2. 中国农业科学院研究生院 北京 100081;
3. 上海海洋大学 水产科学国家级实验教学示范中心 上海 201306;
4. 中国海洋大学三亚海洋研究院 海南省热带水产种质重点实验室 海南 三亚 572000
2. Graduate School of Chinese Academy of Agricultural Sciences, Beijing 100081, China;
3. National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China;
4. Key Laboratory of Tropical Aquatic Germplasm of Hainan Province, Sanya Oceanology Institute, Ocean University of China, Sanya, Hainan 572000, China
紫菜(Neopyropia)是一类生长于潮间带的大型经济红藻,广泛分布于寒带至热带海域,具有较高的经济价值及生态价值(章守宇等, 2019; 江涛等, 2021)。我国是紫菜生产大国,紫菜的栽培规模及产量均居世界之首,年产值约13亿美元(http://www.fao.org/fishery/statistics/global-aquaculture production/zh)。然而,由细菌、卵菌和病毒等引起的病害频繁发生,给养殖户带来了巨大的经济损失(Arasaki, 1947; Migita, 1969; Saito et al, 1972; Kim et al, 2016)。其中,由卵菌病原腐霉(Pythium sp.)引起的赤腐病(red rot disease)是紫菜栽培期最主要的病害,常导致紫菜病烂、空帘而绝收(Arasaki, 1947; Takahashi, 1970; Lee et al, 2015)。目前,生产上经常采用干出、冷藏、酸洗的方法来应对紫菜赤腐病(Fujita et al, 1980; Sakaguch et al, 2001; Akizuki et al, 2007)。将患病紫菜干燥至含水量为30%~40%并在–20℃冷藏1~2周,可使紫菜腐霉菌丝大量死亡,但紫菜腐霉的卵孢子对干燥及冷藏具有较好的耐受性,存活率为10%~30% (Fujita et al, 1980)。用pH 2.0~5.0的无机酸或有机酸处理患病紫菜,在腐霉孢子浓度较低的情况下可有效抑制赤腐病,但在腐霉孢子浓度较高时处理效果不明显(Sakaguchi et al, 2001; Akizuku et al, 2007)。此外,这些物理或化学的处理方法还存在一系列问题,如冷藏设备及场地会大幅度提高栽培成本;酸性杀菌剂不仅污染栽培海区,长期使用还会提高腐霉的耐酸性而影响防治效果(Hwang et al, 2009)。多年来,研究人员一直尝试选育或培育紫菜抗病品系,到目前为止还未得到对赤腐病完全免疫的品系(Polne-Fuller et al, 1984; Park et al, 2014、2015)。
在陆地农作物生产中利用有益的拮抗微生物或其代谢产物进行病害防控是一种绿色、安全、有效的方法(王丁等, 2018),这为经济海藻的病害防控提供了新思路。藻类及其生长环境存在多种多样的微生物,且处在动态变化中,这些微生物分泌的多种活性物质具有抗菌、抗病毒、抗寄生虫等活性(Singh et al, 2014、2015; 阎永伟等, 2022),是筛选生防微生物的良好来源。本研究的目的是从海藻及其生长环境中筛选和鉴定出对紫菜腐霉具有拮抗作用的细菌,为建立紫菜赤腐病的生物防治奠定基础。
1 材料与方法 1.1 供试菌株及培养基紫菜赤腐病病原为腐霉Pythium porphyrae和Pyt. chondricola。本研究所用的Pyt. porphyrae菌株(NBRC 30800、NBRC 33126、NBRC 100633和NBRC 33253)购自日本生物资源库(NITE Biological Resource Center, Department of Biotechnology, National Institute of Technology and Evaluation, Japan),Pyt. chondricola菌株(JS151205、PYTHT201801-1、RZ201902和LS201903)由本实验室分离自患赤腐病紫菜(邱丽萍, 2018; 何礼娟, 2020)。上述菌株保藏于中国水产科学研究院黄海水产研究所养殖生物病害控制与分子病理学研究室,在4℃传代保藏。培养紫菜腐霉的培养基为海水玉米培养基(SCM) (玉米粉2 g/L、酵母粉1 g/L、琼脂15 g/L、盐度为20的海水、pH调为7.0~7.5),常规培养温度为24℃。培养细菌的培养基为2216E海水培养基(MA) (蛋白胨5 g/L、酵母膏1 g/L、FePO4 0.01 g/L、琼脂15 g/L、陈海水),常规培养温度28℃,震荡条件为150 r/min。
1.2 细菌的分离和纯化采集紫菜的苗期贝壳丝状体、海区栽培期的叶状体、海带(Saccharina japonica)苗及相应的栽培水体进行细菌的分离。采集的样品放入无菌采样袋(海博生物)及水样采集袋(海博生物)带回实验室处理。紫菜贝壳丝状体用灭菌海水清洗表面3次,用无菌刮刀刮取约1 cm2贝壳丝状体;紫菜叶状体及海带苗用无菌刷子清除表面附着的杂质,再用灭菌海水清洗3次。所得的藻类样品用无菌研磨棒分别匀浆,用灭菌海水分别将匀浆液进行梯度稀释,取稀释度为10–2、10–3、10–4的稀释液100 μL涂布于MA上。水样也分别稀释至10–1、10–2和10–3,取100 μL涂布于MA上。将所有平板置于28℃培养2 d,挑取形态不同的单菌落进行纯化,–80℃保存在30%甘油里。
1.3 拮抗菌初筛用平板对峙法进行拮抗菌的初筛(Farhaoui et al, 2022)。用接种环挑取细菌菌株在MA上划线,在28℃培养活化2 d备用。供试紫菜腐霉菌株NBRC 33253接种在SCM上,在24℃培养7 d至长满平板后,用打孔器沿边缘菌丝将紫菜腐霉打成5 mm的圆形菌饼,将其接种在新的SCM平板中央,于24℃培养1 d后,以紫菜腐霉菌饼为中心画“十”字作为定位线,挑取细菌菌落在距离紫菜腐霉菌饼中心2 cm、垂直于定位线画1 cm短线,每平板可划4条短线,即接种4株菌株,对照组不接种细菌。将平板置于24℃培养,为高效筛得目标菌株,本实验选用腐霉最适生长温度24℃(何礼娟, 2020),待对照组紫菜腐霉直径长至约为4 cm时,观察接种细菌的周围是否出现抑菌圈。选出对紫菜腐霉有拮抗效果的菌株重复上述实验,在同一平板上接种相同菌株,每组3个平行,用游标卡尺量取定位线上紫菜腐霉边缘与细菌之间的距离。对具有拮抗能力的菌株重复上述筛选2次。
1.4 拮抗菌复筛用含毒介质法进行拮抗菌的复筛(何碧珀等, 2019)。将初筛得到的细菌单菌落接种到100 mL MA液体培养基中,在28℃、150 r/min条件下震荡培养72 h,取30 mL菌液于50 mL离心管中5000 r/min离心10 min,取上清液用0.22 μm膜过滤获得无菌滤液。将灭菌SCM冷却至40℃左右,按无菌滤液∶SCM= 1∶5的比例充分混匀后制备含无菌滤液平板。将紫菜腐霉NBRC 33253制成5 mm菌饼接种在含拮抗菌无菌滤液的平板中央,作为实验组;根据初筛结果选择1株无拮抗效果的菌株制备无菌滤液,用上述方法制备平板,接种紫菜腐霉菌饼作为阴性对照组;在不含无菌滤液的SCM平板上接种紫菜腐霉菌饼作为空白对照组。每组平板设3个平行。将平板置于24℃培养,至空白对照组紫菜腐霉长满平板(约7 d),用菌落分析仪(Synbiosis Protocol 2, 英国)拍摄平板照片,然后用显微镜(OLYMPUS BX53, 日本)的自带软件AJ-VERT的“任意多边形”工具圈取腐霉生长面积,计算抑菌率。重复上述实验2次。
| $ \text { 计算公式:抑菌率 }=\left(A_{\mathrm{b}}-A_{\mathrm{e}}\right) /\left(A_{\mathrm{b}}-A_{\mathrm{i}}\right) \times 100 \% $ |
式中,Ab为空白对照组的腐霉面积,Ae为实验组的腐霉面积,Ai为菌饼面积。
1.5 拮抗谱的测定用平板对峙法测定3株拮抗菌(P3、P6和P19)对8株腐霉的拮抗谱及拮抗能力。具体步骤为:挑取单菌落接种到MA液体培养基培养12 h,按10%的接种量转接至新鲜的MA液体培养基继续培养1~2 h,将细菌培养液5000 r/min离心10 min去除上清液,用灭菌海水悬浮细菌,重复离心、悬浮步骤3次,最后制成108 CFU/mL的菌悬液备用。将5 mm紫菜腐霉菌饼接种在SCM中央,1 d后按1.3中的定位方法,在距离菌饼2 cm处,点种菌悬液2 μL作为实验组,点种2 μL灭菌海水作为空白对照组。每组设3个平行,将所有平板置于15℃。为筛得具有防治赤腐病潜力的菌株,本实验选用温度为实验室腐霉侵染紫菜温度15℃,待空白组腐霉长满平板(10~15 d),计算抑制率,统计方法同1.4。重复上述实验2次。
用打孔器将抑菌圈边缘的腐霉菌丝打成5 mm直径的菌饼,用乳酸酚棉蓝染液(G1600, Solarbio)对腐霉菌饼进行染色,染色完成后用灭菌海水洗去菌饼表面残余的染液后制片,以空白对照组腐霉边缘菌丝为对照,用显微镜观察抑菌圈边缘菌丝的形态变化。
1.6 拮抗细菌的鉴定对细菌进行菌落形态观察、革兰氏染色(G1060, Solarbio)。用通用引物27F/1492R扩增拮抗菌的16S rRNA基因进行细菌鉴定(杨慧超, 2019)。对假交替单胞菌属细菌(Pseudoalteromonas sp.)进一步扩增管家基因dnaA、dnaN、recA基因序列,进行多位点序列分析(multilocus sequence analysis, MLSA)。根据GenBank中假交替单胞菌的dnaA、dnaN、recA序列,利用BioEdit软件(Alzohairy, 2011),识别序列中的高保守区域,设计引物。dnaA的引物序列为PdnaAF (5´-TTTAGCATGTGGGTAAGACC-3´)和PdnaAR (5´-TCTTGGTCTAGCAACTGAAC-3´),dnaN为PdnaNF (5´-TGCAAATAACGATTCCAAGAG-3´)和PdnaNR (T TTGCATCAGAAAGCGTAAA),recA为recA-F/recA-R (Beurmann et al, 2017)。利用MEGA-X软件将16S rRNA (1499 bp)、dnaA (1146 bp)、dnaN (955 bp)和recA (811 bp)基因序列按顺序连接,并进行比对,构建N-J系统发育树。
1.7 数据分析利用Excel和SPSS软件对实验数据进行统计分析,实验结果取平均值±标准差
从紫菜的贝壳丝状体、叶状体、海带及栽培水体中分离到385株细菌用于筛选拮抗菌。用平板对峙法进行初筛时,得到9株对腐霉NBRC 33253具有拮抗作用的菌株(图 1)。2次重复实验显示,拮抗菌的抑菌带宽度在1.65~16.54 mm之间(表 1)。
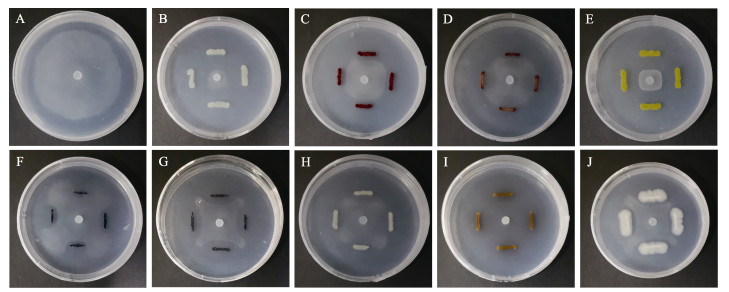
|
图 1 对峙生长法初筛腐霉拮抗菌 Fig.1 Preliminary screening of Pyt. porphyrae antagonistic bacteria by dual culture bioassays A:空白对照;B~J:P3(B)、P4(C)、P5(D)、P6(E)、P7(F)、P8(G)、P12(H)、P19(I)、B1(J)与腐霉的对峙生长 A: Blank control; B~J: Antagonistic growth of P3(B), P4(C), P5(D), P6(E), P7(F), P8(G), P12(H), P19(I), and B1(J) against Pyt. porphyrae |
|
|
表 1 拮抗菌的筛选 Tab.1 Screening of antagonistic bacteria |
用含毒介质法对这9株细菌进行复筛时,有3株菌株(P3、P6和P19)的无菌滤液对腐霉的生长产生了显著的抑制(图 2)。2次重复实验显示,这3株细菌的抑菌率为20.04%~30.09% (表 1)。根据这些结果,选取P3、P6和P19进行下一步实验。
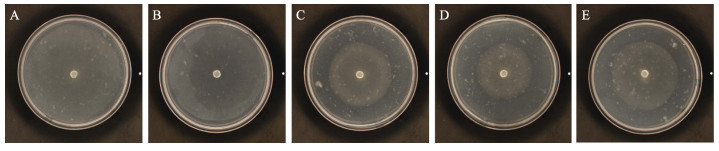
|
图 2 含毒介质法复筛拮抗菌 Fig.2 Secondary screening of antagonistic bacteria by toxic media method A:空白对照;B:阴性对照组;C~E:腐霉在含有P3(C)、P6(D)、P19(E)无菌滤液的培养基上生长 A: Blank control; B: Negative control; C~E: Pyt. porphyrae grew on the medium containing extracellular products from P3(C), P6(D) and P19(E) |
用平板对峙法检测了P3、P6和P19对8株腐霉的拮抗谱和拮抗能力。2次重复实验结果显示,15℃条件下,3株拮抗菌对8株腐霉均具有拮抗作用(图 3),其中,P3的拮抗能力最强,抑菌率达到(52.09%~ 97.95%),其次为P6 (26.81%~78.04%)和P19 (10.47%~ 41.91%) (表 2)。用乳酸棉酚蓝对对峙边缘的腐霉菌丝进行染色观察,结果显示,与对照组的腐霉菌丝相比,与拮抗菌P3和P19对峙的腐霉菌丝密度变稀疏、着色变浅,而与拮抗菌P6对峙的腐霉菌丝无明显变化(图 4)。
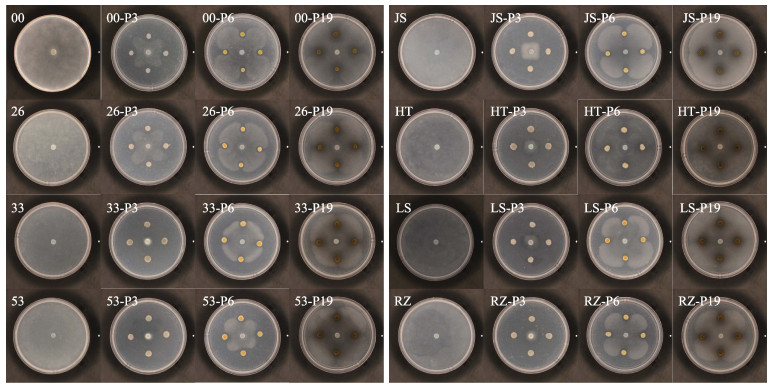
|
图 3 8株腐霉与3株拮抗菌的对峙生长图 Fig.3 Confrontation growth between Pythium and antagonistic bacteria 00、26、33、53、JS、HT、LS、RZ分别为腐霉NBRC 30800、NBRC 33126、NBRC 100633、NBRC 33253、JS151205、PYTHT201801-1、LS201903、RZ201902;00-P3、26-P3、33-P3、53-P3、JS-P3、HT-P3、LS-P3、RZ-P3分别为P3与腐霉NBRC 30800、NBRC 33126、NBRC 100633、NBRC 33253、JS151205、PYTHT201801-1、LS201903、RZ201902的对峙生长;00-P6、26-P6、33-P6、53-P6、JS-P6、HT-P6、LS-P6、RZ-P6分别为P6与腐霉NBRC 30800、NBRC 33126、NBRC 100633、NBRC 33253、JS151205、PYTHT201801-1、LS201903、RZ201902的对峙生长;00-P19、26-P19、33-P19、53-P19、JS-P19、HT-P19、LS-P19、RZ-P19分别为P19与腐霉NBRC 30800、NBRC 33126、NBRC 100633、NBRC 33253、JS151205、PYTHT201801-1、LS201903、RZ201902的对峙生长。 00, 26, 33, 53, JS, HT, LS, RZ represent Pythium NBRC 30800, NBRC 33126, NBRC 100633, NBRC 33253, JS151205, PYTHT201801-1, LS201903, RZ201902, respectively; 00-P3, 26-P3, 33-P3, 53-P3, JS-P3, HT-P3, LS-P3, RZ-P3 represent antagonistic growth of P3 against Pythium NBRC 30800, NBRC 33126, NBRC 100633, NBRC 33253, JS151205, PYTHT201801-1, LS201903, RZ201902, respectively; 00-P6, 26-P6, 33-P6, 53-P6, JS-P6, HT-P6, LS-P6, RZ-P6 represent antagonistic growth of P6 against Pythium NBRC 30800, NBRC 33126, NBRC 100633, NBRC 33253, JS151205, PYTHT201801-1, LS201903, RZ201902, respectively; 00-P19, 26-P19, 33-P19, 53-P19, JS-P19, HT-P19, LS-P19, RZ-P19 represent antagonistic growth of P19 against Pythium NBRC 30800, NBRC 33126, NBRC 100633, NBRC 33253, JS151205, PYTHT201801-1, LS201903, RZ201902, respectively. |
|
|
表 2 拮抗菌对腐霉生长的抑菌率 Tab.2 Growth inhibition rate of antagonistic bacteria against Pythium strains |

|
图 4 拮抗菌和腐霉对峙区域的腐霉菌丝显微观察(乳棉酚蓝染色) Fig.4 Microscopic observation of the Pythium mycelia growing in the confrontation area between Pythium and antagonistic bacteria (lactophenol cotton blue staining) |
菌株P3、P6和P19革兰氏染色均为阴性,其中,P6产黄色色素,P19产棕色色素,P3无色素产生。16S rRNA基因序列分析结果显示,3株拮抗菌均与假交替单胞菌的相似性最高,P3和P6为99.93%,P19为99.86%。将P3、P6和P19的4个基因串联(16S rRNA-dnaA-dnaN-recA)构建多位点序列的系统进化树,分析结果显示,P3和P6与杀鱼假交替单胞菌(Pseudoalteromonas piscicida)聚为一支,P19与解肽假交替单胞菌(Pseudoalteromonas peptidolytica)聚为一支(图 5)。
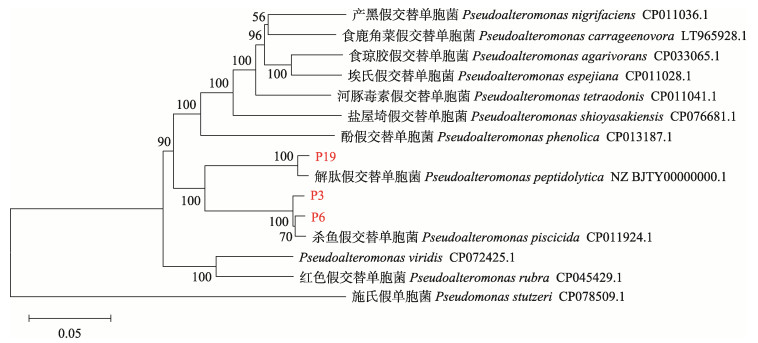
|
图 5 基于拮抗菌P3、P6和P19的16S rRNA-dnaA-dnaN-recA构建的N-J系统发育树 Fig.5 N-J phylogenetic tree based on 16S rRNA-dnaA-dnaN-recA of P3, P6, and P19 |
大型海藻在生长发育过程中会产生多种代谢物质到藻体表面,为微生物定植提供了适宜的基质和营养(Steinberg et al, 2002; Staufenberger et al, 2008; Singh et al, 2013)。附着在海藻表面的微生物群落可产生多种活性物质,这些物质不仅影响海藻的正常形态及生长发育,还可保护宿主免受有害生物的侵害。因此,大型海藻的附着微生物成为生物和医药领域的优选目标(Singh et al, 2014、2015)。本研究从紫菜和海带及其栽培水体分离细菌,通过平板对峙法筛选出9株具有抑制腐霉生长活性的细菌,并通过含毒介质法检测出3株细菌(P3、P6和P19)的胞外产物能够抑制腐霉的生长,表明这3株细菌通过拮抗作用抑制了腐霉的生长。其他6株细菌的胞外产物未检测到抑制腐霉生长的情况,说明这6株细菌通过竞争作用抑制了腐霉的生长。
通过16S rRNA鉴定及多位点序列分析,将P3和P6鉴定为杀鱼假交替单胞菌,将P19鉴定为解肽假交替单胞菌。多个研究表明,从海洋环境分离的假交替单胞菌能分泌具有抗菌、防污、杀藻等作用的活性物质,包括抗生素、胞外酶类物质和胞外毒素等(Holmstrom et al, 1999; Egan et al, 2001; Bowman, 2007; 孙星等, 2018; 练小军等, 2020)。大多数的研究报道了抗细菌活性物质的分离和鉴定,而对抗真菌、抗卵菌的活性物质的报道不多。Moree等(2014)报道了从健康珊瑚分离的一株假交替单胞菌产生的一种酰胺聚酮具有抗真菌活性;Franks等(2005)报道了一株P. tunicata产生的黄色色素具有抗真菌活性,并鉴定为生物碱。分离自海洋环境的一些杀鱼假交替单胞菌菌株被证实能够有效拮抗多种病原菌,包括创伤弧菌(Vibrio vulnificus)、副溶血弧菌(V. parahaemolyticus)、美人鱼发光杆菌(Photobacterium damselae)、金黄色葡萄球菌(Staphylococcus aureus)、白色念珠菌(Candida albicans)及枯草芽孢杆菌(Bacillus subtilis)等(Richards et al, 2017; Eliseikina et al, 2021);一些解肽假交替单胞菌具有淀粉酶、几丁质酶、木质纤维素酶活性(Venkateswaran et al, 2000; Johnson et al, 2021)。但这些分离株产生的抑菌物质尚不清楚。在本研究中,拮抗菌P3和P19与腐霉相互作用区域的腐霉菌丝出现乳酸棉酚蓝着色浅的情况,而与P6相互作用区域的菌丝无明显变化,说明3株细菌可能通过不同方式抑制腐霉生长。当阳性菌株溶解或死亡时,乳酸酚棉蓝染色常呈阴性,说明P3和P19分泌的活性物质有可能降解了腐霉菌丝或影响菌丝活性,导致着色变浅。
在水产养殖中,假交替单胞菌作为益生菌在防治养殖动物的病害中显示了良好的效果(Fjellheim et al, 2010; Goulden et al, 2012; Offret et al, 2019)。本研究筛选得到的拮抗菌P3、P6和P19,对引起紫菜赤腐病的致病性腐霉展示了显著的拮抗能力,显示了其作为益生菌用于防治紫菜赤腐病的生防潜力。在后续的研究中,我们将开展拮抗菌的安全性评价及其在预防和治疗紫菜赤腐病中的生防效果和应用,在此基础上探究拮抗菌的活性物质的鉴定及其生防机制。
AKIZUKI A, TABATA M, KAWAMURA Y. Disinfectant effects of lactic acid on Pythium porphyrae as acid treatment agent. Aquaculture Science, 2007, 55(3): 325-330 |
ALZOHAIRY A M. BioEdit: An important software for molecular biology. GERF Bulletin of Biosciences, 2011, 2(1): 60-61 |
ARASAKI S. Studies on the rot of Porohyra tenera by Pythium. Nippon Suisan Gakkaishi, 1947, 13: 74-90 DOI:10.2331/suisan.13.74 |
BEURMANN S, USHIJIMA B, VIDEAU P, et al. Pseudoalteromonas piratica strain OCN003 is a coral pathogen that causes a switch from chronic to acute Montipora white syndrome in Montipora capitata. PLoS One, 2017, 12(11): e0188319 DOI:10.1371/journal.pone.0188319 |
BOWMAN J P. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Marine Drugs, 2007, 5(4): 220-241 DOI:10.3390/md504220 |
EGAN S, HOLMSTRÖM C, KJELLEBERG S. Pseudoalteromonas ulvae sp. nov., a bacterium with antifouling activities isolated from the surface of a marine alga. International Journal of Systematic and Evolutionary Microbiology, 2001, 51(4): 1499-1504 |
ELISEIKINA M G, BELENEVA I A, KUKHLEVSKY A D, et al. Identification and analysis of the biological activity of the new strain of Pseudoalteromonas piscicida isolated from the hemal fluid of the bivalve Modiolus kurilensis (F. R. Bernard, 1983). Archives of Microbiology, 2021, 203(7): 4461-4473 DOI:10.1007/s00203-021-02432-1 |
FARHAOUI A, ADADI A, TAHIRI A, et al. Biocontrol potential of plant growth-promoting rhizobacteria (PGPR) against Sclerotiorum rolfsii diseases on sugar beet (Beta vulgaris L.). Physiological and Molecular Plant Pathology, 2022, 119: 101829 DOI:10.1016/j.pmpp.2022.101829 |
FJELLHEIM A J, KLINKENBERG G, SKJERMO J, et al. Selection of candidate probionts by two different screening strategies from Atlantic cod (Gadus morhua L. ) larvae. Veterinary Microbiology, 2010, 144(1/2): 153-159 |
FRANKS A, HAYWOOD P, HOLMSTRÖM C, et al. Isolation and structure elucidation of a novel yellow pigment from the marine bacterium Pseudoalteromonas tunicata. Molecules, 2005, 10(10): 1286-1291 DOI:10.3390/10101286 |
FUJITA Y, MIGITA S. Death of parasitic Pythium porphyrae by drying and freeze-preservation of red rot-infected thalli of Porphyra yezoensis. Bulletin of the Faculty of Fisheries Nagasaki University, 1980, 49: 11-16 |
GOULDEN E F, HALL M R, PEREG L L, et al. Identification of an antagonistic probiotic combination protecting ornate spiny lobster (Panulirus ornatus) larvae against Vibrio owensii infection. PLoS One, 2012, 7(7): e39667 DOI:10.1371/journal.pone.0039667 |
HE B P, ZHAO H, LIU H Y, et al. Inhibitory effects, growth-promotion evaluation and identification of two biocontrol bacteria against fungal diseases of sesame. Journal of Henan Agricultural Sciences, 2019, 48(11): 92-98 [何碧珀, 赵辉, 刘红彦, 等. 2株芝麻真菌病害生防菌的抑菌特征、促生评价及鉴定. 河南农业科学, 2019, 48(11): 92-98 DOI:10.15933/j.cnki.1004-3268.2019.11.013] |
HE L J. Isolation, identification and detection of two pathogenic oomycetes of Pyropia. Master´s Thesis of Ocean University of China, 2020 [何礼娟. 紫菜两种病原性卵菌的分离鉴定及检测. 中国海洋大学硕士研究生学位论文, 2020]
|
HOLMSTRÖM C, KJELLEBERG S. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiology Ecology, 1999, 30(4): 285-293 DOI:10.1111/j.1574-6941.1999.tb00656.x |
HWANG E K, CHAN S P, KAKINUMA M. Physicochemical responses of Pythium porphyrae (Oomycota), the causative organism of red rot disease in Porphyra to acidification. Aquaculture Research, 2009, 40(15): 1777-1784 DOI:10.1111/j.1365-2109.2009.02284.x |
JIANG T, FEI F, FANG T. Research progress on the bioactive components of Porphyra and its application. Food Research and Development, 2021, 42(21): 162-167 [江涛, 费帆, 方婷. 紫菜生物活性成分及其应用研究进展. 食品研究与开发, 2021, 42(21): 162-167] |
JOHNSON J, CHOI K Y. Enzymatic utilization of oil and lignocellulosic biomass using halophilic marine bacteria Micrococcus luteus and Pseudoalteromonas peptidolytica. 3 Biotech, 2021, 11(7): 360 DOI:10.1007/s13205-021-02902-9 |
KIM G H, KLOCHKOVA T A, LEE D J, et al. Chloroplast virus causes green-spot disease in cultivated Pyropia of Korea. Algal Research, 2016, 17: 293-299 DOI:10.1016/j.algal.2016.05.023 |
LEE S J, MI S H, PARK M A, et al. Molecular identification of the algal pathogen Pythium chondricola (Oomycetes) from Pyropia yezoensis (Rhodophyta) using ITS and cox1 markers. Algae, 2015, 30(3): 217-222 DOI:10.4490/algae.2015.30.3.217 |
LIAN X J, ZHU K L, ZHANG Q Q, et al. Effects of probiotics-supplemented diets on the growth and survival of Litopenaeus vannamei carrying multiple pathogens. Progress in Fishery Sciences, 2020, 41(2): 121-130 [练小军, 朱开玲, 张庆起, 等. 饲料添加益生菌对多病原阳性的凡纳滨对虾生长与存活的影响. 渔业科学进展, 2020, 41(2): 121-130 DOI:10.19663/j.issn2095-9869.20190316001] |
MIGITA S. Olpidiopsis disease of culture Porphyra. Bulletin of the Faculty of Fisheries Nagasaki University, 1969, 28: 131-145 |
MOREE W J, MCCONNELL O J, NGUYEN D D, et al. Microbiota of healthy corals are active against fungi in a light-dependent manner. ACS Chemical Biology, 2014, 9(10): 2300-2308 DOI:10.1021/cb500432j |
OFFRET C, ROCHARD V, LAGUERRE H, et al. Protective efficacy of a Pseudoalteromonas strain in European Abalone, Haliotis tuberculata, infected with Vibrio harveyi ORM4. Probiotics and Antimicrobial Proteins, 2019, 11: 239-247 DOI:10.1007/s12602-018-9389-8 |
PARK C S, HWANG E K. Biochemical characterization of Pyropia yezoensis-AP1 strain accompanies the resistance reaction to the red rot disease pathogen, Pythium porphyrae. Journal of Applied Phycology, 2015, 27(5): 2149-2156 DOI:10.1007/s10811-015-0527-3 |
PARK C S, HWANG E K. Isolation and evaluation of a strain of Pyropia yezoensis (Bangiales, Rhodophyta) resistant to red rot disease. Journal of Applied Phycology, 2014, 26(2): 811-817 DOI:10.1007/s10811-013-0183-4 |
POLNE-FULLER M, GIBOR A. Developmental studies in Porphyra. I. blade differentiation in Porphyra perforate as expressed by morphology enzymatic digestion, and protoplast regeneration. Journal of Phycology, 1984, 20(4): 609-616 |
QIU L P. Isolation, identification and characterization of Pythium porphyrae JS151205. Master´s Thesis of Ocean University of China, 2018 [邱丽萍. 紫菜腐霉JS151205的分离鉴定及生长特性研究. 中国海洋大学硕士研究生学位论文, 2018]
|
RICHARDS G P, WATSON M A, NEEDLEMAN D S, et al. Mechanisms for Pseudoalteromonas piscicida-induced killing of vibrios and other bacterial pathogens. Applied and Environmental Microbiology, 2017, 83(11): e00175-17 |
SAITO Y, MATSUSATO T, YOSHIKAWA K. On the symptoms of "green spot" and "crape" in Nori (Porphyra) culture. Bulletin of Nansei National Fisheries Research Institute, 1972, 5: 1-9 |
SAKAGUCHI K, PARK C S, KAKINUMA M, et al. Effects of varying temperature, salinity, and acidity in the treatment of Porphyra infected by red rot disease. Japanese Society for Aquaculture Science, 2001, 49(1): 77-83 |
SINGH R P, KUMARI P, REDDY C R K. Antimicrobial compounds from seaweeds-associated bacteria and fungi. Applied Microbiology and Biotechnology, 2015, 99(4): 1571-1586 DOI:10.1007/s00253-014-6334-y |
SINGH R P, MAHENDRA K S, MISHRA A, et al. Bacterial extracellular polymeric substances and their effect on settlement of zoospore of Ulva fasciata. Colloids and Surfaces B: Biointerfaces, 2013, 103: 223-230 DOI:10.1016/j.colsurfb.2012.10.037 |
SINGH R P, REDDY C R K. Seaweed-microbial interactions: Key functions of seaweed-associated bacteria. FEMS Microbiology Ecology, 2014, 88(2): 213-230 DOI:10.1111/1574-6941.12297 |
STAUFENBERGER T, THIEL V, WIESE J, et al. Phylogenetic analysis of bacteria associated with Laminaria saccharina. FEMS Microbiology Ecology, 2008, 64(1): 65-77 DOI:10.1111/j.1574-6941.2008.00445.x |
STEINBERG P D, DE NYS R, KJELLEBERG S. Chemical cues for surface colonization. Journal of Chemical Ecology, 2002, 28(10): 1935-1951 DOI:10.1023/A:1020789625989 |
SUN X, XIANG Y Y, BEN Z Y, et al. Optimization of antimicrobial culture condition of Pseudoalteromonas. SW-1. Chinese Agricultural Science Bulletin, 2018, 34(33): 96-100 [孙星, 向玉勇, 贲宗友, 等. 假交替单胞菌Pseudoalteromonas. SW-1抑菌培养条件的优化. 中国农学通报, 2018, 34(33): 96-100] |
TAKAHASHI M. Identification of genus Pythium. Plant Protection Science, 1970, 24(8): 339-346 |
VENKATESWARAN K, DOHMOTO N. Pseudoalteromonas peptidolytica sp. nov., a novel marine mussel-thread-degrading bacterium isolated from the Sea of Japan. International Journal of Systematic and Evolutionary Microbiology, 2000, 50(2): 565-574 |
WANG D, YANG X, LIU C L, et al. Research progress on biological pesticide. Journal of Green Science and Technology, 2018(24): 179-181 [王丁, 杨雪, 刘春雷, 等. 生物农药研究进展. 绿色科技, 2018(24): 179-181] |
YAN Y W, YANG H C, MO Z L, et al. Epiphytic microbial communities associated with Neopyropia yezoensis with Olpidiopsis disease. Progress in Fishery Sciences, 2022, 43(3): 165-175 [阎永伟, 杨慧超, 莫照兰, 等. 患拟油壶菌病条斑紫菜表面附生菌群分析. 渔业科学进展, 2022, 43(3): 165-175] |
YANG H C. Diseases investigation of Pyropia yezoensis and development of PCR detection method for Pyropia yezoensis green spot disease. Master´s Thesis of Shanghai Ocean University, 2019 [杨慧超. 条斑紫菜(Pyropia yezoensis)的病害调查及其绿斑病病原的PCR检测. 上海海洋大学硕士研究生学位论文, 2019]
|
ZHANG S Y, LIU S R, ZHOU X J, et al. Ecological function of seaweed-formed habitat and discussion of its application to sea ranching. Journal of Fisheries of China, 2019, 43(9): 2004-2014 [章守宇, 刘书荣, 周曦杰, 等. 大型海藻生境的生态功能及其在海洋牧场应用中的探讨. 水产学报, 2019, 43(9): 2004-2014] |



