2. 海南省海洋与渔业科学院 海南省热带海水养殖技术重点实验室 海南 海口 571126;
3. 中国水产科学研究院黄海水产研究所 山东 青岛 266071;
4. 青岛海洋科学与技术试点国家实验室海洋渔业科学与食物产出过程功能实验室 山东 青岛 266071
2. Hainan Provincial Key Laboratory of Tropical Maricultural Technologies, Hainan Academy of Ocean and Fisheries Sciences, Haikou, Hainan 571126, China;
3. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao, Shandong 266071, China;
4. Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, Shandong 266071, China
褐藻(Phaeophyta)具有重要的经济价值,在工农业生产中得到广泛应用(Khan et al, 2009; Rassweiler et al, 2018)。大多数褐藻生活在潮间带和潮下带,受盐度、温度和光照胁迫的波动水平影响(Hurd et al, 2014)。巨藻(Macrocystis pyrifera)是褐藻中较为常见的一种,因其体积大和生长速度快的特点,长期以来被认为是最有希望用于生物量产的大型藻类物种之一(Navarrete et al, 2021)。巨藻在潮下带形成了密集的海藻床和海藻林,产生了大量的生物量,决定了生态系统的结构(Bolton, 2021)。巨藻分布广泛,暴露在各种环境条件下,能够适应各种非生物和生物胁迫(Shukla et al, 2017; Wiencke et al, 2012)。
随着现代工农业的快速发展和矿产资源的开发利用,生态环境,特别是水生环境中的重金属污染日益严重。虽然海水中适量的重金属元素不会对海洋生物产生有害影响, 有些重金属甚至可以作为藻类正常生长和代谢所必需的微量营养物质,但当其含量过高时,就会对藻类造成伤害,产生污染(Maxwell et al, 2000; Jiang et al, 2003; Plekhanov et al, 2003)。目前,重金属对藻类的毒性已成为污染生态学的研究热点之一(Collén et al, 2003)。研究表明,高浓度的金属通过抑制生长、触发氧化损伤、影响基因表达以及破坏光合细胞和线粒体,从而对硅藻(Diatom)产生有害影响(Stauber et al, 1989; Cid et al, 1995; Rijstenbil et al, 2002; Herzi et al, 2013)。在各种金属中,镉(Cd)的毒性特别强,很容易在许多海洋生物中积累,为应优先关注的污染物之一(Blackmore, 1998; Olivier et al, 2002; Chakraborty et al, 2015; 徐莞媛等, 2020)。通常情况下,沉积物和开放海水中的Cd浓度很低(Blackmore, 1998),但由于泄漏或人为排放,在一些近海和河口地区Cd浓度会变高(Simpson, 1981; Villanueva et al, 1992)。Cd可以通过与蛋白质中氨基酸的羟基进行非特异性结合,取代金属酶中的必需金属并造成细胞氧化损伤来发挥生物毒性(Mostofa et al, 2015)。虽然Cd在大型藻类中没有明确的代谢功能,但有研究证明,Cd是威氏海链藻(Thalassiosira weissflflogii)碳酸酐酶的辅助因子(Lane et al, 2000)。
谷胱甘肽S转移酶(glutathione S-transferase, GSTs, EC2.5.1.18)普遍存在于所有生物体中,是由一个大的基因家族编码的多功能蛋白(Han et al, 2018)。GSTs主要通过催化还原型谷胱甘肽(glutathione, GSH)与广泛的疏水和亲电底物结合来解毒外来物质(Frova, 2003)。除了其解毒功能外,GSTs还在其他生理和发育过程中发挥重要作用,包括次级代谢、信号转导、抗紫外线辐射、抗氧化损伤及对重金属的解毒作用(Dixon et al, 2010; Edwards et al, 2011; Sharma et al, 2004)。目前,关于GSTs对重金属解毒作用的研究较多,研究表明GST酶活的增加与重金属耐受性的提高呈正相关,说明GSTs在提高植物重金属耐受性方面发挥了重要作用(Canado et al, 2005; Darko et al, 2004; Dawood et al, 2012)。例如,有报道称,南极冰藻(Chlamydomonas sp. ICE-L)在Cd胁迫下,GST酶活增加(Contreras-Porcia et al, 2011)。
由于目前大型藻类缺乏稳定的遗传转化操作系统,利用大型藻类验证功能尚不明确的基因和蛋白存在较大的困难。本研究克隆巨藻的GST基因,将其构建到细长聚球藻(Synechococcus elongatus PCC7942)的表达载体中并在细长聚球藻中表达,旨在研究巨藻GST在Cd2+耐受中所发挥的作用。
1 材料与方法 1.1 实验材料培养巨藻配子体由中国水产科学研究院黄海水产研究所提供。使用前,所有样品用过滤海水进行冲洗,去除可见的附生异物。然后将样品在10℃的无菌海水中培养24 h。
细长聚球藻PCC7942来自GeneArtTM聚球藻蛋白表达试剂盒(ThermoFisher Scientific, 美国),并在Blue-Green (BG11)培养基中培养7 d,培养温度为(25±2)℃,光照强度为100 µmol photons/(m2·s)。
1.2 RNA的提取和cDNA的合成取100 mg巨藻配子体样品,将水分吸干,用液氮冷冻处理后进行研磨。采用E.Z.N.A.植物RNA提取试剂盒(Omega, 美国)进行RNA的提取。使用2%琼脂糖凝胶电泳和NanoDrop 2000 (ThermoFisher Scientific, 美国)分别检测RNA的完整性和浓度。
使用Evo M-MLV Plus第一链cDNA合成试剂盒(艾科瑞, 中国)反转录RNA,合成cDNA。
1.3 巨藻GST基因cDNA的克隆根据巨藻转录组序列(国家基因库登录号CNP0001061)选取6个GST基因的cDNA序列,设计两端含有酶切位点的基因特异性引物(睿博, 中国) (表 1)用于构建表达载体。以cDNA为模板,添加2×Pro Taq Master Mix(含染料)(艾科瑞, 中国)扩增GST基因。PCR反应条件:94℃预变性30 s;98℃变性10 s,62℃退火30 s,72℃延伸1 min,扩增30个循环,最后72℃延伸2 min。对PCR产物进行测序。
|
|
表 1 本研究使用的引物序列 Tab.1 Primer sequences used in this study |
为了研究巨藻GST基因的功能,根据GeneArtTM聚球藻蛋白表达试剂盒(ThermoFisher Scientific, 美国)的操作说明,构建分别含有6个巨藻GST基因(mpgst1~mpgst6)的重组表达载体,转化至细长聚球藻细胞,并将含mpgst1~mpgst6的转化株分别命名为MG1~MG6。
使用E.Z.N.A. HP植物DNA提取试剂盒(Omega, 美国)提取转化株的基因组DNA。以基因组DNA为模板,添加特异性引物(表 1),使用2×Pro Taq Master Mix (含染料)(艾科瑞, 中国)扩增靶基因。然后对PCR产物进行测序验证。
1.5 转化株酶活的测定取培养至对数期的细长聚球藻藻液,用GST检测试剂盒(科铭, 中国)分析转化株的GST酶活性,以不含酶提取物的混合液作为对照。
1.6 Cd2+胁迫下细长聚球藻生理指标的变化将野生株和转化株用BG11培养基培养至对数期,稀释藻液,使得每个样品750 nm处吸光度值(OD750 nm)为0.01。然后加入CdCl2溶液,使得每个样品中Cd2+的浓度为0.2 mg/L,将等量的处理后的藻液加入到多孔板中(包括未添加Cd2+的对照样品),所有处理重复3次。将多孔板置于恒温培养箱中观察细长聚球藻的生长情况,筛选出有抗性的转化株,培养温度为(25±2)℃,光照强度为100 µmol photons/(m2·s)。
将藻液转移至三角瓶中并重复之前的胁迫处理,然后在恒温培养箱中培养,检测Cd2+胁迫下细长聚球藻的生长、光合色素含量和叶绿素荧光参数。用分光光度计每隔2 d检测细长聚球藻的OD750 nm值,持续12 d,测定其生长情况。按照肖丽等(2008)和Wang等(2020)提到的方法,在培养第12天时从细长聚球藻中提取色素,并按照Lichtenthaler等(2001)提出的方法计算叶绿素a (Chlorophyll a, Chl-a)和类胡萝卜素(Carotenoid, Car)的含量。使用Maxi-Imaging- PAM (Walz, 德国)测量了光系统Ⅱ (photosystem Ⅱ complex, PSⅡ)的最大量子产率(Fv/Fm),采用Zhang等(2021)的方法进行叶绿素荧光参数测定前藻液的处理。最大量子产率(Fv/Fm)的计算遵循Genty等(1989)中的方法。所有实验均重复进行3次。
1.7 数据统计分析采用SPSS软件中单因素方差分析(one-way ANOVA)和双因素方差分析(two-way ANOVA)来分析实验处理组和对照组之间的差异显著性。使用R(3.5.3)和Adobe Illustrator CS6软件来绘制和修改图形。P < 0.05表示存在显著差异(Zar, 1996)。
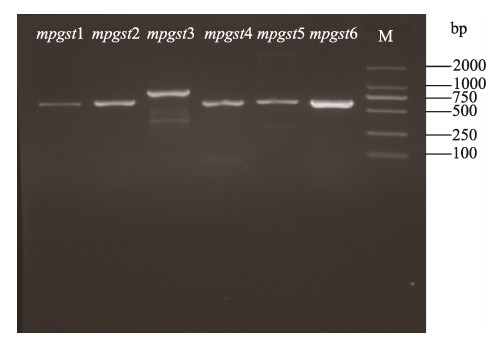
2 结果与分析 2.1 巨藻GST基因克隆采用RT-PCR方法从巨藻中克隆出6个GST基因的cDNA,琼脂糖凝胶电泳的检测结果如图 1所示。结果显示,mpgst3基因大小约800 bp,其余5个基因片段的大小约为700 bp,与目的基因的大小大致相同。这6个mpgst的cDNA序列已储存到GenBank中,登录号为OL362284~OL362289。
|
图 1 巨藻GST基因的PCR扩增 Fig.1 PCR amplification of the GST genes of M. pyrifera M: DL2000 DNA Marker |
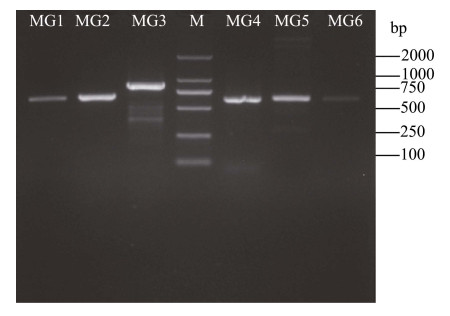
本研究选择细长聚球藻PCC7942来验证巨藻GST基因功能。对细长聚球藻转化株进行基因组DNA的提取,以基因组DNA为模板进行PCR验证。琼脂糖凝胶电泳结果显示(图 2),所有转化株都检测到阳性PCR产物,并且经过测序分析,其序列没有错误,表明mpgst已成功整合到细长聚球藻基因组中。
|
图 2 mpgst的转基因验证 Fig.2 Transgenic verification of mpgst MG1~MG6表示分别以转化株MG1~MG6基因组DNA为模板扩增得到mpgst,M表示DL2000 DNA标记。 MG1~MG6 represent the mpgst amplified by using genomic DNA of transformed strains MG1~MG6 as the template, respectively. M represents the DL2000 DNA Marker. |
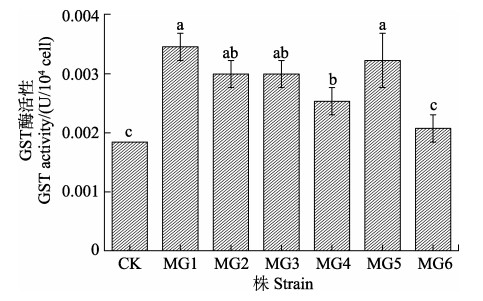
为了进一步证实mpgst的异源表达,测定了生长至对数期的野生株和转化株的GST酶活性,结果如图 3所示。除MG6外,其他转化株的酶活性与野生株均有显著差异(P < 0.05),而MG6的酶活性也略有上升(P > 0.05)。转化株酶活性的增加进一步证实了巨藻GST基因在细长聚球藻中成功表达,为后续检测生理指标提供了依据。
|
图 3 转基因细长聚球藻的GST酶活性 Fig.3 GST activity of genetically modified S. elongatus PCC7942 CK表示野生株,MG1~MG6表示含有mpgst的转化株。误差条表示标准差(SD),n=3,具有不同字母上标的数据间差异显著(P < 0.05),下同。 CK represents the wild strain, MG1~MG6 represent the transformed strains containing mpgst. The error bars indicate standard deviations (SD), n=3. Bar of each column with different small letters mean significant difference (P < 0.05). The same as below. |
经过几天的观察,MG1、MG4、MG6在Cd2+胁迫下正常生长,而野生株和MG2、MG3、MG5不能生长(图 4)。这证明mpgst1、mpgst4、mpgst6能够改善Cd2+对细长聚球藻的不良影响,然而其他3个基因未表现出相应的抗性。为了进一步验证巨藻GST基因对Cd2+的耐受性,选择MG1、MG4、MG6及野生株的生长、光合色素、光合参数等生理指标进行进一步分析。

|
图 4 Cd2+胁迫下细长聚球藻的多孔板培养实验 Fig.4 Multi-well plate culture experiment of S. elongatus PCC7942 under Cd2+ stress a:正常生长的细长聚球藻;b:加入0.2 mg/L Cd2+的细长聚球藻 a: Normally growing S. elongatus PCC7942; b: S. elongatus PCC7942 with 0.2 mg/L Cd2+ added |
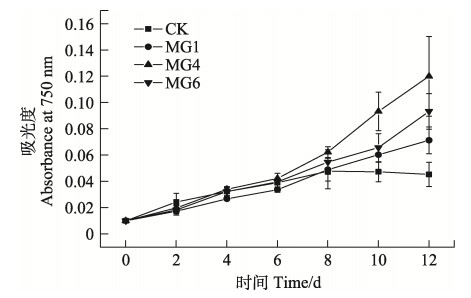
本研究中,除对照组外,所有转化株的吸光度值一直随时间增长,且6 d后转化株吸光度值的增长速度普遍提高,而CK的吸光度值在缓慢增长8 d后开始出现下降(图 5)。结果显示,Cd2+对野生株具有很强的毒性,而对MG1、MG4和MG6转化株的毒性不受暴露时间的影响。MG4生长最好(P < 0.05),这与其表现出的较高的GST酶活性相吻合(图 3),虽然MG1的酶活最高,但MG1的其他生理指标却不是最好,这可能与每一种GST酶的作用机制不同有关。
|
图 5 野生型和转基因细长聚球藻PCC7942在Cd2+胁迫下的生长曲线 Fig.5 The growth curves of wild-type and transformed S. elongatus PCC7942 under Cd2+ stress |
叶绿素a (Chl-a)是主要的光合色素,Chl-a含量的降低会直接导致光合效率的降低。图 6为正常培养和Cd2+胁迫处理下第12天野生株和转化株Chl-a含量的变化。从图 6可以看出,Cd2+处理后,对照组中Chl-a含量下降最为明显,藻体几乎不含Chl-a (P < 0.05),但所有转化株在处理前后的Chl-a含量差异不显著(P > 0.05),这与之前生物量(图 5)及GST酶活性(图 4)的测量所表现出的结果相吻合。转化株MG6的Chl-a含量最高,但都相对于野生株而言显著降低,这可能是因为转化株获得了外源基因,影响了细胞内的代谢,具体原因需要进一步实验进行验证。
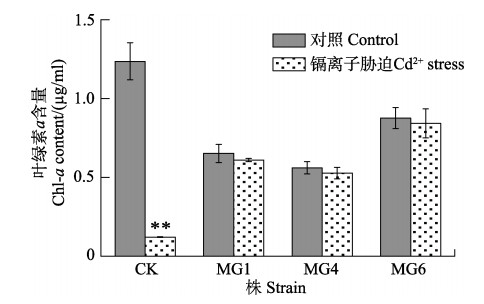
|
图 6 Cd2+胁迫下野生型和转基因细长聚球藻叶绿素a含量 Fig.6 The chlorophyll a content of wild-type and transformed S. elongatus PCC7942 under Cd2+ stress 有星号上标的2组数据间差异显著(P < 0.05),下同。 There was a significant difference between the two groups with asterisks (P < 0.05). The same as below. |
类胡萝卜素(Car)是最重要的捕光色素之一。图 7为正常培养和Cd2+胁迫处理下第12天野生株和转化株Car含量的变化。从图 7可以看出,Cd2+处理后,大部分细长聚球藻的Car含量下降,以对照组表现最明显,藻体几乎不含Car (P < 0.05),这与之前测定的生物量(图 7)和Chl-a含量(图 8)的结果一致。
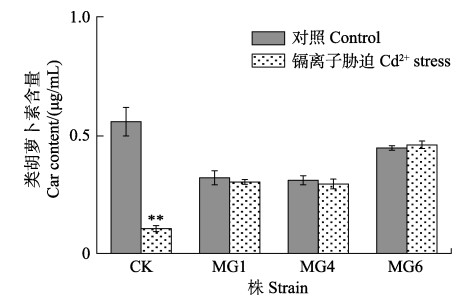
|
图 7 Cd2+胁迫下野生型和转基因细长聚球藻PCC7942的类胡萝卜素含量 Fig.7 The carotenoid content of the wild-type and transformed S. elongatus PCC7942 under Cd2+ stress |
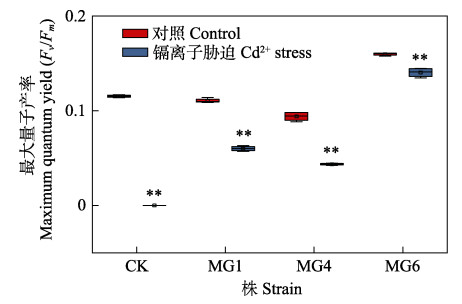
|
图 8 Cd2+胁迫下野生型和转基因细长聚球藻的最大量子产率(Fv/Fm) Fig.8 The maximum quantum yield (Fv/Fm) of wild-type and transformed S. elongatus PCC7942 under Cd2+ stress |
叶绿素荧光参数(Fv/Fm)与光合作用密切相关,是判断胁迫对植物光合作用影响的真实指标(冯力霞, 2006)。图 8为野生株和转化株在正常培养和Cd2+胁迫处理下第12天Fv/Fm的变化。如图 8所示,Cd2+处理后,细长聚球藻的Fv/Fm均明显下降(P < 0.05),对照组最为明显,Fv/Fm为0,藻类已经不显荧光,几乎死亡。这与之前对生物量(图 5)及色素含量(图 6和图 7)的测量结果一致。此外,MG6的Fv/Fm与Chl-a含量变化趋势相同,数值较高且Cd2+胁迫前后差异不大,生长效果较好。光合参数与光合色素含量的结果高度一致。
3 讨论对于海洋藻类来说,非生物胁迫可能是来自光过量或不足、紫外线辐射、温度不适宜、营养限制、厌氧条件、盐度不适宜、干燥或者无机碳的限制(Davison et al, 1996)。此外,还来源于接触汞、镉、铅和铜等重金属物质(Collén et al, 2003)。在高等植物中,Cd2+通过和硫醇基团之间的反应,干扰生长、光合作用、离子和水的运输,以及降低酶活性(Prasad, 1995)。虽然Cd2+可能不是大型藻类生长所必需的,但它很容易被吸收,研究表明,江蓠(Gracilaria tenuistipitata)在光照条件下增强了对Cd2+的吸收(Hu et al, 1996)。相比其他金属离子,Cd2+不会直接影响植物的光合作用,而是干扰植物的其他代谢过程,具有更广泛的毒性作用,引起氧化应激。谷胱甘肽转移酶(GSTs)是一类具有多种功能的超家族蛋白,植物GSTs根据其免疫交叉反应性、蛋白序列相似性、基因结构、底物特异性和特异性残基的保守性可分为14类。由于其功能和种类的多样性,在多种外源性和内源性化合物的细胞解毒过程中发挥着重要作用。GST既具有过氧化物酶的活性,同时,可以直接清除分子结构里带有亲电基团的化合物(孙小雨等, 2021)。因此,研究GST有助于阐明植物生长和抵御胁迫的分子机制,对提高胁迫条件下植物的生存能力具有实际意义(梁志乐等, 2019)。
由于细长聚球藻PCC7942易于培养,基因组较小,易于通过自然转化或接合转移进行基因操作(Atsumi et al, 2009; Ducat et al, 2011; Min et al, 2000)。本研究中,选择细长聚球藻来验证巨藻GST基因的功能。金属离子对藻类细胞生长的影响因其浓度的不同而有所差异。用不同浓度的Cd2+处理小球藻(Chlorella vulgaris)时,0.5 mg/L浓度的Cd2+使藻的生长提高了7%,而更高的Cd2+浓度则降低了藻的生长(El-Nagga et al, 2014)。本研究中,预实验确定0.2 mg/L的实验浓度作为能使野生株死亡但使部分转化株正常生长的Cd2+浓度,之后筛选出具有抗性的转化株MG1、MG4、MG6进行后续实验。预实验中部分转化株不具有抗性,可能是因为转入的这些巨藻GST基因属于不同的GST基因家族从而具有不同的生物学功能。金属胁迫下藻类GST酶活性的增加通常伴随着生长的差异。用Cd2+胁迫处理南极冰藻(Chlamydomonas sp. ICE-L),其GST酶活性增加,生长曲线发生相应的变化(Ding et al, 2005)。本研究分别测定了细长聚球藻的GST酶活性和OD750 nm值的变化,以检测转化株对Cd2+的耐受性。结果表明,转化株的GST酶活性均高于野生株,这也解释了为什么Cd2+处理后野生株的生长在第8天开始下降,而转化株的生长则呈稳定上升趋势。然而,野生株在Cd2+处理初期生长缓慢上升,可能与Cd2+作为辅助因子的作用有关,具体的内在调控机制还需要进一步研究。
叶绿素a (Chl-a)和类胡萝卜素(Car)是大型海藻光合作用的主要色素,Chl-a含量的变化是衡量藻细胞生理状况的重要指标,Car作为一种重要捕光色素的同时也发挥着抗氧化剂的作用。较低的重金属浓度可促进色素含量的增加,而较高的重金属浓度会抑制叶绿素的合成,导致叶绿素含量降低、光合效率降低(Brown et al, 2003)。朱喜锋(2010)研究表明,Cd2+通过损害光合作用器官和结合生物大分子的活性位点(如Cd2+取代叶绿素分子中心的Mg2+从而破坏叶绿素结构)来影响光合作用。对江蓠进行了高浓度的Cd2+处理,发现胁迫处理下江蓠的Chl-a含量明显低于对照组,但Car含量略高于对照组(Collén et al, 2003)。本研究中,测量了Cd2+处理下转化株和野生株的Chl-a及Car含量,并分别设置了转化株的对照组(未添加Cd2+),发现野生株的色素含量在处理前后差异显著,其处理组几乎不含色素,接近死亡,而转化株生长正常,这与生长曲线所表现出的结果一致。
PSⅡ最大光能转化效率(Fv/Fm)又称PSⅡ的光化学最大量子产量,是暗适应下PSⅡ的最大光化学效率,反映了光合自养生物潜在的最大光合作用能力,常作为植物光合性能是否受损的敏感性指标(Kuma et al, 2014)。1 mmol/L的Cd2+可在短期(0.5~2 h)内迅速降低蛋白核小球藻S-39 (Chlorella pyrenoidosa Chick S-39)的光合放氧速率和Fv/Fm值,使PSⅡ光合电子传递过程受到抑制(Plekhanov et al, 2003)。本研究在Cd2+处理下测量转化株和野生株的Fv/Fm值,结果与Chl-a的测定结果基本一致。在Cd2+胁迫下,野生株不显叶绿素荧光,而转化株正常生长,进一步验证了巨藻GST在提高Cd2+的耐受性中的作用。
4 结论本研究从巨藻中获得6个谷胱甘肽S转移酶(GST)基因,并且将其构建到细长聚球藻表达载体,成功将巨藻的gst基因转化到细长聚球藻中,在细长聚球藻PCC7942中验证了它们的功能。结果表明,虽然在6个转化株中GST酶活性都增加,但有3个mpgst基因显著提高了细长聚球藻对Cd2+胁迫的耐受性,说明巨藻中不同的GST具有不同的功能。本研究初步探索了巨藻中GSTs的生理功能,为今后镉污染条件下藻类耐受品系的培养奠定了理论基础。
ATSUMI S, HIGASHIDE W, LIAO J C. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nature Biotechnology, 2009, 27(12): 1177-1180 DOI:10.1038/nbt.1586 |
BLACKMORE G. An overview of trace metal pollution in the coastal waters of Hong Kong. Science of the Total Environment, 1998, 214(1/2/3): 21-48 |
BOLTON J J. The biogeography of kelps (Laminariales, Phaeophyceae): A global analysis with new insights from recent advances in molecular phylogenetics. Helgoland Marine Research, 2021, 64(4): 263-279 |
BROWN M T, NEWMAN J E. Physiological responses of Gracilariopsis longissima (S. G. Gmelin) Steentoft L. M. Iryine and Farnham (Rhodophyceae) to sub-lethal copper concentrations. Aquatic Toxicology, 2003, 64(2): 201-213 DOI:10.1016/S0166-445X(03)00054-7 |
CANADO G M A, DE ROSA V E, FERNANDEZ J H, et al. Glutathione S-transferase and aluminum toxicity in maize. Functional Plant Biology, 2005, 32(11): 1045-1055 DOI:10.1071/FP05158 |
CHAKRABORTY P, RAMTEKE D, CHAKRABORTY S, et al. Relationship between the lability of sediment-bound Cd and its bioaccumulation in edible oyster. Marine Pollution Bulletin, 2015, 100(1): 344-351 DOI:10.1016/j.marpolbul.2015.08.027 |
CID A, HERRERO C, TORRES E, et al. Copper toxicity on the marine microalga Phaeodactylum tricornutum: Effects on photosynthesis and related parameters. Aquatic Toxicology, 1995, 31(2): 165-174 DOI:10.1016/0166-445X(94)00071-W |
COLLÉN J, PIOTO E, PEDERSÉN M, et al. Induction of oxidative stress in the red macroalga Gracilaria tenuistipitata by pollutant metals. Archives of Environmental Contamination and Toxicology, 2003, 45(3): 337-342 |
CONTRERAS-PORCIA L, DENNETT G, GONZÁLEZ A, et al. Identification of copper-induced genes in the marine alga Ulva compressa (Chlorophyta). Marine Biotechnology, 2011, 13(3): 544-556 DOI:10.1007/s10126-010-9325-8 |
DARKO E, AMBRUS H, STEFANOVITS-BANYAI E, et al. Aluminium toxicity, Al tolerance and oxidative stress in an Al-sensitive wheat genotype and in Al-tolerant lines developed by in vitro microspore selection. Plant Science, 2004, 166(3): 583-591 DOI:10.1016/j.plantsci.2003.10.023 |
DAVISON I R, PEARSON G A. Stress tolerance in intertidal seaweeds. Journal of Phycology, 1996, 32(2): 197-211 DOI:10.1111/j.0022-3646.1996.00197.x |
DAWOOD M, CAO F, JAHANGIR M M, et al. Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. Journal of Hazardous Materials, 2012, s209/210(1): 121-128 |
DING Y, MIAO J L, LI G Y, et al. Effect of Cd on GSH and GSH-related enzymes of Chlamydomonas sp. ICE-L existing in Antarctic ice. Journal of Environmental Sciences, 2005, 17(4): 667-671 DOI:10.3321/j.issn:1001-0742.2005.04.030 |
DIXON D P, EDWARDS R. Roles for stress-inducible lambda glutathione transferases in flavonoid metabolism in plants as identified by ligand fishing. Journal of Biological Chemistry, 2010, 285(47): 36322-36329 DOI:10.1074/jbc.M110.164806 |
DUCAT D C, SACHDEVA G, SILVER P A. Rewiring hydrogenase-dependent redox circuits in cyanobacteria. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(10): 3941-3946 DOI:10.1073/pnas.1016026108 |
EDWARDS R, DIXON D P, CUMMINS I, et al. Springer- organic xenobiotics and plants. In: Schröder P and Collins C D (ed) New perspectives on the metabolism and detoxification of synthetic compounds in plants. Plant Ecophysiology, 2011, 125–148
|
EL-NAGGAR A H, SHEIKH H M. Response of the green microalga Chlorella vulgaris to the oxidative stress caused by some heavy metals. Life Science Journal, 2014, 11(10): 1349-1357 DOI:10.7537/marslsj111014.197 |
FENG L X. Effects of environmental stress on the chlorophy fluorescence of 4 microalgal strains. Master´s Thesis of Ocean University of China, 2006 [冯力霞. 环境胁迫对4株微藻叶绿素荧光特性的影响. 中国海洋大学硕士研究生学位论文, 2006]
|
FROVA C. The plant glutathione transferase gene family: Genomic structure, functions, expression and evolution. Acta Physiologiae Plantarum, 2003, 119(4): 469-479 DOI:10.1046/j.1399-3054.2003.00183.x |
GENTY B, BRIANTAIS J M, BAKER N R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta-General Subjects, 1989, 990(1): 87-92 DOI:10.1016/S0304-4165(89)80016-9 |
HAN X M, YANG Z L, LIU Y J, et al. Genome-wide profiling of expression and biochemical functions of the Medicago glutathione S-transferase gene family. Plant Physiology and Biochemistry, 2018, 126(1): 126-133 |
HERZI F, JEAN N, ZHAO H, et al. Copper and cadmium effects on growth and extracellular exudation of the marine toxic dinoflagellate Alexandrium catenella: 3D-fluorescence spectroscopy approach. Chemosphere, 2013, 93(6): 1230-1239 DOI:10.1016/j.chemosphere.2013.06.084 |
HU S, TANG C H, WU M. Cadmium accumulation by several seaweeds. Science of the Total Environment, 1996, 187(2): 65-71 DOI:10.1016/0048-9697(96)05143-1 |
HURD C L, HARRISON P J, BISCHOF K, et al. Seaweed ecology and physiology: Physico-chemical factors as environmental stressors in seaweed biology. 2nd edn. Cambridge University Press, 2014, 294–348
|
JIANG C, GAO H, ZOU Q. Changes of donor and accepter side in photosystem Ⅱ complex induced by iron deficiency in attached-soybean and maize leaves. Photosynthetica, 2003, 41: 267-271 |
KHAN W, RAYIRATH U P, SUBRAMANIAN S, et al. Seaweed extracts as biostimulants of plant growth and development. Plant Growth Regulation, 2019, 28(4): 386-399 |
KUMA K S, DAHMS H U, LEE J S, et al. Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicology and Environmental Safety, 2014, 104(2): 51-71 |
LANE T W, MOREL F M M. A biological function for cadmium in marine diatoms. Proceedings of the National Academy of Sciences, 2000, 97(9): 4627-4631 DOI:10.1073/pnas.090091397 |
LICHTENTHALER H K, BUSCHMANN C. Chlorophylls and carotenoids: Measurements and characterization by UV-Vis spectroscopy. Current Protocols in Food Analytical Chemistry, 2001, F4.3.1-F4.3.8 |
LIANG Z L, SHANG K H, WANG L H, et al. Cloning and expression analysis of the AsGST gene in garlic exposed to salinity stress. Journal of Nuclear Agricultural Sciences, 2019, 33(6): 1088-1095 [梁志乐, 尚珂含, 王立辉, 等. 大蒜谷胱甘肽硫转移酶基因AsGST的克隆及其对盐胁迫的响应. 核农学报, 2019, 33(6): 1088-1095] |
MAXWELL K, JOHNSON G N. Chlorophyll fluorescence: A practical guide. Journal of Experimental Botany, 2000, 51(345): 659-668 DOI:10.1093/jexbot/51.345.659 |
MIN H, GOLDEN S S. A new circadian class 2 gene, opcA, whose product is important for reductant production at night in Synechococcus elongatus PCC 7942. Journal of Bacteriology, 2000, 182(21): 6214-6221 DOI:10.1128/JB.182.21.6214-6221.2000 |
MOSTOFA M G, RAHMAN A, ANSARY M M U, et al. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Scientific Reports, 2015, 5: 14078 DOI:10.1038/srep14078 |
NAVARRETE I A, KIM D Y, WILCOX C, et al. Effects of depth -cycling on nutrient uptake and biomass production in the giant kelp Macrocystis pyrifera. Renewable and Sustainable Energy Reviews, 2021, 141: 110747 DOI:10.1016/j.rser.2021.110747 |
OLIVIER F, RIDD M, KLUMPP D. The use of transplanted cultured tropical oysters (Saccostrea commercialis) to monitor Cd levels in North Queensland coastal waters (Australia). Marine Pollution Bulletin, 2002, 44(10): 1051-1062 DOI:10.1016/S0025-326X(02)00157-1 |
PLEKHANOV S E, CHEMERIS Y K. Early toxic effects of zinc, cobalt, and cadmium on photosynthetic activity of the green alga Chlorella pyrenoidosa chick S-39. Biology Bulletin of the Russian Academy of Sciences, 2003, 30(5): 506-511 DOI:10.1023/A:1025806921291 |
PRASAD M N V. Cadmium toxicity and tolerance in vascular plants. Environmental and Experimental Botany, 1995, 35(4): 525-545 DOI:10.1016/0098-8472(95)00024-0 |
RASSWEILER A, REED D C, HARRER S L, et al. Improved estimates of net primary production, growth, and standing crop of Macrocystis pyrifera in Southern California. Ecology, 2018, 99(9): 2132 DOI:10.1002/ecy.2440 |
RIJSTENBIL J W, GERRINGA L J A. Interactions of algal ligands, metal complexation and availability, and cell responses of the diatom Ditylum brightwellii with a gradual increase in copper. Aquatic Toxicology, 2002, 56(2): 115-131 DOI:10.1016/S0166-445X(01)00188-6 |
SHARMA R, BROWN D, AWASTHI S, et al. Transfection with 4-hydroxynonenal-metabolizing glutathione S-transferase isozymes leads to phenotypic transformation and immortalization of adherent cells. European Journal of Biochemistry, 2004, 271(9): 1690-1701 DOI:10.1111/j.1432-1033.2004.04067.x |
SHUKLA P, EDWARDS M S. Elevated pCO2 is less detrimental than increased temperature to early development of the giant kelp, Macrocystis pyrifera (Phaeophyceae, Laminariales). Phycologia, 2017, 56(6): 638-648 DOI:10.2216/16-120.1 |
SIMPSON W R. A critical review of cadmium in the marine environment. Progress in Oceanography, 1981, 10(1): 1-70 DOI:10.1016/0079-6611(81)90007-0 |
STAUBER J L, FLORENCE T M. The effect of culture medium on metal toxicity to the marine diatom Nitzschia closterium and the freshwater green alga Chlorella pyrenoidosa. Water Research, 1989, 23(7): 907-911 DOI:10.1016/0043-1354(89)90016-X |
SUN X Y, WANG Y Z, LI Y, et al. Effects of polychlorinated biphenyls (PCB153) on the physiological ecology and ultrastructure of Dicrateria zhanjiangensis. Progress in Fishery Sciences, 2021, 42(4): 158-167 [孙小雨, 王祎哲, 李旸, 等. 多氯联苯(PCB153)对湛江叉鞭金藻生理生态和超微结构的影响. 渔业科学进展, 2021, 42(4): 158-167] |
VILLANUEVA S, BOTELLO A V. Metales pesados en la zona costera del Golfo de México y Caribe Mexicano: una revisión. Revista Internacional de Contaminación Ambiental, 1992, 8(1): 47-61 |
WANG Y, FAN X, GAO G, et al. Decreased motility of flagellated microalgae long-term acclimated to CO2-induced acidified waters. Nature Climate Change, 2020, 10(6): 561-567 DOI:10.1038/s41558-020-0776-2 |
WIENCKE C, BISCHOF K. Seaweed biology: Novel insights into ecophysiology, ecology and utilization. Springer, 2012
|
XIAO L, GAO R F, SUI F G. Effects of chloride stress on the photosynthesis and chlorophyll content of Chinese cabbage seedlings. Soil and Fertilizer Sciences in China, 2008, 2(2): 44-47 [肖丽, 高瑞凤, 隋方功. 氯胁迫对大白菜幼苗叶绿素含量及光合作用的影响. 中国土壤与肥料, 2008, 2(2): 44-47 DOI:10.3969/j.issn.1673-6257.2008.02.011] |
XU W Y, MA H K, SUN J Q, et al. Biological functional analysis of MBL gene in resistance to cadmium stress in Exopalaemon carinicauda. Progress in Fishery Sciences, 2020, 41(4): 174-180 [徐莞媛, 马杭柯, 孙金秋, 等. 脊尾白虾甘露糖结合凝集素(MBL)基因在抗镉胁迫中的生物学功能分析. 渔业科学进展, 2020, 41(4): 174-180] |
ZAR J. Biostatistical analysis. Prentice-Hall Inc, Englewood Cliffs, New Jersey, USA, 1996
|
ZHANG Y, GU Z, REN Y, et al. Integrating transcriptomics and metabolomics to characterize metabolic regulation to elevated CO2 in Chlamydomonas reinhardtii. Marine Biotechnology, 2021, 23(2): 255-275 DOI:10.1007/s10126-021-10021-y |
ZHU X F. Studies on the toxic effects of mercury, copper and cadmium to three economic marine algae. Master´s Thesis of Shantou University, 2010 [朱喜锋. 重金属汞、铜和镉对三种大型经济海藻毒性效应的研究. 汕头大学硕士研究生学位论文, 2010]
|



