2. 福建省水产生物育种与健康养殖工程研究中心 福建省发展和改革委员会 福建 厦门 361021;
3. 农业农村部东海健康养殖重点实验室 福建 厦门 361021
2. Fujian Engineering Research Center of Aquatic Breeding and Healthy Aquaculture; Fujian Provincial Development and Reform Commission, Xiamen, Fujian 361021, China;
3. Key Laboratory of Healthy Mariculture for the East China Sea, Ministry of Agriculture and Rural Affairs, Xiamen, Fujian 361021, China
紫菜(Neoporphyra或Neopyropia)是我国沿海传统的大宗水产养殖种类,富含人体必需的氨基酸、矿物质及维生素等营养物质(Cao et al, 2016)。2020年全国紫菜养殖总产量约为22.2万t,其中,坛紫菜(Neoporphyra haitanensis)产量占70%以上(中国渔业统计年鉴, 2021)。由于丰富的营养价值和生态效益,坛紫菜的市场需求持续增加。然而,随着栽培规模的扩大,良种匮乏的问题日益凸显。目前,大部分栽培品系是由岩礁上采集的野生坛紫菜驯化而来,加之多年自养自留,使得坛紫菜种质质量下降,抗逆性减弱(Yan et al, 2010)。通过国家审定的坛紫菜新品种虽然已有5个,但仍不足以满足多变的海域环境和市场的需求。因此,培育出更多符合生产需求的新品系仍是当前坛紫菜育种的重要任务。
目前,紫菜新品种的选育技术主要包括选择育种、杂交育种、诱变育种等,其中,选择育种和杂交育种存在周期长、效率低等难题(丁洪昌等, 2019),而诱变育种操作简易、方便处理大批量的样品,且有利于筛选出一些具有重要研究价值和应用价值的材料(常金华等, 2002)。诱变育种在紫菜遗传育种中已被广泛应用,Lee等(2019)通过γ射线诱变得到的甘紫菜(Pyropia tenera)色素突变体,不仅生长性状优于野生型,还具有较强的耐高温能力。Ding等(2019)利用60Co-γ诱变处理紫菜新品系HR-5后,成功筛选出一株长度、重量、耐高温性等性状优于HR-5的突变株。除此之外,我国研究人员利用诱变育种等方法培育出了4个坛紫菜新品种(“申福1号”、“申福2号”、“闽丰1号”和“闽丰2号”)(梁志强, 2004; 王长青等, 2011; 梁艳, 2009; 梁艳等, 2009)。由此可见,诱变育种是选育紫菜优良新品种(系)的重要手段。
随着坛紫菜基因组测序的完成(Chen et al, 2021),对坛紫菜重要性状调控基因的功能解析越来越迫切。但是,由于遗传操作技术体系不成熟、特异突变体缺乏等原因,坛紫菜关键农艺性状的遗传调控机理尚不明确。通过诱变方法构建突变体库是研究基因功能、发现新基因以及解析目标性状调控机制的有效方法(梁英等, 2020)。研究人员已在模式植物拟南芥(Arabidopsis)(Greene et al, 2003)、小麦(袁凯等, 2021; 张婷等, 2021)、大豆(张小明等, 2016; 齐波等, 2019)、水稻(Oryza sativa L.) (叶俊, 2006)中构建了具有丰富性状的突变体库,并通过正向遗传学,从特定表型性状变化的个体中找到对应的突变基因,并揭示其功能(Zhang et al, 2017; Gao et al, 2021)。因此,为了满足紫菜遗传学和育种学的需要,实现对基因功能的深入研究,开展紫菜诱变和突变体性状研究具有重要意义。
表型性状鉴定是紫菜育种工作中最基础的工作,紫菜的表型性状主要有数量性状和质量性状2种类型,数量性状主要受多个微效基因调控,极易受环境影响(孔繁玲, 2006),比如紫菜的长度、宽度、重量、厚度等性状(Xu et al, 2015)。而紫菜的扭曲、叶型等属于质量性状,主要受单基因控制,不易受环境影响(邵元健, 2006)。因而,考察藻体各类性状,分析其遗传多样性,有利于改良紫菜性状。通过计算表型变异系数和遗传多样性指数,可为今后挖掘优质坛紫菜资源及选配杂交亲本提供理论参考。
本研究以纯化的坛紫菜野生品系NSD35为实验材料,利用γ射线诱变获得不同类型突变体,经过体细胞克隆技术获得各突变体的纯系,对子代叶状体进行培育、观察和鉴定,以期为解析坛紫菜经济性状的遗传调控机理以及选育优良品种提供基础材料。
1 材料与方法 1.1 实验材料与培养本实验所用材料均来自福建省坛紫菜种质资源库的NSD35品系,该品系采自福建牛山岛,并通过酶解纯化获得纯系,以自由丝状体形式保存。取一定量NSD35丝状体在温度为27~29℃、光照强度为10~ 30 μmol/(m2·s)、光周期为9 L∶15 D的条件下促熟,每隔10 d更换1/3丝状体培养液。当丝状体形成壳孢子囊枝时,挑选状态良好的藻丝,放入500 mL的锥形瓶中充气培养,在温度为(21.0±0.5)℃、光强度为40~50 μmol/(m2·s)、光周期为12 L∶12 D的条件下培养。每2 d更换2/3培养液。培养促放出的壳孢子至小苗,待藻体长至3~4 cm时,挑选形态完整的叶状体进行人工诱变处理。
1.2 诱变处理于2019年11月在浙江省辐照中心利用岛津UV-1800进行诱变,γ射线放射源为铯-137,采用重铬酸钾(银)剂量计测定辐照处理实际计量值。设置5个梯度的辐射剂量分别为700、900、1100、1300和1500 Gy,剂量率分别为35、45、55、65和75 Gy/h。
1.3 诱变后的细胞显微观察及突变体分离 1.3.1 诱变后细胞的显微观察将辐射后的藻体进行黑暗恢复培养24 h,之后观察细胞死亡率。24 h后将光照强度恢复至20~30 μmol/(m2·s)并弱充气培养藻体7 d,显微观察统计叶状体变异细胞的种类和数量(20倍镜下,每1 cm随机取10~15个视野)。
1.3.2 突变体分离分别选取诱变后的藻体基部、中部和尖端各2 cm2,利用海螺酶进行酶解。
1.3.3 突变体初步筛选对颜色、形态等性状有所变异的单克隆苗或细胞团进行筛选。
测量单色突变体色色度值:将藻体平摊置于白色湿润磁盘上,利用分光测色剂(CM-700d),垂直于藻体随机测量藻体3个点,取色度值a值和b值,a值越大藻体偏红色,a值越小藻体偏绿;b值越大表示藻体偏黄色,b值越小表示偏蓝色。
1.3.4 突变体F1代叶状体获取对初筛出的突变株(色块)进行第2次酶解,获得它们的遗传纯合丝状体,诱导丝状体成熟放散壳孢子,培养放散出的壳孢子至小苗,用于后续F1代叶状体的性状测定。
1.4 突变株F1代叶状体数量性状的测定生长相关参数测定:随机筛选30株3.8~4.2 cm健康完整的幼苗,每2株分别置于1000 mL的锥形瓶中充气培养,培养条件:温度为(21.0±0.5)℃,光强为40~50 μmol/(m2·s),光周期12 L∶12 D。培养期间,每3 d更换1次培养液,培养15 d后观察并测量其长(L)、宽(W)和鲜重(FW)。
厚度测定:随机取12株(25±5) cm藻体,用刀片横向切取藻体1/4 (基部)、1/2 (中部)和3/4 (尖端)处的单层细胞,在40倍镜下进行观察测量,并将其测量结果与对照组进行比对,数值显著大于对照组的为厚叶型突变体,反之则为薄叶型突变体。
生长速率测定:根据以上测量结果计算长度特定生长率(LGR)、宽度特定生长率(WGR)和鲜重特定生长率(FWGR),计算公式如下:
| $\text { LGR }=\left(\ln L_n-\ln L_0\right) / n \times 100 \% $ |
| $ \mathrm{WGR}=\left(\ln W_n-\ln W_0\right) / n \times 100 \% $ |
| $\mathrm{FWGR}=\left[\ln \left(\mathrm{FW}_n\right)-\ln \left(\mathrm{FW}_0\right)\right] / n \times 100 \% $ |
式中,n为培养天数;Ln为培养n天藻体长度(cm);L0为初始藻体长度(cm);Wn为培养n天藻体宽度(cm);W0为初始藻体宽度(cm);FWn为培养n天藻体鲜重(g);FW0为初始藻体鲜重(g)。
1.5 突变株F1代叶状体质量性状的赋值情况对于坛紫菜叶状体颜色、藻体叶形、藻体基部形态、藻体锯齿等质量性状采用目测法,观察其形态特征,并按照谢小雪(2014)中的方法,将质量性状数字化。其中,参照颜色色谱表将叶状体颜色分为12个等级;参照张学成等(2005)编著的《海藻遗传学》,将叶型、基部形状分别划分为2个和3个等级;将培养15 d的叶状体按基部锯齿大小分为4个等级,按叶状体扭曲比例大小分为4个等级,按藻体老化程度分为2个等级。具体赋值情况见表 1。
|
|
表 1 质量性状的赋值情况 Tab.1 The assignment value of quality traits |
获得的原始数据先用Excel 2016计算平均值(mean)和标准差(SD)等,将21个突变株系F1代叶状体的14个性状数据导入SPSS 22软件进行聚类分析。其中,性状表型值以平均值表示,将各性状的数值进行Z得分标准化,并转换度量为绝对值,采用间连接组间连接法,区间为欧式距离进行聚类。
1.7 数据分析利用Excel 2010和SPSS 22软件进行实验数据的统计分析,F1代藻体的性状数值均与对照组(未经诱变的NSD35藻体)的性状数值作进行对比。其中,假定判断方法采用单样本Kolmogorov-Smirnov检验;性状相关性分析采用Pearson分析方法;根据变异系数(CV)和变异系数差判定突变体变异情况,计算公式:
| $\begin{gathered} \mathrm{CV}=\text { 标准差/平均值 } \times 100 \% \\ 变异系数差 =\mathrm{F}_1 代变异系数 - 对照组变异系数 \end{gathered}$ |
γ射线辐照对坛紫菜的叶状体存活影响较大,不同剂量的γ射线均造成坛紫菜叶状体细胞一定程度的死亡,且藻体细胞的死亡量随着诱变剂量的增加而升高。在700~900 Gy辐照强度下,NSD35叶状体只有少量细胞死亡,细胞存活率为90%以上;当诱变剂量增加至1100 Gy时,NSD35品系叶状体细胞间隙增大,中部少部分细胞开始出现死亡,尖端细胞无明显变化。经高剂量(1300 Gy以上)辐照后,藻体中部细胞间隙变大,细胞受损严重,大部分细胞形态由不规则形变为球形,细胞死亡率约为40%,尖端细胞色素析出(图 1A)。就叶状体不同部位而言,基部细胞死亡率最高,尖端细胞死亡率次之,中部细胞死亡率最低(图 1B)。
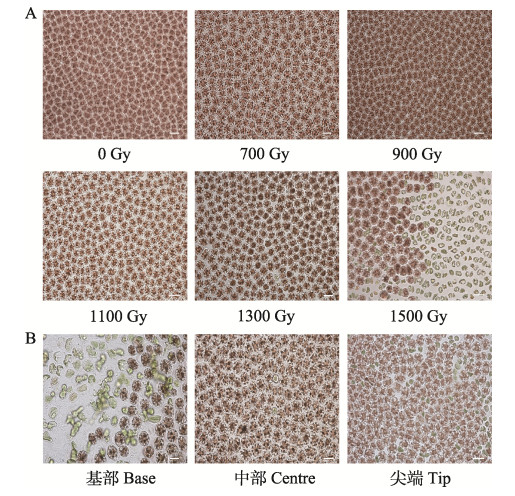
|
图 1 γ射线辐照坛紫菜叶状体显微观察图(标尺=20 μm) Fig.1 The microscopic observation of leaf shape of N. haitanensis irradiated by γ ray (bar =20 μm) A:不同剂量处理后的细胞状态;B:叶状体不同部位细胞状态 A: Status of cell after treatment with different doses of NSD35; B: Status of cell in different parts of blade |
与未辐照的叶状体相比,经辐照后的叶状体颜色变深、生长速度变慢。恢复培养7 d后,变异细胞与周围细胞出现明显的分界线,变异细胞块主要有橙色、橙红色、红色、绿色和紫色等色块(图 2A)。以辐照剂量1100 Gy为界,辐照低于1100 Gy剂量的藻体色素突变的细胞较少,存活率高;而辐照高于1100 Gy剂量的藻体细胞出现多个形态接近球形的细胞,部分色素突变细胞濒临死亡。随着诱变剂量的增加,NSD35色素突变细胞个数呈先上升后下降的趋势,在1300 Gy时达到峰值(图 2B)。
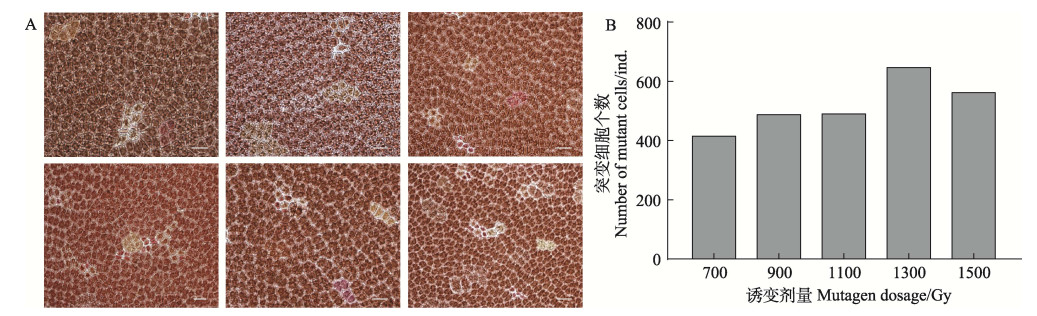
|
图 2 γ射线辐照过的NSD35叶状体上形成的变异细胞块 Fig.2 The color-mutated cell-clusters formed on the γ ray irradiated blades of NSD35 A:NSD35不同颜色的细胞(标尺=20 μm);B:经不同剂量辐照后色素变异的细胞数目 A: Cells of different colors of and NSD35 (bar = 20 μm); B: The number of cells with pigment variation after irradiation at different doses |
将诱变叶状体的中部藻块进行酶解获得单离体细胞,进行个体再生培养,通过对再生体的颜色、形态、生长等特性进行筛选和鉴定。NSD35品系初选获得67个突变株系。突变株包含色素突变体45个,形态突变体51个,其他类型突变11个,部分突变株有2个及2个以上的突变性状,表型突变具体情况见表 2。
|
|
表 2 初步筛选坛紫菜突变体的表型统计 Tab.2 Phenotypic statistics of mutation types of N. haitanensis |
未经诱变处理的坛紫菜叶状体为野生色(图 3A),基部呈绿色,逐渐过渡成红棕色。在单克隆苗中,色素突变较为丰富,主要包括黄色、红色、浅绿色、褐绿色、紫色、野生红色、野生绿色等突变(图 3B~F)。进一步检测突变体的色度值,结果显示,不同颜色藻体的色度值差异显著(图 3G)。
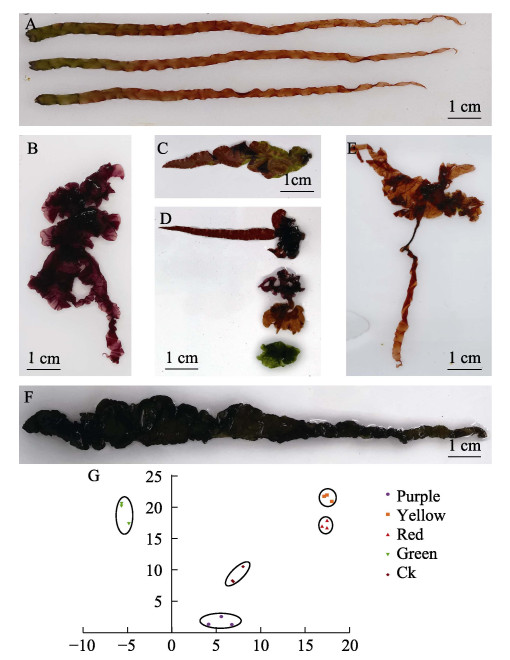
|
图 3 色素突变体藻体 Fig.3 Gametophytic blades of pigmentation mutant strains A:未经诱变处理的NSD35叶状体;B:紫色的叶状体;C:红色和绿色相嵌合的叶状体;D:从上往下:翠绿色的叶状体、黄色的叶状体、紫色的叶状体、红色的叶状体;E:黄色的叶状体;F:褐绿色的叶状体;G:色素突变体的色度值(横坐标为a值;纵坐标为b值)。 A: Gametophytic blades of NSD35 without treatment; B: Gametophytic blades of purple; C: Gametophytic blades of red and green. D: From top to bottom: Gametophytic blades of emerald green, yellow, purple, red; E: Gametophytic blades of yellow; F: Gametophytic blades of brownish green; G: Value of chroma between the pigment mutants (Abscissa represent the a value; Ordinate represents the b value). |
形态突变体有宽度突变体、锯齿突变体、基部形状突变体、厚度突变体等,共51份突变体。其中,厚度突变体21个,包括薄叶型藻体17个、厚叶型藻体4个。未经诱变的NSD35藻体中部厚度为(23.54±1.39) μm (图 4C、D),而经过诱变处理后获得的薄叶型突变体的叶状体中部厚度减少了4~8 μm (图 4E、F),但厚叶型突变体的叶状体中部厚度增加了4~10 μm (图 4A、B)。由于藻体是单层细胞,所以可以通过测定细胞厚度判断藻体厚度,如图 4B、D、F所示,依次为厚叶型突变体、对照组NSD35、薄叶型突变体。

|
图 4 厚度突变体藻体 Fig.4 Gametophytic blades of thickness mutant strains isolated from NSD35 A:厚叶型突变体叶状体;B:厚叶型突变体单层细胞厚度;C:对照组叶状体;D:对照组单层细胞厚度;E:薄叶型突变体叶状体;F:薄叶型突变体单层细胞厚度。 A: Blade of thick leaf mutant; B: Thickness of cell from thick leaf mutant; C: Blade without mutagenic treatment; D: Thickness of cell without mutagenic treatment; E: Blade of thin leaf mutant. F: Thickness of cell from thin leaf mutant. |
宽度突变体有22个,包括宽叶型藻体16个、窄叶型藻体有6个,宽叶和窄叶的藻体表型对比明显(图 5)。未经处理的NSD35藻体宽度可达1 cm,经过诱变后宽叶型突变体的宽度为2~3 cm (图 5A、B、C),而极端窄叶型突变体的宽度不足0.1 cm (图 5D)。
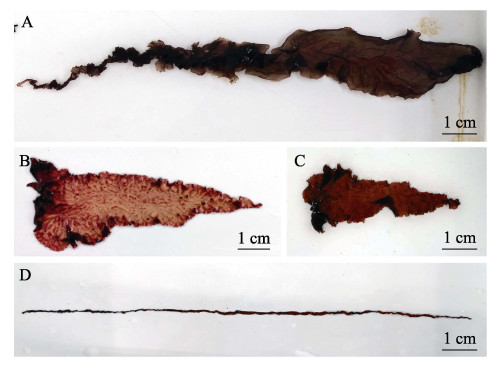
|
图 5 宽度突变体藻体 Fig.5 Gametophytic blades of width mutant strains isolated from NSD35 A~C:宽叶型突变体;D:窄叶型突变体A~C: Wide leaf mutant; D: Narrow leaf mutant |
如表 3所示,从叶状体表型性状的平均值来看,F1代的长、宽、鲜重和厚度数值均小于对照组,偏向于负向变异,而长宽比、颜色、基部形状和扭曲程度均大于对照组,偏向于正向变异。变异系数在8.74%~59.49%之间,其中,变异系数最小的是尖端厚度,仅有8.74%;而颜色、宽度、基部形状和锯齿变异系数较大,并依次递减,F1代藻体的上述4项表型性状的变异系数分别为59.49%、44.45%、43.03%和40.83%。此外,老化程度的变异系数差为负值,这说明F1代的老化程度小于对照组,突变株不易老化。从最大值和最小值来看,F1代藻体的变异幅度大于对照组。
|
|
表 3 坛紫菜表型性状及Kolmogorov-Smirnov检验结果 Tab.3 Phenotypic character and Kolmogorov-Smirnov test results of N. haitanensis |
通过相关性分析发现,F1代藻体的长度和日增长率、日增宽率、日增重率、长宽比呈极显著正相关(P < 0.01),相关系数分别为0.982、0.536、0.688和0.695。宽度与鲜重、日增宽率、叶型呈现极显著正相关,相关系数分别为0.834、0.595和0.561;宽度与日增重率呈显著正相关(P < 0.05),相关系数为0.489;而宽度与长宽比呈极显著负相关,相关系数为0.743。鲜重与日增宽率、日增重率呈极显著正相关,相关系数分别为0.739和0.724;与颜色的相关系数为0.520,呈显著负相关。日增长率与日增宽率、日增重率及长宽比呈极显著正相关,相关系数分别为0.572、0.712和0.675。日增宽率与日增重率相关系数为0.908,呈极显著正相关。日增宽率与叶形态呈显著正相关,与叶状体中部厚度呈显著负相关。日增重率与藻体叶型呈显著正相关。叶状体不同部位之间的厚度呈极显著正相关,尖端厚度与叶形态呈显著负相关;而藻体尖端厚度与锯齿、扭曲呈显著相关,相关系数分别为0.364和0.464。藻体的叶型与藻体质量性状中的锯齿程度呈极显著负相关,相关系数为0.553。藻体的扭曲程度与藻体老化程度呈正相关,相关系数为0.457。藻体的基部形状与各性状间均没有显著相关性(图 6)。总体而言,质量性状与数量性状之间的显著相关性较低,数量性状之间显著相关性较高。
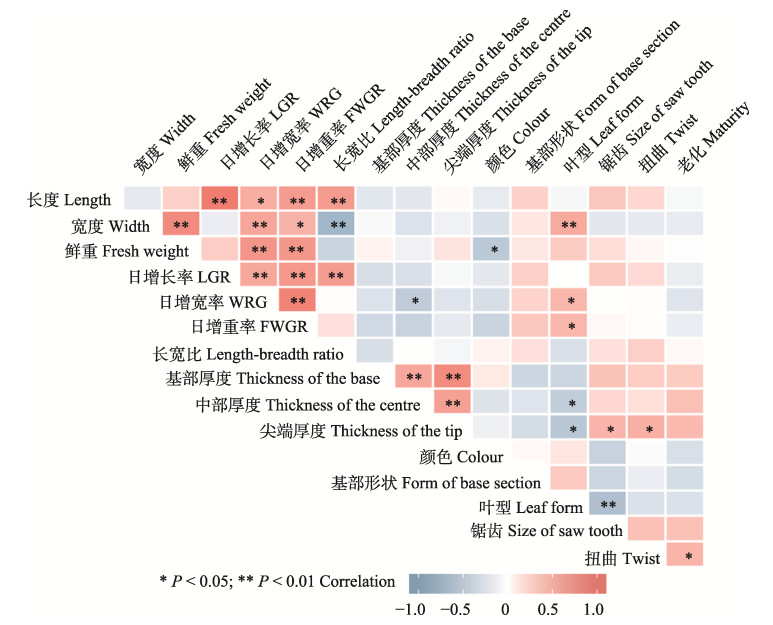
|
图 6 坛紫菜突变体叶状体表型性状的相关性分析 Fig.6 Correlation of phenotypic character of blades of N. haitanensis *表示差异显著(P < 0.05);**表示差异极显著(P < 0.01) * indicates significant difference at P < 0.05; ** indicates significant difference at P < 0.01 |
对F1代藻体表型性状进行z-score标准化处理后,采用组间连接法,区间欧式距离进行系统聚类。由图 7可知,坛紫菜叶状体F1代在遗传距离为20处,可将21个单株聚类为4个类群。第Ⅰ类有5株,其特点是藻体颜色偏红;第Ⅱ类有6株,特点是叶状体较宽、生物量大;第Ⅲ类有2株,特点是日均增长快、藻体较薄;第Ⅳ类有8株,特点是长而窄的叶状体。
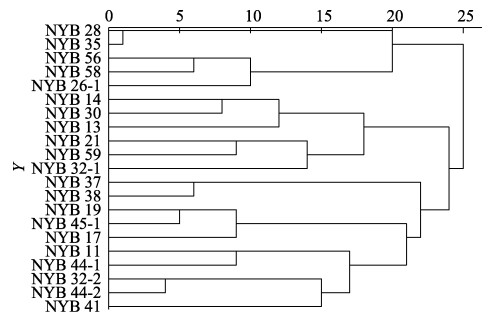
|
图 7 坛紫菜突变体F1代单株聚类分析树状图 Fig.7 Cluster analysis of F1 generation of N. haitanensis |
人工诱变育种可以快速获得大量突变体,是新种质创制的有效途径之一。其中,γ射线诱变方法易操作、安全,已被广泛应用于紫菜育种中(付峰等, 2018)。选择最佳诱变条件是利用γ射线诱变从而成功获得突变体的关键。本研究以5个辐照强度(700、900、1100、1300和1500 Gy),对坛紫菜不同藻体进行诱变,通过对细胞死亡率及细胞突变率进行综合分析,以确定最适诱变剂量。结果显示,色素变异细胞的数量随诱变剂量的增加呈先增加后降低的趋势(图 2),与严兴洪等(2005)的研究结果相似。此外,突变体F1代变异幅度大于对照组,且除尖端厚度以外,F1代其他性状的变异系数均大于10%而小于90%,属于中度变异(刘继龙等, 2018),这说明γ射线诱变能获得较好的诱变效果,且诱变效果强于紫外线诱变(Chen et al, 2016)和化学诱变(李勇斌, 2006),且无需添加任何化学品、无二次污染,具有高效、清洁、低成本、操作方便等优点,因此,γ射线诱变方法在紫菜未来的诱变育种中仍有较广阔的应用前景(丁洪昌等, 2019)。
通过诱变进行育种的首要目标是获得差异明显的表型性状,表型性状鉴定是育种工作中的基础工作(张丰收等, 2020)。相较高等植物而言,紫菜叶状体外形简单,其形态特征主要包括叶长、叶宽、叶厚及叶色(童冠文, 2010; Xu et al, 2012; 黄林彬等, 2017; 仵燕青等, 2016; 柳佩娟等, 2008)。藻体长度、宽度和厚度等性状和紫菜的产量密切相关,而藻体颜色是影响其品质的主要指标之一(Hwang et al, 2019)。本研究通过对坛紫菜突变体F1代中21个株系的长、宽、鲜重、厚度、颜色等14个表型性状进行分析发现,突变群体中诱变效应最为明显的性状是颜色,其变异系数达59.49%。这与以往研究结果一致,多个研究组利用不同的诱变方式获得的紫菜突变体大多都是色素突变体(赵爽等, 2019; 张倩等, 2019; 李琳等, 2006),紫菜易产生色素突变体,说明调控藻体颜色的基因经过诱变后最易发生突变。而紫菜的叶状体颜色主要与藻红蛋白、藻蓝蛋白和叶绿素a的含量及比例相关(Zhang et al, 2014),往往藻胆蛋白含量高的藻体颜色深、鲜亮,符合市场需求(羌玺等, 2021)。此外,由于坛紫菜的减数分裂发生在壳孢子的前2次分裂期间,之后最初4个细胞发育成了线性四分体,导致形成嵌合叶片,而不同色素突变体可以作为遗传标记来区分嵌合叶片的每个部分,从而便于获得纯系藻体。
与颜色性状突变不同,坛紫菜F1代的长、宽、鲜重和厚度数值均小于对照组,偏向于负向变异。例如,经过诱变后共获得薄叶型藻体17个、厚叶型藻体4个。紫菜藻体的厚度影响其口感,并限制其加工方法,进而影响紫菜附加值的提升(Ding et al, 2019; 全汉锋等, 2012)。条斑紫菜藻体薄,更适合自动化加工,其初级产品可进行附加值更高的二次加工(如寿司),而坛紫菜藻体厚而宽,一般用于半机械化或手工生产附加值低的产品,如紫菜饼(何培民等, 2018)。因此,选育藻体薄的坛紫菜新品系是育种的主要目标之一(Ding et al, 2020)。本研究获得的突变体中,藻体薄的品系的中部厚度仅有16 μm左右,相较于新品种“闽丰2号”(藻体中部厚度约为21 μm)更薄(梁艳等, 2009)。作为对比,一些传统养殖品种在第3次采收时藻体厚度已达到60 μm,严重影响其口感和后期加工(何培民等, 2018)。研究发现,藻体的厚度性状遗传较稳定,藻体的厚度主要与细胞壁厚度和胶质腔厚度相关(Gao et al, 2021; 童冠文等, 2010)。魏鹏华(2021)研究表明,NAD糖依赖性差向异构酶(GME)基因的表达量与琼胶含量显著相关,进而调控坛紫菜藻体的厚度,但其具体调控机制仍有待深入研究。而本研究筛选的厚度突变体为坛紫菜厚度性状的遗传调控机制解析提供了难得的实验材料。此外,不同突变体表型性状之间具有非常紧密的关联性,对不同性状进行相关性分析,从中找出变异规律,可辅助坛紫菜的育种工作,例如,选择长和薄的藻体就有可能选育出高产、优质的坛紫菜新品系。
4 结论本研究通过γ射线照射处理坛紫菜叶状体获得不同突变体,之后利用酶解单克隆技术获得再生突变体,最终筛选出67个突变体株系。并基于14个表型性状,对其中21个株系的F1代进行了相关性和聚类分析,以期为坛紫菜遗传育种研究以及优良新品种选育提供材料支撑。
CAO J, WANG J P, WANG S C, et al. Porphyra species: A mini-review of its pharmacological and nutritional properties. Journal of Medicinal Food, 2016, 19(2): 111-119 DOI:10.1089/jmf.2015.3426 |
CHANG J H, LI Y H. The applying the technology of artificial induced mutation in plant improvement. Journal of Hebei Agricultural University, 2002, 25(S1): 14-16 [常金华, 李玉华. 诱变技术在植物遗传改良中的应用. 河北农业大学学报, 2002, 25(S1): 14-16] |
CHEN H M, CHU J S, CHEN J J, et al. Insights into the ancient adaptation to intertidal environments by red algae based on a genomic and multiomics investigation of Neoporphyra haitanensis. Molecular Biology and Evolution, 2021, 39(1): msab315 |
CHEN S S, DING H C, YAN X H. Isolation and characterization of an improved strain of Porphyra chauhanii (Bangiales, Rhodophyta) with high-temperature resistance. Journal of Applied Phycology, 2016, 28(5): 3031-3041 DOI:10.1007/s10811-016-0803-x |
DING H C, LIU C J, YAN X H. Selection and characterization of an improved strain (ST-2) of Pyropia haitanensis by 60Co-γ ray irradiation. Aquaculture Research, 2019, 50(9): 2388-2397 DOI:10.1111/are.14120 |
DING H C, WANG T G, ZHANG P, et al. High-temperature resistance of the thin-blade strain of Pyropia haitanensis and its pilot cultivation in mariculture farm. Journal of Applied Phycology, 2020, 32(4): 2261-2270 DOI:10.1007/s10811-019-01885-8 |
DING H C, YAN X H. Advances in Pyropia (formerly Porphyra) genetics and breeding. Journal of Fishery Sciences of China, 2019, 26(3): 592-603 [丁洪昌, 严兴洪. 紫菜遗传育种研究进展. 中国水产科学, 2019, 26(3): 592-603] |
Fishery administration of Ministry of Agriculture and Rural Affairs. China Fishery Statistics Yearbook 2021. Beijing: China Agriculture Press, 2021 [农业农村部渔业渔政管理局. 2021中国渔业统计年鉴. 北京: 中国农业出版社, 2021]
|
FU F, SUI Z H, SUN L Q, et al. Research advance on the algal mutation breeding technologies. Biotechnology Bulletin, 2018, 34(10): 58-63 [付峰, 隋正红, 孙利芹, 等. 藻类诱变育种技术研究进展. 生物技术通报, 2018, 34(10): 58-63] |
GAO J, YANG S, TANG K, et al. GmCCD4 controls carotenoid content in soybeans. Plant Biotechnology Journal, 2021, 19(4): 801-813 DOI:10.1111/pbi.13506 |
GREENE E A, CODOMO C A, TAYLOR N E, et al. Spectrum of chemically induced mutations from a large-scale reverse- genetic screen in Arabidopsis. Genetics, 2003, 164(2): 731-740 DOI:10.1093/genetics/164.2.731 |
HE P M, ZHANG Z Y, ZHANG X C, et al. Seaweed cultivation. Beijing: Science Press, 2018 [何培民, 张泽宇, 张学成, 等. 海藻栽培学. 北京: 科学出版社, 2018]
|
HUANG L B, YAN X H. Genetic analysis of major economic traits in Pyropia yezoensis using double haploid population. Journal of Fisheries of China, 2017, 41(12): 1847-1857 [黄林彬, 严兴洪. 条斑紫菜双单倍体群体主要经济性状的遗传分析. 水产学报, 2017, 41(12): 1847-1857 DOI:10.11964/jfc.20161210641] |
HWANG E K, YOTSUKURA N, PANG S J, et al. Seaweed breeding programs and progress in eastern Asian countries. Phycologia, 2019, 58(5): 484-495 DOI:10.1080/00318884.2019.1639436 |
KONG F L. Plant quantitative genetics. China Agricultural University Press, 2006 [孔繁玲. 植物数量遗传学. 北京: 中国农业大学出版社, 2006]
|
LEE H J, CHOI J I. Enhancing temperature tolerance of Pyropia tenera (Bangiales) by inducing mutation. Phycologia, 2019, 58(5): 496-503 DOI:10.1080/00318884.2019.1623547 |
LI L, YAN X H. Isolation and characterization of a green type mutant in Porphyra haitanensis (Bangiales, Rhodophyta). Journal of Shanghai Fisheries University, 2006, 15(1): 30-35 [李琳, 严兴洪. 坛紫菜绿色突变体的分离与特性分析. 上海水产大学学报, 2006, 15(1): 30-35] |
LI Y B. Effects of MNNG on growth and development in thalli of Porphyra haitanensis and analysis of the different protein expression in the mutants. Masterxs Thesis of Xiamen University, 2006 [李勇斌. MNNG对坛紫菜叶状体细胞生长发育的影响与坛紫菜突变品系间的差异蛋白质研究. 厦门大学硕士研究生学位论文, 2006]
|
LIANG Y, XU Y, CHEN C S, et al. Research on the main economic traits of the new strain of high quality Porphyra haitanensis. Progress in Fishery Sciences, 2009, 30(4): 108-116 [梁艳, 徐燕, 陈昌生, 等. 坛紫菜优质新品系(Q-1)主要经济性状的研究. 渔业科学进展, 2009, 30(4): 108-116 DOI:10.3969/j.issn.1000-7075.2009.04.017] |
LIANG Y, YAN Y Y, LAI Q X, et al. Researching advances in microalgal mutation breeding. Periodical of Ocean University of China (Natural Science), 2020, 50(6): 19-32 [梁英, 闫译允, 赖秋璇, 等. 微藻诱变育种研究进展. 中国海洋大学学报(自然科学版), 2020, 50(6): 19-32] |
LIANG Y. Research on main economic traits and DNA sequence analysis of the new pure Porphyra haitanensis Lines. Masterxs Thesis of Jimei University, 2009 [梁艳. 坛紫菜新品系纯系主要经济性状的研究及DNA序列分析. 集美大学硕士研究生学位论文, 2009]
|
LIANG Z Q. Preliminary study on genetic breeding of Porphyra haitanensis. Masterxs Thesis of Shanghai Ocean University, 2004 [梁志强. 坛紫菜遗传育种的初步研究. 上海海洋大学硕士研究生学位论文, 2004]
|
LIU J L, LIU L, MA X Y, et al. Spatial variability of soil salt in different soil layers at different scales. Journal of Basic Science and Engineering, 2018, 26(2): 305-312 [刘继龙, 刘璐, 马孝义, 等. 不同尺度不同土层土壤盐分的空间变异性研究. 应用基础与工程科学学报, 2018, 26(2): 305-312] |
LIU P J, XIE C T, CHEN C S, et al. Preliminary study on different pigment mutants of Porphyra haitanensis. Progress in Fishery Sciences, 2008, 29(4): 78-83 [柳佩娟, 谢潮添, 陈昌生, 等. 坛紫菜不同色素突变体的初步研究. 海洋水产研究, 2008, 29(4): 78-83] |
QI B, RU X X, JIA Z Z, et al. Radiation intensities affect important agronomic characters of soybean M1 generation. Chinese Agricultural Science Bulletin, 2019, 35(12): 40-45 [齐波, 汝玄玄, 贾召召, 等. 不同辐射强度对大豆M1代重要农艺性状的影响. 中国农学通报, 2019, 35(12): 40-45] |
QIANG X, WANG L J, NIU J F, et al. Research progress of phycobiliproteins from seaweed. Science and Technology of Food Industry, 2021 [羌玺, 王立军, 牛建峰, 等. 海藻来源藻胆蛋白研究进展. 食品工业科技, 2021 DOI:10.13386/j.issn1002-0306.2021080019] |
QUAN H F, WANG X C, ZHAN Z Y, et al. Studies on the improvement of full-automatic Prophyra haitanensis processing equipment and its technology optimization. Progress in Fishery Sciences, 2012, 33(1): 122-128 [全汉锋, 王兴春, 詹照雅, 等. 坛紫菜全自动加工设备及工艺的改进. 渔业科学进展, 2012, 33(1): 122-128] |
SHAO Y J. Analysis of definitions about qualitative and quantitative trait. Chinese Journal of Oil Crop Sciences, 2006(4): 55-57 [邵元健. 质量性状和数量性状含义的辨析. 生物学杂志, 2006(4): 55-57] |
TONG G W. Research progress on economic traits of Porphyra yezoensis. Modern Agricultural Science and Technology, 2010(11): 343-345 [童冠文. 条斑紫菜经济性状研究进展. 现代农业科技, 2010(11): 343-345] |
WANG C Q, YAN X H, HUANG L B, et al. Characterization of an improved strain (SF-2) of Porphyra haitanensis (Bangiales, Rhodophyta) and its pilot cultivation in mariculture farm. Journal of Fisheries of China, 2011, 35(11): 1658-1667 [王长青, 严兴洪, 黄林彬, 等. 坛紫菜优良品系"申福2号"的特性分析与海区中试. 水产学报, 2011, 35(11): 1658-1667] |
WEI P H. Preliminary study on regulation mechanism of algal thickness in Pyropia haitanensis. Masterxs Thesis of Jimei University, 2021 [魏鹏华. 坛紫菜藻体厚度调控机理的初步研究. 集美大学硕士研究生学位论文, 2021]
|
WU Y Q, XIE C T, XIE X X, et al. Analysis on the variation patter of phenotypic traits of Pyropia haitanensis germplasms. Journal of Fisheries Research, 2016, 38(3): 173-180 [仵燕青, 谢潮添, 谢小雪, 等. 坛紫菜种质品系表型性状的变异规律分析. 渔业研究, 2016, 38(3): 173-180] |
XIE X X. Construction of Pyropia haitanensis core germplasm repositories. Masterxs Thesis of Jimei University, 2014 [谢小雪. 坛紫菜核心种质库的构建. 集美大学硕士研究生学位论文, 2014]
|
XU Y, HUANG L, JI D H, et al. Construction of a dense genetic linkage map and mapping quantitative trait loci for economic traits of a doubled haploid population of Pyropia haitanensis (Bangiales, Rhodophyta). BMC Plant Biology, 2015, 15(1): 228 |
XU Y, XIE C T, CHEN C S, et al. Genetic analyses of six quantitative traits of a doubled haploid population of Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). Journal of Applied Phycology, 2012, 24(1): 89-96 |
YAN X H, LIANG Z Q, SONG W L, et al. Induction and isolation of artificial pigmentation mutants in Porphyra haitanensis (Bangiales, Rhodophyta). Journal of Fisheries of China, 2005, 29(2): 166-172 [严兴洪, 梁志强, 宋武林, 等. 坛紫菜人工色素突变体的诱变与分离. 水产学报, 2005, 29(2): 166-172] |
YAN X H, LV F, LIU C J, et al. Selection and characterization of a high-temperature tolerant strain of Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). Journal of Applied Phycology, 2010, 22(4): 511-516 |
YE J. The screening of mutants and construction of rice mutant population for variety '9311' of Rice (Oryza sativa L. ). Masterxs Thesis of Zhejiang University, 2006 [叶俊. 水稻"9311"突变体的筛选和突变体库的构建. 浙江大学硕士研究生学位论文, 2006]
|
YUAN K, ZHANG W, WEN H W, et al. EMS-induced mutagenesis and phenotypic variation analysis in wheat variety Jimai 22. Journal of Plant Genetic Resources, 2021, 22(4): 979-988 [袁凯, 张伟, 温宏伟, 等. EMS诱导济麦22突变体库的构建及表型变异分析. 植物遗传资源学报, 2021, 22(4): 979-988] |
ZHANG F S, WANG Q. Research progresses in the plant breeding of radiation mutation. Journal of Henan Normal University (Natural Science), 2020, 48(6): 39-49 [张丰收, 王青. 植物辐射诱变育种的研究进展. 河南师范大学学报(自然科学版), 2020, 48(6): 39-49] |
ZHANG Q, DING H C, YAN X H, et al. Isolation and characterization of pigmentation mutants of Pyropia crispata (Bangiales, Rhodophyta). Journal of Shanghai Ocean University, 2019, 28(4): 526-534 [张倩, 丁洪昌, 严兴洪, 等. 皱紫菜色素突变体的分离与特性分析. 上海海洋大学学报, 2019, 28(4): 526-534] |
ZHANG T, LI J F, MA F, et al. Study of photosynthetic characteristics of the Pyropia yezoensis Thallus during the cultivation process. Journal of Applied Phycology, 2014, 26(2): 859-865 |
ZHANG T, LI Y F, MA L, et al. Lateral floret 1 induced the three-florets spikelet in rice. Proceedings of the National Academy of Sciences, 2017, 114(37): 9984-9989 |
ZHANG T, WEN H W, YUAN K, et al. Construction of ethyl methane sulfonate mutant library of Jinmai 47 and preliminary analysis of quality characteristics of high generation mutants. Journal of Nuclear Agricultural Sciences., 2021, 35(8): 1731-1739 [张婷, 温宏伟, 袁凯, 等. 晋麦47 EMS突变体库的构建及高代突变材料品质性状的初步分析. 核农学报, 2021, 35(8): 1731-1739] |
ZHANG X M, XUE Y G. Research progress of soybean mutant library construction. Soybean Science, 2016, 35(2): 345-350 [张小明, 薛永国. 大豆突变体库构建研究进展. 大豆科学, 2016, 35(2): 345-350] |
ZHANG X C, QIN S, MA J H, et al. The genetics of marine algae. Beijing: China Agriculture Press, 2005 [张学成, 秦松, 马家海, 等. 海藻遗传学. 北京: 中国农业出版社, 2005]
|
ZHAO S, DING H C, LIU Z J, et al. Induction and isolation of pigmentation mutant in Pyropia suborbiculata kjellman (Bangiales, Rhodophyta). Haiyang Xuebao, 2019, 41(2): 114-122 [赵爽, 丁洪昌, 刘长军, 等. 圆紫菜人工色素突变体的诱导与分离. 海洋学报, 2019, 41(2): 114-122] |



