牡蛎(Crassostrea gigas)幼虫的大规模死亡,对这一世界性海洋主养贝类养殖产业造成严重打击(Barbosa-Solomieu et al, 2015; Hine et al, 1992; Nicolas et al, 1992)。目前,尚不清楚1972年病例疱疹样病毒粒子与OsHV-1是否为同一种病毒。产业健康发展的需求刺激多国科研机构围绕OsHV-1开展了病原学、流行病学、病原生态学和防控手段等众多领域的研究(Alfaro et al, 2019; Rodgers et al, 2019; Rosani et al, 2019; Whittington et al, 2016; 白昌明等, 2021),OsHV-1为首个被正式命名、系统分类地位明确的无脊椎动物疱疹病毒。与常见脊椎动物疱疹病毒普遍表现较高的宿主特异性不同,OsHV-1宿主范围较广,目前已证实被OsHV-1感染的物种有10余种,分属双壳纲(Bivalvia)、牡蛎目(Osteroida)、珍珠贝目(Pterioida)、蚶目(Arcoida)、帘蛤目(Veneroida)下不同的科和属(白昌明等, 2021)。同时,有多种双壳贝类以及荷兰、挪威等国的养殖贝类仅有PCR检测OsHV-1核酸阳性的结果,不能作为发生病毒感染的直接证据(Barbieri et al, 2019; Gittenberger et al, 2016; Mortensen et al, 2016)。这些易感性存疑的结果为流行病学监测计划的制定、动物检疫和疫病防控措施的实施造成障碍。
目前已开发出多种OsHV-1检测方法,如PCR (Batista et al, 2007)、原位杂交(Lipart et al, 2002)、原位PCR (李亚楠等, 2019)、组织病理、透射电镜、环介导等温核酸扩增(LAMP) (Ren et al, 2009; Zaczek-Moczydlowska et al, 2020)和重组酶聚合酶扩增(RPA) (Gao et al, 2018)等。根据世界动物卫生组织(OIE)水生动物手册的建议,OsHV-1感染的确诊需要核酸特异性检测阳性结果,结合组织病理和透射电镜影像才能做出诊断;单独使用核酸扩增技术对病原进行检测的方法不能用于感染案例的确诊(OIE, 2019)。透射电镜如果直接观察到处于不同时期的疱疹样病毒粒子,也可以作为病毒感染确诊的证据;但透射电镜检测的灵敏度和特异性低,尚需要PCR等特异性检测手段确认观察到的疱疹样病毒粒子是OsHV-1。原位杂交同时具备核酸检测特异性和组织原位观察的优势,是确诊病毒感染的一种重要技术手段(Lipart et al, 2002)。原位杂交虽然特异性强,但存在灵敏度不高、检测程序复杂、步骤繁琐等缺点(Muro-Cacho, 1997)。受限于透射电镜和原位杂交技术的使用限制,PCR仍然是目前最广泛使用的OsHV-1流行病学调查方法,这也导致出现很多核酸阳性病例未得到确诊的物种和地区。OsHV-1原位PCR检测方法作为原位杂交技术的延伸,提高了原位杂交的灵敏度(李亚楠等, 2019),但仍然存在操作程序复杂、需要高值专用设备(如组织切片PCR工作站等)的问题。本研究拟根据原位LAMP技术原理,建立一套兼具高灵敏度、简便快捷的OsHV-1检测方法,并利用该方法对2019年以来采集的发病贝类样本进行检测,为OsHV-1潜在易感宿主的鉴定提供依据;同时解析该病毒在新宿主中的分布规律和组织亲嗜性。
1 材料与方法 1.1 样本采集与保存魁蚶病料采集于2018年9月,用于原位LAMP反应条件的摸索和检测方法的建立。长牡蛎、福建牡蛎(Crassostrea angulata)、栉孔扇贝(Chlamys farreri)、虾夷扇贝(Mizuhopecten yessoensis)、毛蚶(Scapharca subcrenata)和菲律宾蛤仔(Ruditapes philippinarum)样本于2019—2021年采集自不同海区和养殖场非正常死亡案例(表 1)。采集的发病贝类样本普遍出现沉底(幼虫)、双壳闭合不全、反应迟钝、外套膜萎缩等临床症状。从表现临床症状的群体中选择受刺激时闭壳反应弱、内脏团完好、无异味的个体。现场或低温运回实验室后,分别采集发病贝类外套膜、鳃、肝胰腺和闭壳肌等组织器官,切取约5 mm厚,立即浸入至少10倍于采样组织体积的Davidson´s AFA固定液中,固定24~48 h后,直接进行组织切片或将固定的组织块儿移入70%乙醇溶液中室温存放。从各解剖个体剩余组织中剪取约30 mg外套膜组织,置于1.5 mL离心管中,直接用于DNA提取或–40℃保存备用。
|
|
表 1 OsHV-1原位LAMP反应体系所用引物 Tab.1 Primers for the OsHV-1 in situ LAMP reaction system |
使用海洋动物组织基因组DNA提取试剂盒(DP324) (北京天根生化科技有限公司)提取各贝类样本组织总DNA,按照试剂盒说明书操作。利用TaqMan实时定量PCR (qPCR)技术对各样本中OsHV-1核酸载量进行定量(Martenot et al, 2010)。反应引物和探针分别为:上游引物BF: 5′-GTCGCATCTTTGGATTTAACA A-3′、下游引物B4: 5′-ACTGGGATCCGACTGACAA C-3′,探针及生物素标记B-6FAM-TGCCCCTGTCAT CTTGAGGTATAGACAATC-BHQ-1。引物和探针由生工生物工程(上海)股份有限公司合成。PCR使用Fast Start Essential DNA Probes Master试剂盒(Roche, Cat. No.06402682001),反应体系:12.5 µL 2×PCR mix,上、下游引物(10 µmol/L)各1 µL,探针(10 µmol/L) 0.5 µL,DNA模板2 µL,添加水(试剂盒配备) 8 µL至总反应体系为25 µL。反应程序:95℃预变性10 min;95℃ 10 s,60℃ 20 s,40个循环,反应程序在Bio-Rad CFX Connect Real-Time PCR Detection System (Bio-Rad)仪器控制下完成。每批次qPCR均使用10倍梯度稀释(1×106、1×105、1×104、1×103、1×102、1×101拷贝/µL)的标准品进行定量分析。
1.3 LAMP反应引物的优化与验证根据Zaczek-Moczydlowska等(2020)研究中的OsHV-1 LAMP检测方法,外侧上、下游引物分别为OsHV-LP-F:TTTCAGTTCGTGCTCGGATT,OsHV-LP-B:TCCGGAACAATTGACCAAGC。内侧上、下游引物分别由2部分组成,上游引物OsHV-LP-FIP为F1c (CCAGTTGTATTTGGGCATGCCC)和F2 (GA GGCACATTCCATTTCCCT),中间由4个T (Thymine,胸腺嘧啶)连接(F1c+TTTT+F2);下游引物OsHV-LP-BIP为B1c (TCAGGCAGGCATTCAATTTGGC)和B2 (CCCCAG AAACAGGTCACAG),中间由4个T (Thymine, 胸腺嘧啶)连接(B1c+TTTT+B2)。在借鉴Zaczek-Moczydlowska等(2020)研究的内、外引物信息的基础上,为提高LAMP的反应速度和稳定性,使用LAMP引物设计软件(PrimerExplorer, http://primerexplorer.jp/lampv5e/index.html)设计4对环引物,并筛选得到扩增效果最好的1对环引物OsHV-LP-LF:5′-AGATA CAGTAGTTGGG AGTCTTACT-3′和OsHV-LP-LB:5′-ATGGTTCACGG GAGTGTATCC-3′,以便适应在切片上进行LAMP扩增时出现的复杂多变的扩增环境。
为避免开盖导致的气溶胶污染风险,通过核酸检测试纸条和一次性检测装置,直接检测LAMP扩增效果,为满足扩增结束后,直接进行核酸试纸条检测的需求,分别使用Biotin和6-FAM标记内引物OsHV-LP-FIP和外引物OsHV-LP-F的5′端。每个LAMP反应体系共25 μL,包括如下组分:10×Thermopol反应缓冲液2.5 μL,MgSO4 (100 mmol/L) 3.5 μl,引物OsHV-LP-FIP、OsHV-LP-BIP (20 μmol/L)各2 μL、OsHV-LP-LF、OsHV-LP-LB (20 μmol/L)各1 μL、OsHV-LP-F、OsHV-LP-B (10 μmol/L)各0.5 μL、dNTPs (10 mmol/L) 3.5 μL,甜菜碱(1.2 mol/L) 6 μL,8 U Bst DNA聚合酶(New England Biolabs),已提取的DNA模板2 μL。反应在PCR仪中进行,反应温度为65℃,反应时间为60 min,最后,85℃灭活5 min。
1.4 OsHV-1原位LAMP检测步骤常规方法进行切片、展片后,使用粘附载玻片捞片,随后在烤片机(Leica HI1220)上烘烤约12 h,使组织牢固地粘附在切片上,防止缓冲液浸洗等原位LAMP复杂操作引起的组织脱片。OsHV-1原位LAMP检测参考Chen等(2019)研究中的步骤,具体操作如下:
1.4.1 脱蜡与水化首先,将充分烘烤粘附的切片浸泡在杂交缸中,常温下进行常规脱蜡水化:二甲苯5 min (3次),无水乙醇5 min (2次),95%乙醇5 min,85%乙醇5 min,70%乙醇5 min,50%乙醇5 min。
1.4.2 消化与变性0.2 mol/L HCl室温浸浴20 min;蛋白酶K (20 μg/mL) 37℃消化15 min;95℃变性5 min,然后,置于4℃冷却5 min。
1.4.3 原位LAMP扩增将切片置于湿盒中,按照上述1.2节所述配制LAMP反应体系[使用带有地高辛标记的dNTP (Roche)],每张切片滴加LAMP反应体系150 µL,盖上盖玻片,在杂交炉中65℃扩增60 min。杂交完成后移去盖玻片,按如下步骤依次使用SSC梯度缓冲液浸洗:2×SSC室温5 min (2次),1×SSC 37℃ 5 min (2次),0.5×SSC 42℃ 15 min,0.1×SSC 42℃ 15 min,清除非特异性信号。
1.4.4 抗体孵育向每张切片滴加封闭剂(MAB∶10% BMB∶山羊血清原液=7∶2∶1) 1 mL,室温下封闭1 h。吸去预杂交液,滴加500 μL抗体孵育液[碱性磷酸酶标记抗地高辛抗体(Roche)∶封闭剂=1∶1000]孵育90 min。孵育完成后,将切片转移到杂交缸中,首先使用BufferⅠ(Tris-HCl 100 mol/L、NaCl 150 mol/L)浸洗10 min (2次),随后,使用BufferⅡ(Tris-HCl 100 mol/L、NaCl 100 mol/L、MgCl2 250 mol/L)浸洗5 min。
1.4.5 显色与复染吸取200 μL配制好的碱性磷酸酶底物显色剂(40 μL 25×NBT加入到1 mL 1×AP反应缓冲液)滴加在切片组织样本上,湿盒内室温显色(显色时间优化:30 min、45 min、60 min),苯胺棕复染2 min。复染完成后,用BufferⅢ(Tris-HCl 100 mol/L、EDTA 10 mol/L)浸洗30 min终止反应,并清洗切片。
1.4.6 脱水与封片将切片浸泡在杂交缸中,常温下进行酒精梯度脱水:50%乙醇5 min,70%乙醇5 min,85%乙醇5 min,95%乙醇5 min,无水乙醇5 min (2次),二甲苯5 min (3次),二甲苯透明,树脂封片。显微镜(Nikon Eclipse E80i)下观察组织切片的杂交信号并拍照。
1.5 基于原位LAMP检测方法的OsHV-1易感宿主调查应用OsHV-1原位LAMP检测方法对2019—2021年间收集的长牡蛎、福建牡蛎、栉孔扇贝、虾夷扇贝、毛蚶和菲律宾蛤仔的发病贝类样本进行检测(表 1)。选择这些物种的原则是国内外曾报道过OsHV-1核酸检测阳性案例,但我国近年来在相关物种(特定生物阶段)尚缺乏OsHV-1感染确诊的证据。采集样本时已经利用qPCR方法对每批次样本感染率以及阳性样本携带OsHV-1核酸拷贝数进行测定,并挑选部分样本进行组织固定、制作石蜡切片和病理检测。为验证本研究所开发OsHV-1原位LAMP检测方法的稳定性和特异性,重新对保存蜡块进行切片、原位LAMP扩增、抗体孵育,最后使用碱性磷酸酶显色剂显色后,光镜下观察蓝紫色阳性信号在不同组织中的分布情况并拍照。每批原位LAMP操作过程中均使用已知感染和未感染OsHV-1的魁蚶组织切片作为阳性和阴性对照。使用Kappa统计量检验qPCR检测方法与原位LAMP检测方法对样本检测结果的一致性。
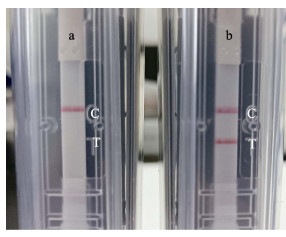
2 结果 2.1 LAMP反应引物的优化与验证最终使用的外侧、内侧以及环引物对见表 1。核酸试纸检测结果显示,感染OsHV-1的魁蚶检测结果为阳性(图 1b),而未感染OsHV-1魁蚶的反应结果均为阴性(图 1a),表明该LAMP体系可应用于后续原位LAMP测试。
|
图 1 核酸试纸条测试LAMP扩增结果 Fig.1 LAMP amplification results detected by nucleic acid strips a:阴性对照;b:阳性样本;C:质控线;T:测试线。 a: Negative control; b: Positive sample; C: Quality control line; T: Test line. |
本研究在建立OsHV-1原位LAMP检测方法过程中,参考Chen等(2019)报道的十足目虹彩病毒1 (Decapod iridescent virus 1, DIV1)原位LAMP检测方法,以及本团队在开发鲍疱疹病毒(Haliotid herpesvirus 1, HaHV-1)原位LAMP检测技术过程中积累的经验,最终形成如前所述的OsHV-1原位LAMP检测具体步骤。按照该技术流程进行操作,最终观察到的病毒信号显色清晰、易分辨(图 2B)。不加抗体的阴性对照未出现病毒信号(图 2C)。图 2为使用已知感染OsHV-1的魁蚶组织,进行原位LAMP检测时得到结果的示例。从组织病理HE染色结果可以看出,组织损伤、血淋巴渗出和浸润主要发生在结缔组织(图 2A),肌肉组织无炎症反应。与病理观察结果相一致,病毒阳性信号集中出现在发生组织损伤和炎症反应的结缔组织中。根据细胞形态及其分布,推测出现病毒阳性信号的细胞为血淋巴细胞和成纤维细胞(图 2D)。

|
图 2 OsHV-1原位LAMP检测方法开发结果示例
Fig.2 An example of the test results during the development of OsHV-1 in situ LAMP method
A:HE染色;B:原位LAMP检测;C:原位LAMP检测不加抗体阴性对照;D:图B中黑色方框区域的局部放大 m:肌肉组织;c:结缔组织;*:血细胞浸润;黑色箭头示病毒杂交信号。 A: HE staining; B: In situ LAMP detection; C: Negative control (without antibody) of OsHV-1 in situ LAMP tests; D: High magnification graphic of regions labeled by rectangular boxes in Fig.2B m: Muscle; c: Connective tissue; *: Haemocyte infiltration; Black arrows indicate viral hybridization signals. |
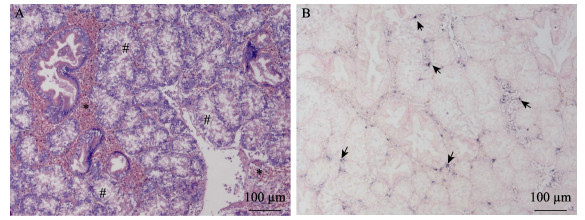
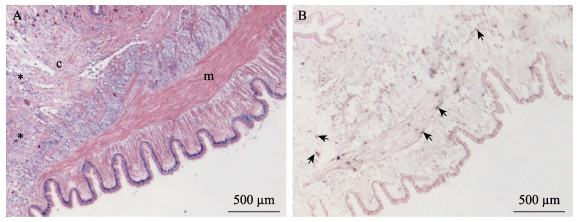
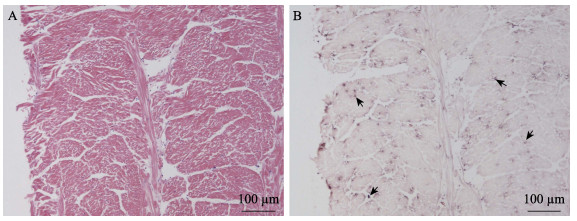
使用新开发的原位LAMP方法对保存的蜡块样本进行检测。结果显示,几个批次的长牡蛎、福建牡蛎、栉孔扇贝、虾夷扇贝和菲律宾蛤仔样本未检测到病毒感染,从多个批次的毛蚶样本中检测到OsHV-1感染杂交信号(表 2)。原位LAMP检测方法与qPCR检测方法对样本的检测结果经Kappa一致性检验,具有高度一致性,K值为0.638 (表 3)。OsHV-1杂交信号在毛蚶内脏团多个器官中浸润的血淋巴细胞内被观察到,说明血淋巴细胞对该病毒非常易感。肝胰腺小管出现不同程度的上皮细胞坏死、组织损伤,但病毒信号主要出现在结缔组织、肝胰腺小管间浸润的血淋巴细胞中(图 3)。外套膜结缔组织出现损伤和坏死,肌肉组织偶见损伤和血细胞浸润。根据这些器官原位LAMP检测结果,推测结缔组织中被感染的细胞类型主要为成纤维细胞和浸润血淋巴细胞;肌肉组织被感染的为肌细胞的细胞核(图 4)。肌细胞携带病毒杂交信号的现象在富含肌肉纤维的斧足和闭壳肌中也经常被观察到(图 5和图 6),这些结果说明,毛蚶的肌细胞对OsHV-1的亲嗜性较强。部分个体鳃丝样本出现轻度肿胀,肿胀的鳃丝中有血淋巴细胞浸润,并观察到病毒阳性信号,推测杂交信号正是来源于浸润的血淋巴细胞(图 7)。肝胰腺器官的组织病变和病毒杂交信号在各发病个体中稳定出现,推荐肝胰腺作为毛蚶感染OsHV-1组织病理、原位LAMP检测的首选目标器官。
|
|
表 2 OsHV-1原位LAMP检测2019—2021年间发病贝类样本列表 Tab.2 Samples collected from 2019 to 2021 for verification of OsHV-1 in situ LAMP method |
|
|
表 3 OsHV-1原位LAMP检测与qPCR检测结果Kappa一致性分析 Tab.3 Analysis of Kappa concordance between OsHV-1 in situ LAMP assay and qPCR results |
|
图 3 肝胰腺原位LAMP检测结果
Fig.3 In situ LAMP detection in the hepatopancreas
A:HE染色;B:原位LAMP检测 *:血细胞浸润区;#:肝胰腺小管上皮坏死;黑色箭头被OsHV-1感染的细胞。 A: HE staining; B: In situ LAMP detection *: Infiltrated haemocytes; #: Epithelial cell and tissue necrosis of the hepatopancreatic tubules; Black arrows show OsHV-1 signals. |
|
图 4 外套膜原位LAMP检测结果
Fig.4 In situ LAMP detection in the mantle
A:HE染色;B:原位LAMP检测 m:肌肉组织;c:结缔组织;*:血细胞浸润;黑色箭头示病毒杂交信号。 A: HE staining; B: In situ LAMP detection m: Muscle; c: Connective tissue; *: Infiltrated haemocytes; Black arrows show OsHV-1 signals. |
|
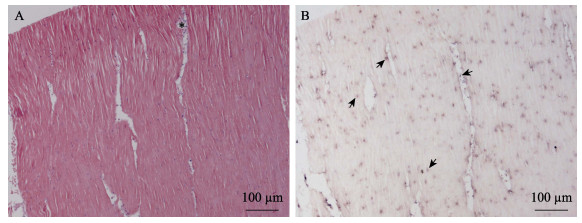
图 5 斧足原位LAMP检测结果
Fig.5 In situ LAMP detection in the axe foot
A:HE染色;B:原位LAMP检测 图B中的黑色箭头示被OsHV-1感染的细胞。 A: HE staining; B: In situ LAMP detection Black arrows in Fig.B show OsHV-1 signals. |
|
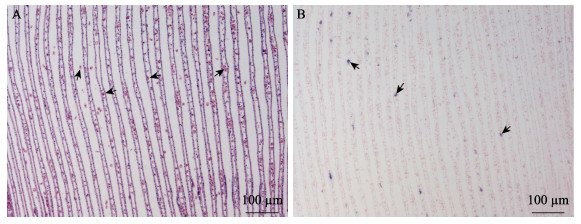
图 6 闭壳肌原位LAMP检测结果
Fig.6 In situ LAMP detection in the muscle
A:HE染色;B:原位LAMP检测 *:血细胞浸润;黑色箭头示被OsHV-1感染的细胞。 A: HE staining; B: In situ LAMP detection *: Haemocyte infiltration; Black arrows show OsHV-1 signals. |
|
图 7 鳃原位LAMP检测结果 Fig.7 In situ LAMP detection in the gill A:HE染色(黑箭头示异常增多的血淋巴细胞);B:原位LAMP检测(黑色箭头示被OsHV-1感染的细胞) A: HE staining (Black Arrows show increased haemocytes); B: In situ LAMP detection (Black arrows show OsHV-1 signals) |
贝类养殖产业是我国海水养殖产业的重要组成部分,特别是20世纪80年代,以扇贝养殖为驱动力掀起的我国海水养殖第三次浪潮,引领了我国海水养殖产业的跨越式发展。自20世纪90年代以来,贝类养殖产量在海水养殖总产量中的占比稳居70%以上(阙华勇等, 2016)。随着贝类养殖产业总体规模的不断扩大,养殖病害问题日益严重。据统计,2020年中国贝类养殖产业因疾病造成的经济损失高达120.0亿元(中国水生动物卫生状况报告)。OsHV-1是近年来我国和世界贝类养殖产业面临的一种主要病毒性病原,其具有宿主范围广、变异株频繁出现等特点(Arzul et al, 2017; 白昌明等, 2021; 张淑敏等, 2020),这给该病毒的流行病学调查和防控工作带来了极大挑战。对重要病原微生物易感宿主的准确认定,是制定有效疫病防控措施的前提。2014年,OIE对水生动物疾病易感宿主的标准进行了修订,至少满足以下3个原则才能被认定为易感宿主:病例来源于自然感染或无创人工感染实验(混养、浸浴或投喂),通过注射等侵害性人工感染方式、浸浴时病原浓度高于自然环境或水体理化条件异于自然水体(如高温)等条件下发生的病例不能用于易感性评估;病原微生物的种类得到充分鉴定;有明确证据表明病原微生物在宿主体内发生复制,或病原微生物寄生部位伴有感染发生(OIE, 2021)。这些原则给OsHV-1流行病学调查过程中适宜采用的技术手段提供了参考。原位杂交技术作为一项集OsHV-1分子水平种特异性与病毒组织分布原位检测于一体的技术手段,为OsHV-1感染的确诊提供了一个解决方案(Lipart et al, 2002)。但由于该方法操作繁琐、耗时较长,不适合开展大规模流行病学调查工作。
原位LAMP技术自2003年被提出以来(Maruyama et al, 2003),已被应用于包括传染性皮下及造血组织坏死病毒(infectious hypodermal and hematopoietic necrosis virus, IHHNV) (Jitrakorn et al, 2016)和十足目虹彩病毒1 (Decapod iridescent virus 1, DIV1) (Chen et al, 2019)等水生动物病原在内的多种病原微生物检测(Hashimoto et al, 2018; Ye et al, 2011)。与传统原位杂交和原位PCR检测技术相比,原位LAMP具有核酸扩增反应温度低、对组织破坏小,恒温扩增不需要特殊实验设备以及实验操作更方便、快捷等优点(Maruyama et al, 2003)。本研究在结合已有OsHV-1 LAMP特异性引物的基础上,补充设计环引物,构建OsHV-1原位LAMP检测方法。应用该方法对2019年来本团队收集的非正常死亡贝类样本进行检测,首次确认毛蚶是OsHV-1的易感宿主。在毛蚶外套膜、肝胰腺结缔组织,以及富含肌肉细胞的斧足和闭壳肌中也发现较多病毒杂交信号,推测部分肌肉组织中的病毒杂交信号来自肌细胞的细胞核。在毛蚶鳃丝中也发现病毒杂交信号,根据信号出现的位置和细胞形态推测其来源于血淋巴细胞。
聚合酶链式反应(PCR)与荧光定量(qPCR)检测技术由于其灵敏度高、特异性强,并且可以进行定量检测的技术优势,已被广泛用于OsHV-1等多种水生动物病原感染的筛查和确诊研究中。本研究使用的OsHV-1 qPCR检测方法的检测灵敏度为6病毒拷贝/mg组织(Martenot et al, 2010),同时,液相PCR在密闭环境下进行,具有稳定性强的优势。本研究所使用的LAMP检测方法灵敏度为103病毒拷贝/反应(Zaczek-Moczydlowska et al, 2020),同时,反应在开放的载破片上进行,稳定性较差。但qPCR的检测需要提取病毒DNA作为模板,提取过程破坏了病毒感染的组织,且易受污染,造成假阳性。原位LAMP检测技术与之相比虽然灵敏度较低,但规避了qPCR无法定位病毒在组织分布的缺点。本研究结果表明,qPCR适合开展OsHV-1的大规模流行病学筛查,原位LAMP适合开展OsHV-1感染确诊研究。
20世纪90年代末,栉孔扇贝曾是我国北方最重要的海水养殖贝类之一,年产量最高时达60万t以上,在海水养殖产业中占有极其重要的地位(张福绥等, 1999)。由于大规模、高密度的单一品种养殖,海区生态系统失衡,导致病害暴发,引起栉孔扇贝发生大规模死亡(宋微波等, 2001; 王崇明等, 2002)。对从死亡栉孔扇贝中纯化的疱疹样病毒粒子的基因组测序和系统发育关系分析的结果显示,该疱疹病毒与OsHV-1为同种病毒的不同变异株,我国学者根据其致病特点将其命名为扇贝急性病毒性坏死病毒(acute viral necrosis virus, AVNV)。AVNV与OsHV-1参考株基因组相似度为97.0%。受海区生态环境失衡和AVNV病害的影响,我国栉孔扇贝养殖产业迅速萎缩,年产量最低时跌至不足20万t (Guo et al, 2016)。对非正常死亡栉孔扇贝开展的被动流行病学调查显示,2001—2007年AVNV感染率(> 60%)和感染强度(> 104拷贝/ng总DNA)普遍较高(2000年之前的样本缺失),2008—2013年感染率(< 50%)和感染强度(< 103拷贝/ng总DNA)普遍下降,2014—2020年未检测到确诊病例(Bai et al, 2015)。本研究使用新开发的OsVH-1原位LAMP检测方法,对近年来采集的栉孔扇贝样本进行检测的结果也为阴性。
长牡蛎原产于西北太平洋中韩日俄海域,因其生长快、抗逆性强,被引进到全球各地广泛养殖(Guo, 2009; 张国范等, 2020)。从全球范围来看,长牡蛎也是受OsHV-1病害影响最严重的物种,主要引起幼虫和幼贝的大规模死亡(Barbosa Solomieu et al, 2015)。我国长牡蛎感染OsHV-1的案例最早报道于2009年,但目前因OsHV-1感染引起的大规模死亡案例仅发生于幼虫阶段。福建牡蛎是长牡蛎的暖水姊妹亚种,在欧洲又被称为葡萄牙牡蛎(张国范等, 2020)。西班牙和葡萄牙学者从当地福建牡蛎样本中检测到OsHV-1核酸(López Sanmartín et al, 2016a、2016b),但尚无确诊案例报道。本研究利用qPCR从福建牡蛎种贝中检测到低载量的OsHV-1 DNA,但原位LAMP检测结果为阴性。2个批次的福建牡蛎样本采集自育苗场,同期曾发生过长牡蛎幼虫感染OsHV-1死亡案例。推测由于场区未做好生物安保措施,水体或环境中存在OsHV-1污染,从而导致qPCR检测福建牡蛎种贝呈弱阳性。
OsHV-1病害自20世纪90年代在我国发生以来,其宿主范围不断扩大,并随着海区生态环境等因素发生变化,给该病毒病的防控造成困难。近年来,我国长牡蛎三倍体人工育种和养殖规模不断扩大,OsHV-1感染引起的幼虫死亡案例频繁发生。目前,我们对我国大宗养殖贝类与常见野生贝类对OsHV-1的易感性尚不完全了解,本研究基于原位LAMP技术建立了OsHV-1原位LAMP检测方法,并应用到OsHV-1的易感宿主调查工作中;为后续开展OsHV-1流行病学、易感宿主调查和组织亲嗜性等研究提供技术支撑。
ALFARO A C, NGUYEN T V, MERIEN F. The complex interactions of Ostreid herpesvirus 1, Vibrio bacteria, environment and host factors in mass mortality outbreaks of Crassostrea gigas. Reviews in Aquaculture, 2019, 11(4): 1148-1168 DOI:10.1111/raq.12284 |
ARZUL I, CORBEIL S, MORGA B, et al. Viruses infecting marine molluscs. Journal of Invertebrate Pathology, 2017, 147: 118-135 DOI:10.1016/j.jip.2017.01.009 |
BAI C M, WANG C M, XIA J Y, et al. Emerging and endemic types of Ostreid herpesvirus 1 were detected in bivalves in China. Journal of Invertebrate Pathology, 2015, 124: 98-106 DOI:10.1016/j.jip.2014.11.007 |
BAI C M, XIN L S, WANG C M. Malacoherpesviruses and their associated damages to mollusk aquaculture industry. Progress in Fishery Sciences, 2021, 42(1): 214-226 [白昌明, 辛鲁生, 王崇明. 软体动物疱疹病毒及其对贝类养殖产业的危害. 渔业科学进展, 2021, 42(1): 214-226 DOI:10.19663/j.issn2095-9869.20200527001] |
BARBIERI E S, MEDINA C D, Vázquez N, et al. First detection of Ostreid herpesvirus 1 in wild Crassostrea gigas in Argentina. Journal of Invertebrate Pathology, 2019, 166: 107222 DOI:10.1016/j.jip.2019.107222 |
BARBOSA SOLOMIEU V, RENAULT T, TRAVERS M A. Mass mortality in bivalves and the intricate case of the Pacific oyster, Crassostrea gigas. Journal of Invertebrate Pathology, 2015, 131: 2-10 DOI:10.1016/j.jip.2015.07.011 |
BATISTA F M, ARZUL I, PEPIN J F, et al. Detection of ostreid herpesvirus 1 DNA by PCR in bivalve molluscs: A critical review. Journal of Virological Methods, 2007, 139(1): 1-11 DOI:10.1016/j.jviromet.2006.09.005 |
CHEN X, QIU L, WANG H, et al. Susceptibility of Exopalaemon carinicauda to the infection with shrimp hemocyte iridescent virus (SHIV 20141215), a strain of decapod iridescent virus 1 (DIV1). Viruses, 2019, 11(4): 387 DOI:10.3390/v11040387 |
FARLEY C A, BANFIELD W G, KASNIC Jr G, et al. Oyster herpes-type virus. Science, 1972, 178(4062): 759-760 DOI:10.1126/science.178.4062.759 |
GAO F, JIANG J Z, WANG J Y, et al. Real-time quantitative isothermal detection of Ostreid herpesvirus-1 DNA in Scapharca subcrenata using recombinase polymerase amplification. Journal of Virological Methods, 2018, 255: 71-75 DOI:10.1016/j.jviromet.2018.02.007 |
GITTENBERGER A, VOORBERGEN-LAARMAN M A, ENGELSMA M Y. Ostreid herpesvirus OsHV-1 mu Var in Pacific oysters Crassostrea gigas (Thunberg 1793) of the Wadden Sea, a UNESCO world heritage site. Journal of Fish Diseases, 2016, 39(1): 105-109 DOI:10.1111/jfd.12332 |
GUO X M, FORD S E. Infectious diseases of marine molluscs and host responses as revealed by genomic tools. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 2016, 371: 1689 |
GUO X M. Use and exchange of genetic resources in molluscan aquaculture. Reviews in Aquaculture, 2009, 1(3/4): 251-259 |
HASHIMOTO M, SAKAMOTO H, IDO Y, et al. In situ loop-mediated isothermal amplification (LAMP) for identification of Plasmodium species in wide-range thin blood smears. Malaria Journal, 2018, 17: 235 DOI:10.1186/s12936-018-2381-7 |
HINE P, WESNEY B, HAY B. Herpesviruses associated with mortalities among hatchery-reared larval Pacific oysters, Crassostrea gigas. Diseases of Aquatic Organisms, 1992, 12(2): 135-142 |
JITRAKORN S, ARUNRUT N, SANGUANRUT P, et al. In situ DIG-labeling, loop-mediated DNA Amplification (ISDL) for highly sensitive detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV). Aquaculture, 2016, 456: 36-43 DOI:10.1016/j.aquaculture.2016.01.023 |
LI Y N, BAI C M, LIU J L, et al. Establishment and application of indirect in situ polymerase chain reaction for detection of Ostreid herpesvirus-1 (OsHV-1). Journal of Fisheries of China, 2019, 43(3): 679-687 [李亚楠, 白昌明, 刘金兰, 等. 牡蛎疱疹病毒(OsHV-1)间接原位杂交PCR检测方法的建立与初步应用. 水产学报, 2019, 43(3): 679-687] |
LIPART C, RENAULT T. Herpes-like virus detection in infected Crassostrea gigas spat using DIG-labelled probes. Journal of Virological Methods, 2002, 101(1/2): 1-10 |
LÓPEZ SANMARTÍN M, LÓPEZ FERNÁNDEZ J R, CUNHA M E, et al. Ostreid herpesvirus in wild oysters from the Huelva coast (SW Spain). Diseases of Aquatic Organisms, 2016a, a, 120(3): 231-240 |
LÓPEZ SANMARTÍN M, POWER D M, DE LA HERRÁN R, et al. Evidence of vertical transmission of ostreid herpesvirus 1 in the Portuguese oyster Crassostrea angulata. Journal of Invertebrate Pathology, 2016b, b, 140: 39-41 |
MARTENOT C, ODEN E, TRAVAILLÉ E, et al. Comparison of two real-time PCR methods for detection of ostreid herpesvirus 1 in the Pacific oyster Crassostrea gigas. Journal of Virological Methods, 2010, 170(1/2): 86-89 |
MARUYAMA F, KENZAKA T, YAMAGUCHI N, et al. Detection of bacteria carrying the stx2 gene by in situ loop-mediated isothermal amplification. Applied and Environmental Microbiology, 2003, 69(8): 5023-5028 DOI:10.1128/AEM.69.8.5023-5028.2003 |
MORTENSEN S, STRAND A, BODVIN T, et al. Summer mortalities and detection of Ostreid herpesvirus microvariant in Pacific oyster Crassostrea gigas in Sweden and Norway. Diseases of Aquatic Organisms, 2016, 117(3): 171-176 DOI:10.3354/dao02944 |
MURO-CACHO C A. In situ PCR. Overview of procedures and applications. Frontiers in Bioscience, 1997, 2(3): 15-29 |
NICOLAS J, COMPS M, COCHENNEC N. Herpes-like virus infecting Pacific-oyster larvae, Crassostrea gigas. Bulletin of the European Association of Fish Pathologists, 1992, 12(1): 11-13 |
OIE. Criteria for listing species as susceptible to infection with a specific pathogen. In: OIE. Aquatic animal health code. OIE Publications Unit Paris, 2021, 1–4
|
OIE. Infection with Ostreid herpesvirus 1 micro variants. In: OIE. Manual of diagnostic tests for aquatic animals. OIE Publications Unit Paris, 2019, 1–14
|
QUE H Y, ZHANG G F. Status and trend of molluscan mariculture techniques in China. Studia Marina Sinica, 2016(1): 69-76 [阙华勇, 张国范. 我国贝类产业技术的现状与发展趋势. 海洋科学集刊, 2016(1): 69-76] |
REN W C, WANG C M, CAI Y Y. Loop-mediated isothermal amplification for rapid detection of acute viral necrobiotic virus in scallop Chlamys farreri. Acta Virologica, 2009, 53(3): 161-167 |
RODGERS C, ARZUL I, CARRASCO N, et al. A literature review as an aid to identify strategies for mitigating ostreid herpesvirus 1 in Crassostrea gigas hatchery and nursery systems. Reviews in Aquaculture, 2019, 11(3): 565-585 |
ROSANI U, YOUNG T, BAI C M, et al. Dual analysis of virus-host interactions: The case of Ostreid herpesvirus 1 and the cupped oyster Crassostrea gigas. Evolutionary Bioinformatics, 2019, 15: 1-8 |
SONG W B, WANG C M, WANG X H, et al. New research progress on massive mortality of cultured scallop Chlamys farreri. Marine Science, 2001, 25(12): 23-26 [宋微波, 王崇明, 王秀华, 等. 栉孔扇贝大规模死亡的病原研究新进展. 海洋科学, 2001, 25(12): 23-26] |
WANG C M, WANG X H, SONG X L, et al. Purification and ultrastructure of a spherical virus in cultured scallop Chlamys farreri. Journal of Fisheries of China, 2002, 26(2): 180-184 [王崇明, 王秀华, 宋晓玲, 等. 栉孔扇贝一种球形病毒的分离纯化及其超微结构观察. 水产学报, 2002, 26(2): 180-184] |
WHITTINGTON R, HICK P, EVANS O, et al. Pacific oyster mortality syndrome: A marine herpesvirus active in Australia. Microbiology Australia, 2016, 37(3): 126-128 |
YE Y, WANG B, HUANG F, et al. Application of in situ loop-mediated isothermal amplification method for detection of Salmonella in foods. Food Control, 2011, 22(3/4): 438-444 |
ZACZEK-MOCZYDLOWSKA M A, MOHAMED-SMITH L, TOLDRÀ A, et al. A single-tube HNB-based loop-mediated isothermal amplification for the robust detection of the Ostreid herpesvirus 1. International Journal of Molecular Sciences, 2020, 21: 6605 |
ZHANG F S, YANG H S. Analysis of the causes of mass mortality of farming Chlamys farreri in summer in coastal areas of Shandong, China. Marine Science, 1999(1): 44-47 [张福绥, 杨红生. 山东沿岸夏季栉孔扇贝大规模死亡原因分析. 海洋科学, 1999(1): 44-47] |
ZHANG G F, LI L, QUE H Y. An evolution of oyster mariculture industry in China: New knowledge, variety and product. Oceanologia et Limnologia Sinca, 2020, 51(4): 740-749 [张国范, 李莉, 阙华勇. 中国牡蛎产业的嬗变——新认知、新品种和新产品. 海洋与湖沼, 2020, 51(4): 740-749] |
ZHANG S M, BAI C M, XIN L S, et al. Gene cloning and expression of Ostreid herpesvirus 1 envelope protein (ORF111). Progress in Fishery Sciences, 2020, 41(2): 183-190 [张淑敏, 白昌明, 辛鲁生, 等. 牡蛎疱疹病毒囊膜蛋白(ORF111)的基因克隆及表达. 渔业科学进展, 2020, 41(2): 183-190] |



