2. 中国水产科学研究院黄海水产研究所 青岛海洋科学与技术试点国家实验室深蓝渔业工程联合实验室 山东 青岛 266071
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Joint Laboratory for Open Sea Fishery Engineering, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, Shandong 266071, China
许氏平鲉(Sebastes schlegelii)属鲉形目(Scorpaeniformes)、平鲉科(Sebastidae)、平鲉属(Sebastes),是我国近海常见的冷温性底层卵胎生鱼类(冯昭信, 2003; Feng et al, 2014),也是我国北方重要海水养殖经济鱼类之一(万瑞景等, 1988),具有较高的商业及休闲垂钓价值,同时也是一种非常适宜海洋牧场增殖的优质无远距离洄游鱼类。近年来,由于野生资源的过度捕捞和海洋环境的变化,许氏平鲉自然资源不断下降(曾慧慧等, 2012; Zhang et al, 2015)。为了养护衰退的许氏平鲉自然资源,伴随着我国海洋牧场建设的快速发展,我国山东、河北、辽宁等多个沿海省份都开展了大规模的许氏平鲉增殖放流活动,每年在海洋牧场等近海水域放流数千万尾许氏平鲉苗种,对自然资源的养护和修复起到了积极的促进作用。标记放流是评估水生生物增殖效果的重要手段,为准确评估许氏平鲉的放流效果,学者们采用耳石荧光标记(Lü et al, 2014)或定制网捕获等方法分析了其对资源增殖的效果,为许氏平鲉科学放流提供了参考依据。然而,目前已报道的可用于许氏平鲉增殖放流的标记技术仅有耳石荧光标记技术,且耳石染色(朱亚华等, 2020)存在标记不易检测、易消褪等问题,有必要开发新型的高效标记技术,支撑许氏平鲉增殖放流效果的精准评估。
金属编码标签(coded-wire tag, CWT)具有体积小、易操作、精确度与回收率高且规模化操作价格低等优点,是评价鱼类放流效果重要标记技术之一(Guy et al, 1996),国内外已在金头鲷(Sparus aurata)、大西洋鲑鱼(Salmo salar)、拉萨裸裂尻鱼(Schizopygopsis younghusbandi Regan, 1905)、大黄鱼(Larimichthys crocea)、牙鲆(Paralichthys olivaceus)、七鳃鳗(Entosphenus tridentatus)、欧洲鳗(Anguilla anguilla)等多种标记放流鱼类中得到应用(Sánchez-Lamadrid et al, 2001; Crozier et al, 2002; Zhu et al, 2016; 黄建华等, 2016; 徐永江等, 2017; Hanson et al, 2017; Simon et al, 2020),取得了较好的标记效果,但尚未见有关标记对鱼类生理指标的影响的研究报道。目前,CWT对许氏平鲉苗种的标记放流研究尚未见报道。本研究以许氏平鲉为对象,测试CWT标签对不同规格许氏平鲉苗种不同标记部位的标记效果,记录存活率、脱标率以及生长指标。放流苗种的标记操作对鱼类是一种胁迫,为了从生理学角度深入揭示CWT标记操作对放流苗种的生理胁迫与适应机制,本研究还通过测定免疫相关酶——超氧化物歧化酶(SOD)、过氧化氢酶(CAT)的活性变化和生长及应激相关基因(IGF-1和HSP70)的表达变化评价苗种对CWT标记的生理适应特性,以期为许氏平鲉放流适宜标记方式选择与增殖效果评估提供技术支撑。
1 材料与方法 1.1 实验材料本实验于2020年8—10月在青岛贝宝海洋科技有限公司开展,所需许氏平鲉苗种为该公司培育的人工苗种,苗种规格整齐且摄食游泳行为良好。小规格苗种全长为(8.25±0.84) cm,体重为(9.99±2.75) g;大规格苗种全长为(11.13±0.67) cm,体重为(20.95±2.99) g (表 2)。实验场地为该公司育苗车间的6个容积为4 m3的水泥池,实验期间的养殖条件:盐度为29~31,溶解氧为6~7 mg/L,pH为7.8~8.0,水温为21~23℃,日换水率为200%。投喂配合饲料,日投喂2次,饱食投喂。
|
|
表 1 许氏平鲉标记苗种实时荧光定量PCR引物序列 Tab.1 Primer sequences used for RT-qPCR amplification of IGF-1, HSP70 from S. schlegeli |
|
|
表 2 各实验组许氏平鲉苗种脱标率、死亡率及生长情况 Tab.2 The tag shedding rate, mortality and growth of S. schlegeli juveniles after tagged with CWT |
选择实验鱼鳃部鄂弓提肌(levator arcus palatine muscle)和背部肌肉作为标记位点。不同规格苗种和不同标记位置设置4个实验组和2个对照组:小规格鳃鄂弓提肌标记组(SLM)、小规格背部肌肉标记组(SDM)、大规格鳃鄂弓提肌标记组(LLM)、大规格背部肌肉标记组(LDM)、小规格对照组(无标记)(SCN)和大规格对照组(LCN)。每个实验组设置1个重复。每组各使用120尾鱼进行实验,实验开始前和结束时,每组随机选取30尾进行体重、全长等指标测量并记录(表 2)。暂养期间,每隔12 h进行投喂,并在投喂1 h后清理池底。
1.2 CWT标记操作实验所用CWT标记设备及探测器购自美国西北海洋技术公司。CWT标签的直径为0.25 mm,长度为1 mm,利用配套的标记器进行标记操作,利用探测器进行标签的扫描识别(图 1)。所有实验鱼在标记开始前提前24 h停止投喂,对照组鱼苗不进行标记处理,实验组苗种以MS-222进行麻醉,小规格和大规格苗种的MS-222麻醉剂量分别为30和40 mg/L。由专业人员将麻醉后的苗种置于铺有湿毛巾的平面上进行CWT标记操作,左手戴手套用于固定鱼体,右手持标记枪进行操作,分别标记在鳃盖肌肉处和背部肌肉处(图 2)。鳃鄂弓提肌位于眼右后方和鳃盖后缘中间的位置,背部肌肉选择肌肉较厚的位置(背鳍基部下方4~5个鳞片处)进行标记,将标记枪头呈45°插入肌肉,标记方向与鳞片同向,深度为2~3 mm,轻轻推入标签,按动开关剪断标签后拔出。标记后,使用探测器(图 1下)扫描实验鱼被标记部位,听到声响后确认标签植入成功。苗种标记后,迅速置于洁净的海水中复苏。
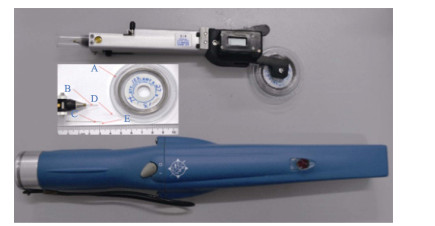
|
图 1 CWT标记器(上)、标签系统(中)和探测器(下) Fig.1 The CWT injector (upper), CWT system (middle), and detector (bottom) |
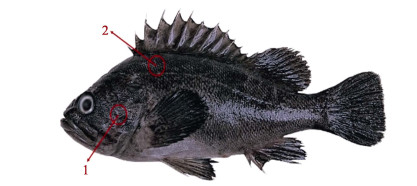
|
图 2 许氏平鲉苗种标记位置 Fig.2 The schematic diagram of CWT tagging position in S. schlegelii 1:鳃鄂弓提肌标记位置;2:背部标记位置。 1: Gill levator arcus palatine muscle tagging; 2: Dorsal muscle tagging. |
标记完成后,各实验组和对照组分别选取120尾鱼在池内养殖,养殖时间为30 d。标记苗种养殖期间,观察标记苗种的游泳与摄食行为。在5 d、15 d和实验结束时,使用探测器检测各实验组的CWT脱标情况,及时拣出脱标的苗种,记录脱标率。每天上午和下午投喂配合饲料,投喂量为苗种体重的3%,日换水量为200%。实验结束时,随机选择30尾实验鱼,测量并记录其体重和全长等数据,特定生长率(SGR)的计算参照以下公式:
| $ \mathrm{SGR}=\frac{\ln W_2-\ln W_1}{\Delta t} \times 100$ |
式中,W1为起始个体体重,W2为实验结束后个体体重,Δt为实验持续时间。
实验开始前后,每实验组采集6尾实验鱼的脑和肝脏样品,液氮保存,并转移至–80℃冰箱保存,用于RNA的提取。同时,取肝脏组织样品,置于冻存管中于–20℃保存,用于SOD和CAT酶活性的测定。
1.4 酶活性测定使用南京建成生物工程有限公司(南京, 中国)的CAT和SOD试剂盒,按照使用说明测定酶活性。利用BCA蛋白浓度测定试剂盒测量酶液中的蛋白浓度。CAT酶活性单位定义为:在37℃条件下,每毫克组织蛋白每秒钟分解1 μmol的H2O2的量为1个活性单位。SOD酶活性单位定义为:每100 mg组织在1 mL反应液中SOD抑制率达50%时所对应的酶量为1个SOD活性单位(U/mL)。
1.5 基因表达分析 1.5.1 总RNA提取和第一链cDNA合成利用RNAiso Plus (TaKaRa,日本)提取样品总RNA,通过NanoDrop 2000C分光光度计(Thermo,美国)测定RNA的纯度和浓度,以1%琼脂糖凝胶电泳检验RNA完整性。使用PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time)反转录试剂盒(TaKaRa,日本)合成第一链cDNA。将反转录产物于−20℃冰箱保存备用。
1.5.2 实时荧光定量PCR检测参照NCBI数据库中许氏平鲉IGF-1 (AF481856)、HSP70 (KC172645)和β-actin (JN226153)设计各目基因的特异性实时荧光定量PCR (RT-qPCR)引物(表 1)。使用SYBR Premix Ex TaqTMⅡ (TaKaRa,日本)试剂盒以不同处理组的cDNA为模板进行基因定量表达分析,β-action为内参基因。RT-qPCR反应体系为20 μL,包括10 μL 2× SYBR®Premix Ex Taq™Ⅱ、0.8 μL上下游引物(引物浓度均为10 μmol/L)、1 μL cDNA模板以及7.4 μL无菌水,利用LightCycler® 96 real-time PCR仪(Roche,德国)进行荧光定量检测。每个样品3个平行,采用三步PCR法:95℃ 5 s;56℃ 20 s,72℃ 10 s,共40个循环,反应结束后进行熔解曲线分析。所有目的基因和参考基因的标准曲线相关系数(r2)和扩增效率(E):0.99 < r2 < 0.999,0.9 < E < 1.1。基因相对表达量参照2–ΔΔCt法计算,结果以平均值±标准误(Mean±SE)表示。
1.6 数据处理实验数据进以平均值±标准差(Mean±SD)表示。利用IBM Statistic SPSS 25.0软件对结果进行统计分析,运用单因素方差分析(one-way ANOVA)和Duncan检验法对各实验组的荧光定量数据和酶活数据进行显著性差异分析和多重比较,当显著性水平P < 0.05时认为差异显著。
2 结果 2.1 CWT标记对苗种摄食状态的影响在苗种被麻醉标记后,不同规格的苗种在清洁海水中均能保证3 min内复苏并正常游动。SLM和LLM组苗种在标记1~5 d摄食情况良好(30 min后无剩余残饵),6~30 d摄食情况积极(5 min后无剩余残饵);SDM和LDM组苗种在标记1~5 d摄食情况一般(30 min后有剩余残饵),6~30 d摄食情况积极(5 min后无剩余残饵)。
2.2 CWT标记苗种死亡率、脱标率及生长情况CWT标记后苗种经过30 d的暂养,成活率均为100%。各实验组的脱标率、全长、体重和特定生长率变化情况见表 2。SLM组和LLM组苗种脱标率分别为13%和3%;SDM和LDM组苗种均未出现脱标现象。同等规格各实验组标记鱼的体长和体重与各自对应的对照组实验鱼差异显著(P < 0.05)。同等规格的标记苗种特定生长率均低于各自对照组苗种,且具有显著差异(P < 0.05)。
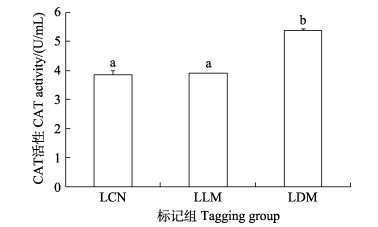
2.3 CWT标记对肝脏CAT、SOD酶活性的影响暂养30 d后,SLM组实验鱼肝脏CAT酶活性(5.272 7 U/mL)显著高于对照组(3.572 1 U/mL) (P < 0.05),而SDM组实验鱼肝脏中CAT酶活性略高于对照组(图 3)。LLM组实验鱼在暂养30 d后,肝脏组织CAT酶活性(4.552 1 U/mL)与对照组未有显著差异,而LDM组实验鱼肝脏CAT酶活性(5.366 1 U/mL)显著高于对照组(P < 0.05)和LLM组(3.915 3 U/mL) (P < 0.05) (图 4)。

|
图 3 CWT标记30 d小规格苗种肝脏中CAT酶活性 Fig.3 The activity of CAT in the liver of CWT labeled small-sized juveniles at 30 days post tagging SCN:小规格对照组;SLM:小规格鳃鄂弓提肌标记组;SDM:小规格背部肌肉标记组。图 5、7和9同。不同字母表示差异显著(P < 0.05)。下同。 SCN: Small-sized control group; SLM: Small-sized levator arcus palatine muscle marking group; SDM: Small-sized dorsal muscle marking group. The same as in Fig. 5, 7 and 9. Different letters indicated significant difference (P < 0.05). The same as below. |
|
图 4 CWT标记30 d大规格苗种肝脏中CAT酶活性 Fig.4 The activity of CAT in the liver of CWT labeled large-sized juveniles at 30 days post tagging LCN:大规格对照组;LLM:大规格鳃鄂弓提肌标记组;LDM:大规格背部肌肉标记组。图 6、8和10同。 LCN: Large-sized control group; LLM: Large-sized levator arcus palatine muscle marking group; LDM: Large-sized dorsal muscle marking group. The same as in Fig. 6, 8 and 10. |
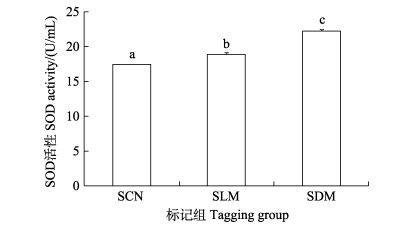
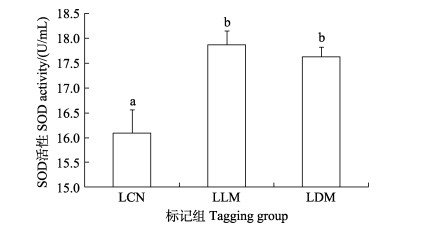
暂养30 d后,SLM与SDM组实验鱼肝脏组织SOD酶活性(22.251 8、18.903 0 U/mL)均显著高于对照组(17.451 8 U/mL) (图 5)。LLM与LDM组实验鱼肝脏组织SOD酶活性(17.865 7、17.625 6 U/mL)均显著高于对照组(16.087 7 U/mL) (P < 0.05) (图 6)。
|
图 5 CWT标记30 d小规格苗种肝脏中SOD酶活性 Fig.5 The activity of SOD in the liver of CWT labeled small-sized juveniles at 30 days post tagging |
|
图 6 CWT标记30 d大规格苗种肝脏中SOD酶活性 Fig.6 The activity of SOD in the liver of large-sized juveniles at 30 days post tagging |
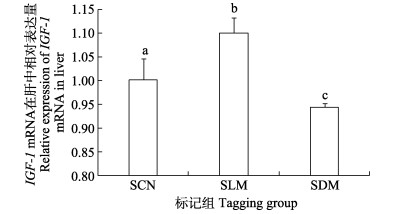
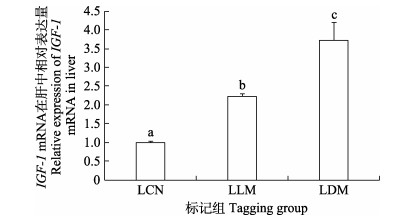
暂养30 d后,SLM组实验鱼肝脏IGF-1 mRNA表达水平显著高于对照组,而SDM组实验鱼肝脏IGF-1 mRNA表达水平显著低于SCN组与SLM组(P < 0.05) (图 7)。LLM组和LDM组实验鱼肝脏IGF-1 mRNA表达水平均显著高于对照组(P < 0.05),且以LDM组实验鱼肝脏IGF-1 mRNA表达水平为最高(图 8)。
|
图 7 CWT标记30 d小规格苗种肝脏中IGF-1 mRNA表达 Fig.7 IGF-1 mRNA expression in liver of CWT labeled small-sized juveniles at 30 days post tagging |
|
图 8 CWT标记30 d大规格苗种肝脏中IGF-1 mRNA表达 Fig.8 IGF-1 mRNA expression in liver of CWT labeled large-sized juveniles at 30 days post tagging |
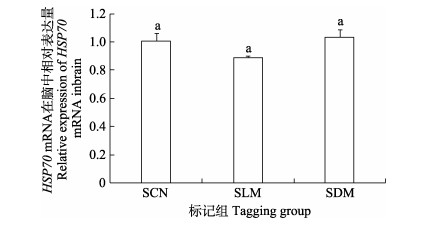
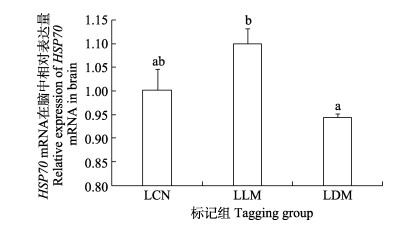
暂养30 d后,小规格实验鱼与对照组脑组织HSP70 mRNA表达水平无显著差异(图 9),而大规格实验鱼在CWT标记暂养30 d后,LLM组实验鱼脑中HSP70 mRNA表达水平显著高于LDM组(P < 0.05) (图 10)。
|
图 9 CWT标记30 d小规格苗种脑中HSP70 mRNA表达 Fig.9 HSP70 mRNA expression in the brain of CWT labeled small-sized juveniles at 30 days post tagging |
|
图 10 CWT标记30 d大规格苗种脑中HSP70 mRNA表达 Fig.10 HSP70 mRNA expression in the brainof CWT labeled large-sized juveniles at 30 days post tagging |
金属编码标签是一种小型且可高效标记的内部标记,作为评价标记鱼类放流效果的一种重要标记技术已在多种鱼类中成功对不同的部位进行标记(Simon et al, 2020),在放流鱼种群密度、种群迁徙、鱼种生长等研究中广泛应用(Crum et al, 2018; Beacham et al, 2019、2020)。
3.1 CWT标记30 d后对生长的影响RASH等(2018)通过实验证实,CWT标签丢失主要是由于操作方法失误、标记部位不当造成的,可见标记部位的选择对成功标记至关重要。利用CWT标记不同规格欧洲鳗背部肌肉,是因为其具有全身弯曲游泳的特性,在快速游泳时容易造成标签的脱落(Hanson et al, 2017; Simon et al, 2020)。张彬等(2007)利用CWT标记稚鳜鱼(Siniperca chuatsi)背部肌肉、腹壁、尾柄等部位后,经过一定时间的暂养发现背部肌肉标记效果最佳。Rash等(2018)对褐鳟(Salmo trutta)的颊部、鼻软骨处进行CWT标记一段时间后发现,其颊部标记效果更佳。大西洋鲑通常在吻部会获得较好的标记效果,但标记回收时必须从鱼头处取出(Goulette et al, 2016)。杨晓鸽等(2013)研究发现,利用CWT标记达乌尔鳇(Huso dauricus)背部肌肉可以获得较高的标签保持率。徐永江等(2017)利用CWT标记不同规格牙鲆背部肌肉和鳃鄂弓提肌,均获得了较好的标记效果。本研究将不同规格的许氏平鲉背部肌肉和鳃鄂弓提肌作为CWT标记部位,背部肌肉标记组标签保持率为100%,获得了较好的标记效果;大规格鳃鄂弓提肌标记组标签保持率为97%,小规格鳃鄂弓提肌标记组标签保持率为87%,就不同标记部位比较而言,背部肌肉更适合作为许氏平鲉标记的理想部位;就不同规格比较而言,大规格标记效果更佳。
标记效果会受到鱼的种类和大小、标记操作方法、标记部位等因素的影响,鱼类的游泳行为也会对标签的保持率产生影响(Guy et al, 1996),死亡率会受到由CWT标记针规格和标记鱼体厚度之间的比值构成的最小阈值影响(Meeuwig et al, 2007)。徐永江等(2017)研究利用CWT标记不同规格牙鲆苗种时发现,6 cm以下规格苗种出现较高的死亡率,6 cm以上规格的苗种标记成功率较高。本研究标记不同规格许氏平鲉苗种的鳃鄂弓提肌和背部肌肉后暂养30 d,苗种成活率为100%,说明标记苗种规格大小适宜。对不同规格标记苗种及其对照组苗种生长数据比较分析发现,标记组苗种特定生长率显著低于对照组,短期内标记的苗种生长受到抑制,但SLM、LLM和LDM组实验鱼肝脏IGF-I mRNA表达水平显著升高,表明CWT标记后暂养30 d的苗种虽然生长受到一定程度的影响,但生长轴的补充调控作用已启动。在今后苗种标记放流时,应适当延长标记苗种暂养驯化时间。这一结果与其他鱼类的研究结果具有一致性,如CWT标记太平洋七鳃鳗短期内其生长受到抑制,且其生理机制受到影响(Johnson et al, 2016)。本研究建立的标记操作方法可以应用到许氏平鲉苗种标记放流中,如适当延长标记后苗种的暂养时间,则会获得更好的标记效果。
3.2 CWT标记30 d后对肝脏CAT和SOD酶活性的影响当鱼类受到环境胁迫时,会产生过量的活性氧导致氧化应激加剧,造成一定程度的生理胁迫,从而使鱼类的生长代谢受到影响(Valavanidis et al, 2006; Amit et al, 2015; 常志成等, 2018)。而SOD和CAT可通过清除过多的O2–以及中间产物H2O2,保护鱼体免受损伤(Sui et al, 2016; 靳晓敏等, 2006),该酶可以为机体起到抗氧化防御的作用。本研究结果表明,CWT标记在体内存在30 d后,仍然会造成一定的生理胁迫,致使鱼体内由于脂质过氧化现象产生的活性氧积聚消散较慢。这与一些鱼类胁迫后体内组织或血清酶活性变化结果相一致。黄建华等(2016)研究CWT标记对大黄鱼血清酶活性的影响时发现,标记操作后CAT和SOD酶活性表现为快速升高,以免受因操作胁迫而产生的活性氧自由基的侵害。环境胁迫时也会引起体内氧自由基的升高,如低温胁迫后的银鲳(Pampus argenteus)幼鱼通过血清及肝脏组织中SOD和CAT酶活性的升高来调节应激产生的氧自由基(谢明媚等, 2015)。而军曹鱼(Rachycentron canadum)受到低氧胁迫后肝脏组织中抗氧化酶活性降低,表现出低氧造成的损伤无法恢复(郭志雄等, 2020)。鱼体受到胁迫后体内生理变化复杂,多种因素相互作用,通过体内SOD和CAT活性的升高来消除产生的应激和过量的活性氧。说明标记苗种在暂养30 d后存在一定的生理应激,为获得较好的标记放流后回捕效果,应该适当增加标记鱼暂养时间。或者依赖于野生环境中的慢慢修复,但这种生理胁迫对于放流后苗种存活与生长的影响值得关注。
3.3 CWT标记30 d后HSP70的表达变化研究表明,当动物机体受到应激刺激时HSP70表达量迅速增加(Liu et al, 2012),提升细胞的抗损伤能力,使其快速适应应激环境,是机体自我保护的物质基础。金头鲷和虹鳟(Oncorhynchus mykiss)在急性饥饿胁迫条件下,短时间内HSP70基因的表达表现为上调趋势(CARA et al, 2005)。罗伟等(2016)通过对奥尼罗非鱼(Oreochromis niloticus ♀ × Oreochromis aureus ♂)幼鱼低温、饥饿及恢复的长时间监测发现,35 d后幼鱼肝脏组织中HSP70的表达会恢复至初始水平。本研究苗种标记30 d后,肝脏组织中的HSP70基因的表达与对照组无差异(P > 0.05),暂养30 d后标记苗种可稳定生长,对环境的适应性与对照组无差异,表明在基因表达层面,CWT标记后苗种可快速适应环境。研究发现,CWT标记太平洋七鳃鳗、欧洲鳗,短时间内鱼的生长及生理机制会受到一定影响,但经过长时间标记重捕实验监测发现,其获得了较好的标记效果(Hanson et al, 2017; Simon et al, 2020),表明针对不同鱼类,CWT标记后经不同的暂养时间可获得不同的标记效果。
综上所述,大小规格不同标记部位的许氏平鲉标记苗种标记30 d后标签保持率高,无死亡发生,生长状态良好,是许氏平鲉苗种标记放流的合适标记方式;标记后苗种肝脏组织抗氧化酶活性有所升高,生长基因表达与对照组差异显著(P < 0.05),生理受到一定的胁迫,生长受到一定程度的抑制。在标记研究时,应适当延长标记苗种的标记周期和室内暂养时间,并在标记操作完成后将标记鱼苗进行体表消毒,以期为放流苗种提供准确的生理适应机制的评价,为苗种放流的大规模应用提供参考。
AMIT KUMAR S, HAMADA A, GAURAV Z, et al. Nutritional status as the key modulator of antioxidant responses induced by high environmental ammonia and salinity stress in European sea bass (Dicentrarchus labrax). PLoS One, 2015, 10(8): e0135091 DOI:10.1371/journal.pone.0135091 |
BEACHAM T D, JONSEN K, MCINTOSH B, et al. Large-scale parentage-based tagging and genetic stock identification applied in assessing mixed-stock fisheries and hatchery brood stocks for coho salmon in British Columbia, Canada. Canadian Journal of Fisheries and Aquatic Sciences, 2020, 77(9): 1505-1517 DOI:10.1139/cjfas-2020-0035 |
BEACHAM T D, WALLACE C, JONSEN K, et al. Variation in migration pattern, broodstock origin, and family productivity of coho salmon hatchery populations in British Columbia, Canada, derived from parentage-based tagging. Ecology and Evolution, 2019, 9(17): 9891-9906 DOI:10.1002/ece3.5530 |
CARA J B, ALURU N, MOYANO F J, et al. Food-deprivation induces HSP70 and HSP90 protein expression in larval gilthead sea bream and rainbow trout. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2005, 142(4): 426-431 DOI:10.1016/j.cbpb.2005.09.005 |
CHANG C Z, WEN H S, ZHANG M Z, et al. Effects of dissolved oxygen levels on oxidative stress response and energy utilization of juvenile Chinese seabass (Lateolabrax japonicus) and associate physiological mechanisms. Periodical of Ocean University of China (Natural Science), 2018, 48(7): 20-28 [常志成, 温海深, 张美昭, 等. 溶解氧水平对花鲈幼鱼氧化应激与能量利用的影响及生理机制. 中国海洋大学学报(自然科学版), 2018, 48(7): 20-28 DOI:10.16441/j.cnki.hdxb.20170296] |
CROZIER W W, KENNEDY G J A. Impact of tagging with coded wire tags on marine survival of wild Atlantic salmon (Salmo salar L.) migrating from the R. Bush, Northern Ireland. Fisheries Research, 2002, 59(1/2): 209-215 |
CRUM K P, BALOUSKUS R G, TARGETT T E. Growth and movements of mummichogs (Fundulus heteroclitus) along armored and vegetated estuarine shorelines. Estuaries and Coasts, 2018, 41(1): 131-143 |
FENG J R, LIU L M, JIANG H B, et al. Histological observation of germ cell development and discovery of spermatophores in ovoviviparous black rockfish (Sebastes schlegeli, Hilgendorf) in reproductive season. Journal of Ocean University of China, 2014, 13(5): 830-836 DOI:10.1007/s11802-014-2265-6 |
FENG Z X. Ichthyology. Beijing: China Agriculture Press, 2003 [冯昭信. 鱼类学. 北京: 中国农业出版社, 2003]
|
GOULETTE G S, LIPSKY C A. Nonlethal batch identification of Atlantic salmon using coded wire tags. North American Journal of Fisheries Management, 2016, 36(5): 1084-1089 DOI:10.1080/02755947.2016.1198283 |
GUO Z X, ZENG Z Q, HUANG J S, et al. Effects of acute hypoxia on oxidative stress, energy utilization and carbohydrate metabolism in liver of large-sized juvenile cobia (Rachycentron canadum). Journal of Guangdong Ocean University, 2020, 40(3): 134-140 [郭志雄, 曾泽乾, 黄建盛, 等. 急性低氧胁迫对大规格军曹鱼幼鱼肝脏氧化应激、能量利用及糖代谢的影响. 广东海洋大学学报, 2020, 40(3): 134-140] |
GUY C S, SCHULTZ R D, CLOUSE C P. Coded wire tag loss from paddlefish: A function of study location. North American Journal of Fisheries Management, 1996, 16(4): 931-934 DOI:10.1577/1548-8675(1996)016<0931:CWTLFP>2.3.CO;2 |
HANSON K C, BARRON J M. Evaluation of the effects of marking Pacific lamprey ammocoetes with visual implant elastomer, coded wire tags, and passive integrated transponders. Transactions of the American Fisheries Society, 2017, 146(4): 626-633 DOI:10.1080/00028487.2017.1290681 |
HUANG J H, SHEN B, HUANG X T, et al. The effects of coded wire tagging on activities of five serum enzymes of large yellow croaker. Journal of Zhejiang Ocean University (Natural Science), 2016, 35(6): 483-488 [黄建华, 沈斌, 黄晓婷, 等. 金属线码标记操作对大黄鱼5种血清酶活力的影响. 浙江海洋学院学报(自然科学版), 2016, 35(6): 483-488] |
JIN X M, WU Y, YANG S, et al. Effects of two kinds of prethrin pesticide on CAT and SOD in serum of common carp. Journal of Agro-Environment Science, 2006, 25(3): 615-618 [靳晓敏, 吴垠, 杨松, 等. 两种菊酯类农药对鲤血清CAT和SOD的影响. 农业环境科学学报, 2006, 25(3): 615-618] |
JOHNSON N S, BRENDEN T O, SWINK W D, et al. Survival and metamorphosis of larval sea lamprey (Petromyzon marinus) residing in Lakes Michigan and Huron near river mouths. Journal of Great Lakes Research, 2016, 42(6): 1461-1469 DOI:10.1016/j.jglr.2016.09.003 |
LIU T, DANIELS C K, CAO S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacology and Therapeutics, 2012, 136(3): 354-374 DOI:10.1016/j.pharmthera.2012.08.014 |
LÜ H J, ZHANG X M, XI D, et al. Use of calcein and alizarin red S for immersion marking of black rockfish Sebastes schlegelii juveniles. Chinese Journal of Oceanology and Limnology, 2014, 32(1): 88-98 DOI:10.1007/s00343-014-3022-9 |
LUO W, GAO Y, LI W H. Effects of low temperature and starvation stress, recovery on expression of HSP70 gene in hybrid tilapia juveniles. Genomics and Applied Biology, 2016, 35(10): 2640-2646 [罗伟, 高扬, 李文红. 低温、饥饿胁迫及恢复对奥尼罗非鱼幼鱼HSP70基因表达的影响. 基因组学与应用生物学, 2016, 35(10): 2640-2646] |
MEEUWIG M H, PULS A L, BAYER J M. Survival and tag retention of Pacific lamprey larvae and macrophthalmia marked with coded wire tags. North American Journal of Fisheries Management, 2007, 27(1): 96-102 DOI:10.1577/M06-074.1 |
RASH J M, GOODFRED D W, JONES JR E M. Evaluation of coded wire tag retention in brown trout (Salmo trutta) fingerlings tagged at three anatomical locations. Journal of Freshwater Ecology, 2018, 33(1): 503-509 |
SÁNCHEZ-LAMADRID A. Effectiveness of four methods for tagging juveniles of farm-reared gilthead sea bream, Sparus aurata, L. Fisheries Management and Ecology, 2001, 8(3): 271-278 |
SIMON J, WICKSTRÖM H. Long-term retention of alizarin red S marks and coded wire tags in European eels. Fisheries Research, 2020, 224: 105453 |
SUI Y M, HUANG X Z, KONG H, et al. Physiological responses to salinity increase in blood parrotfish (Cichlasoma synspilum ♀ × Cichlasoma citrinellum ♂). SpringerPlus, 2016, 5(1): 1246 |
VALAVANIDIS A, VLAHOGIANNI T, DASSENAKIS M, et al. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Environmental Safety, 2006, 64(2): 178-189 |
WAN R J, CHEN R S. Reproductive behavior and morphology of early development of Sebastodes fuscescens (Houttuyu) in the Bohai Sea. Marine Fisheries Research, 1988(9): 213-220 [万瑞景, 陈瑞盛. 黑鲪的生殖习性及早期形态. 海洋水产研究, 1988(9): 213-220] |
XIE M M, PENG S M, ZHANG C J, et al. Effects of acute temperature stress on antioxidant enzyme activities and immune indexes of juvenile Pampus argenteus. Marine Fisheries, 2015, 37(6): 541-549 [谢明媚, 彭士明, 张晨捷, 等. 急性温度胁迫对银鲳幼鱼抗氧化和免疫指标的影响. 海洋渔业, 2015, 37(6): 541-549] |
XU Y J, LIU X Z, ZHANG K, et al. Tagging juvenile Japanese flounder (Paralichthys olivaceus) with coded wire tags. Progress in Fishery Sciences, 2017, 38(1): 168-174 [徐永江, 柳学周, 张凯, 等. 编码金属标签对牙鲆(Paralichthys olivaceus)苗种标记的效果. 渔业科学进展, 2017, 38(1): 168-174] |
YANG X G, WEI Q W, DU H, et al. Primary study on tag performance tagged with visible implant of Kaluga sturgeon (Huso dauricus) elastomer and coded wire tag. Freshwater Fisheries, 2013, 43(2): 43-47 [杨晓鸽, 危起伟, 杜浩, 等. 可见植入荧光标记和编码金属标对达乌尔鳇标志效果的初步研究. 淡水渔业, 2013, 43(2): 43-47] |
ZENG H H, XU B D, XUE Y, et al. Study on fish species composition and seasonal variation in the shallow waters of Jiaozhou Bay. Periodical of Ocean University of China (Natural Science), 2012, 42(1/2): 67-74 [曾慧慧, 徐宾铎, 薛莹, 等. 胶州湾浅水区鱼类种类组成及其季节变化. 中国海洋大学学报, 2012, 42(1/2): 67-74] |
ZHANG B, LI Z J. Tagging juvenile mandarin fish Siniperca chuatsi (Basilewsky) with coded wire tag. Journal of Fishery Sciences of China, 2007, 14(7): 53-58 [张彬, 李钟杰. 微型金属标标记鳜稚鱼. 中国水产科学, 2007, 14(7): 53-58] |
ZHANG Y, XU Q, ALÓS J, et al. Short-term fidelity, habitat use and vertical movement behavior of the black rockfish Sebastes schlegelii as determined by acoustic telemetry. PLoS One, 2015, 10(8): e0134381 |
ZHU T B, GUO W, WU X B, et al. Effects of visible implant elastomer and coded wire tags on growth and survival of Schizopygopsis younghusbandi Regan, 1905. Journal of Applied Ichthyology, 2016, 32(1): 110-112 |
ZHU Y H, JIANG T, CHEN X B, et al. The marking characteristics of otoliths of larval grass carp (Ctenopharyngodon idellus) by immersion in alizarin complexone. Progress in Fishery Sciences, 2020, 41(6): 28-36 [朱亚华, 姜涛, 陈修报, 等. 草鱼仔鱼耳石茜素络合物标记特征研究. 渔业科学进展, 2020, 41(6): 28-36] |



