2. 农业农村部海水养殖病害防治重点实验室 中国水产科学研究院黄海水产研究所 山东 青岛 266071;
3. 青岛海洋科学与技术试点国家实验室海洋渔业科学与食品产出过程功能实验室 山东 青岛 266071
2. Key Laboratory of Maricultural Organism Disease Control, Ministry of Agriculture and Rural Affairs, Yellow Sea Fisheries Research Institute, Chinese Academic of Fishery Sciences, Qingdao 266071, China;
3. Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266071, China
进入21世纪以来,我国的海水养殖产业发展迅猛,养殖规模不断扩大,产量不断增加,但随之而来的养殖密度过高、水环境恶化等诸多问题导致病害频发,给产业造成了严重的经济损失。其中,细菌性疾病成为海水养殖中最常见的问题之一,哈维氏弧菌(Vibrio harveyi)、副溶血弧菌(Vibrio parahaemolyticus)、大菱鲆弧菌(Vibrio scophthalmi)、鳗弧菌(Vibrio anguillarum)和美人鱼发光杆菌美人鱼亚种(Photobacterium damselae subsp. damselae)是近年来频繁见于文献报道的几种重要的海水养殖动物致病菌,可感染海水鱼类(张飞等, 2012; 陈吉祥等, 2005)、对虾(陈月忠等, 2000; 李翠苹等, 2020)和贝类(Hidalgo et al, 2008)等多种海水经济动物发病。这些病原菌具有致病性强、流行面积广、致死率高等显著特点,对水产养殖生产危害很大,加强对这些病原菌的快速检测技术研究,有助于有效防控其传播和感染,减少养殖业的经济损失。
荧光定量PCR技术因在检测通量、精准性、灵敏度以及成本方面的突出优势而得到迅速发展(何军霞等, 2021),被广泛应用于食品安全(雷庆等, 2021)、医学诊断(Fidalgo et al, 2021)和畜牧兽医(张若楠等, 2020)等众多领域。微流控技术(microfluidics)又被称为芯片实验室,是一种以在微米尺度空间对流体进行操控为主要特征的科学技术(陈京等, 2020)。将微流控技术与荧光定量PCR技术相结合,形成的微流控荧光定量芯片检测是利用微流控器件热质量小和比面积高等特点实现快速传热,从而缩短PCR扩增过程中稳定温度需要的时间,与常规的实验室荧光定量PCR仪器相比,微流控荧光定量PCR技术具有检测所需样品量少、升降温速度快、耗时短、成本低、便于携带、环境条件要求低等显著特点,在疾病临床诊断(王可可等, 2018)、食品检测(吴姗等, 2009)和畜牧兽医(王楷宬等, 2021)等多个领域已经得到广泛应用。
本研究基于微流控荧光定量PCR技术,针对哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种设计特异性引物,通过优化反应体系和反应条件,进行这5种病原菌在微流控检测芯片的集成应用,实现重要海水养殖病原菌的微流控荧光定量PCR一步式快速检测,研究结果将为开发在水产养殖现场应用的病原菌多重微流控检测技术奠定基础。
1 材料与方法 1.1 实验菌株本研究使用15种细菌,共计28株。其中,灿烂弧菌(Vibrio splendidus)标准菌株购自中国科学院微生物研究所中国普通微生物菌种保藏管理中心(China General Microbiological Culture Collection Center, CGMCC),坎贝氏弧菌(Vibrio campbelii)等11株标准菌株购自自然资源部第三海洋研究所海洋微生物菌种保藏管理中心(Marine Culture Collection of China, MCCC),哈维氏弧菌等17株菌株由本实验室分离自不同的患病水产动物。菌株具体信息见表 1。
|
|
表 1 实验所用菌株 Tab.1 The strains for testing |
本研究选用UF-150微流控荧光定量PCR仪(GENECHECKERTM Ultra-Fast Real-time PCR System),由韩国Genesystem公司生产,其微流控反应芯片由聚甲基丙烯酸甲酯材料制成,尺寸大小为38 mm×25 mm× 6 mm (图 1)。该芯片共有10个反应通道且都标有孔号,奇数孔号在芯片顶部,偶数孔号在芯片底部,加样孔位于孔号一侧,每个加样孔对面是通气孔,反应通道内的空气和加样时的过量液体会通过通气孔排出。每个反应通道的容量为10 μL,将荧光染料预混液、引物和待测样品混匀后加入不同反应通道后,用封膜胶带密封每个孔,以防止在热循环期间样品蒸发和污染。反应结束后,可通过查看反应通道的荧光信号强弱和扩增曲线来判定结果。

|
图 1 微流控芯片结构图与实物 Fig.1 Microfluidic chip structure diagram and product (a)结构图;(b)实物;①、⑤:芯片翼;②:孔号;③:加样孔;④:通气孔。 (a) Structure of the chip; (b) Product of the chip; ①, ⑤: Chip wing; ②: Hole numbers; ③: Loading hole; ④: Vent hole. |
首先将保存于–80 ℃的28株细菌接种于TSB培养基,28 ℃培养18 h活化后,挑取单菌落转接至新的TSB平板培养基,在28 ℃下纯化培养18 h,然后挑取1~2个单菌落于100 μL的无菌水中混匀,100 ℃金属浴15 min,再12 000 r/min离心5 min,收集上清液即为细菌的DNA模板,保存于–20 ℃备用。
1.4 引物设计及特异性验证根据GenBank中登录的哈维氏弧菌vhhA基因、副溶血弧菌toxR基因和大菱鲆弧菌luxR基因的保守序列,用Primer Premier 5.0软件设计特异性引物,再通过NCBI中Blast验证引物的特异性。鳗弧菌empA基因和美人鱼发光杆菌美人鱼亚种Mcp基因的特异性引物分别参考本实验室已有研究结果(刘智超, 2012; Zhang et al, 2021)。设计好的引物序列交由生工生物工程(上海)股份有限公司合成,引物序列见表 2。
|
|
表 2 5株病原菌的特异性引物序列 Tab.2 The specific primer sequences of 5 pathogen strains |
以哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种作为检测目标菌,坎贝氏弧菌等其他10种细菌作为对照菌,无菌水作为空白对照,应用实验室常规实时荧光定量PCR仪(Eppendorf AG 22331 Hamburg, 德国)进行扩增,验证各引物的特异性。哈维氏弧菌、副溶血弧菌、大菱鲆弧菌反应体系为2×Taq Pro Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd) 10 μL,Primer F/R各0.4 μL,DNA模板1 μL,ddH2O 8.2 μL。反应条件为95 ℃预变性2 min,95 ℃变性5 s,60 ℃退火30 s,扩增40个循环。鳗弧菌和美人鱼发光杆菌美人鱼亚种反应体系与反应条件参考刘智超(2012)和Zhang等(2021)。
将哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种分别应用UF-150微流控荧光定量PCR仪进行微流控荧光定量PCR扩增,验证引物在微流控仪器上应用的可行性和特异性,反应总体系设定为10 μL:2×Taq Pro Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd) 5 μL,Primer F/R各1 μL,DNA模板2 μL,ddH2O 1 μL。反应条件为95 ℃预变性30 s,95 ℃变性5 s,退火时间30 s,退火温度与普通实时荧光定量PCR一致,扩增30个循环。
1.5 标准曲线的建立将上述特异性引物分别进行普通PCR扩增,扩增产物切胶回收后连接至PMD-19T载体,转化至DH-5α感受态细胞,增菌培养进行菌液PCR扩增后测序筛选出阳性菌作为标准品。将标准品增菌培养后提取质粒,按10倍梯度稀释6个梯度分别进行荧光定量PCR扩增并绘制标准曲线。
1.6 多病原微流控荧光定量PCR快速检测条件优化分别用哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种的DNA模板,比较单重微流控荧光定量PCR的退火温度,分别设定反应温度为58、59、60、61、62和63 ℃进行扩增,最终筛选5株菌最佳的共同退火温度。同时分别设反应循环数为25、30、35、40,验证最佳反应循环数。
1.7 集成芯片引物交叉反应分析将设计好的哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种的特异性引物集成到微流控芯片上,并用制备好的5株菌的DNA模板在集成的芯片上进行多重微流控荧光定量PCR扩增,验证引物间有无交叉反应。反应总体系设定为10 μL:2×Taq Pro Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd) 5 μL,Primer F/R各1 μL,DNA模板2 μL,ddH2O 1 μL。反应条件为95 ℃预变性30 s,95 ℃变性5 s,60 ℃退火30 s,扩增30个循环。
1.8 多病原微流控荧光定量PCR快速检测灵敏度分析按1.3章节相同的方法,将哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种划线接种于TSB平板培养基进行纯化培养。取适量纯化好的菌苔用无菌PBS溶液分别配制成4.0× 106、2.0×106、2.0×106、5.0×106和2.0×106 CFU/mL浓度的菌悬液,按10倍梯度依次稀释6个梯度,涂布计数以验证其浓度的准确性。将不同浓度梯度的菌悬液按照1.3章节相同的方法制备细菌DNA模板,以灭菌的去离子水为阴性对照,在优化后的反应条件下分别进行多重微流控荧光定量PCR反应,确定该方法的最低检测限。
1.9 多病原微流控荧光定量PCR快速检测技术的应用效果选取大菱鲆(Scophthalmus maximus)、凡纳滨对虾(Litopenaeus vannamei)、仿刺参(Apostichopus japonicus)为实验对象,分别取大菱鲆的肌肉、鳃、肝组织,凡纳滨对虾的肌肉、鳃组织,仿刺参的呼吸树、肠道组织各0.1 g,分别加入100 μL哈维氏弧菌(1.0× 106 CFU/mL)、副溶血弧菌(1.2×106 CFU/mL)、大菱鲆弧菌(1.6×106 CFU/mL)、鳗弧菌(2.3×106 CFU/mL)和美人鱼发光杆菌美人鱼亚种(8.0×106 CFU/mL)的菌液,同时,以未加菌液的组织作为对照组,将组织研磨匀浆后取10 μL加入100 μL裂解液(Vazyme Biotech Co., Ltd)混匀,100 ℃金属浴15 min后加入100 μL稳定液(Vazyme Biotech Co., Ltd)混匀,12 000 r/min离心5 min,取上清液作为模板,在已经优化好的反应条件下进行多重微流控荧光定量PCR扩增,验证检测效果。
2 结果 2.1 引物特异性验证应用所设计的引物分别进行普通荧光定量PCR和微流控荧光定量PCR反应,引物特异性验证的结果显示,哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种DNA出现特异性扩增曲线,其他细菌DNA及阴性对照无扩增曲线出现(图 2),表明所设计的5种菌的引物特异性良好。

|
图 2 引物特异性验证 Fig.2 Primer specificity validation a:普通实时荧光定量PCR特异性实验;b:微流控荧光定量PCR特异性实验;Ⅰ:哈维氏弧菌;Ⅱ:副溶血弧菌;Ⅲ:大菱鲆弧菌;Ⅳ:鳗弧菌;Ⅴ:美人鱼发光杆菌美人鱼亚种。 a: Conventional real-time quantitative PCR specificity test; b: Microfluidic fluorescence quantitative PCR specific test; Ⅰ: V. harveyi; Ⅱ: V. parahaemolyticus; Ⅲ: V. scophthalmi; Ⅳ: V. anguillarum; Ⅴ: P. damselae subsp. damselae. |
在单重微流控荧光定量PCR反应条件的基础上,对多重微流控荧光定量PCR方法的反应条件进行优化。最佳反应条件下的反应结果如图 3所示,最终确定多重微流控荧光定量PCR反应体系为2×Taq Pro Universal SYBR qPCR Master Mix 5 μL,Primer F/R各1 μL,DNA模板2 μL,ddH2O 1 μL。反应条件为95 ℃ 30 s,95 ℃ 5 s,61 ℃ 30 s,扩增30个循环。
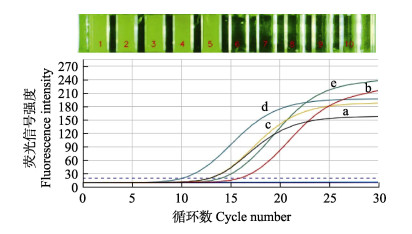
|
图 3 退火温度61 ℃时微流控荧光定量PCR反应结果 Fig.3 Microfluidic fluorescence quantitative PCR result at annealing temperature 61 ℃ 1、6:哈维氏弧菌引物;2、7:鳗弧菌引物;3、8:美人鱼发光杆菌美人鱼亚种引物;4、9:副溶血弧菌引物;5、10:大菱鲆弧菌引物;6~10:空白对照;a:哈维氏弧菌;b:鳗弧菌;c:美人鱼发光杆菌美人鱼亚种;d:副溶血弧菌;e:大菱鲆弧菌。 1, 6: Primers of V. harveyi; 2, 7: Primers of V. anguillarum; 3, 8: Primers of P. damselae subsp. damselae; 4, 9: Primers of V. parahaemolyticus; 5, 10: Primers of V. scophthalmi; 6~10: Negative control; a: V. harveyi; b: V. anguillarum; c: P. damselae subsp. damselae; d: V. parahaemolyticus; e: V. scophthalmi. |
将哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种的质粒标准品分别按10倍梯度稀释6个梯度,进行荧光定量PCR扩增,并绘制标准曲线。结果如图 4所示,哈维氏弧菌的标准曲线在2.42×104~2.42×109copies/μL浓度范围内有良好的线性相关性(y= –2.972x+6.73, R2=0.999),副溶血弧菌的标准曲线在3.83×104~3.83×109 copies/μL浓度范围内有良好的线性相关性(y= –3.287x+7.48, R2= 0.998),大菱鲆弧菌在2.87×104~2.87×109 copies/μL浓度范围内有良好的线性相关性(y= –3.549x+8.14, R2= 0.998),鳗弧菌在1.39×104~1.39×109 copies/μL浓度范围内有良好的线性相关性(y= –3.912x+7.83, R2= 0.999),美人鱼发光杆菌美人鱼亚种在2.20×104~ 2.20×109 copies/μL浓度范围内有良好的线性相关性(y= –3.969x+7.07, R2=0.992)。
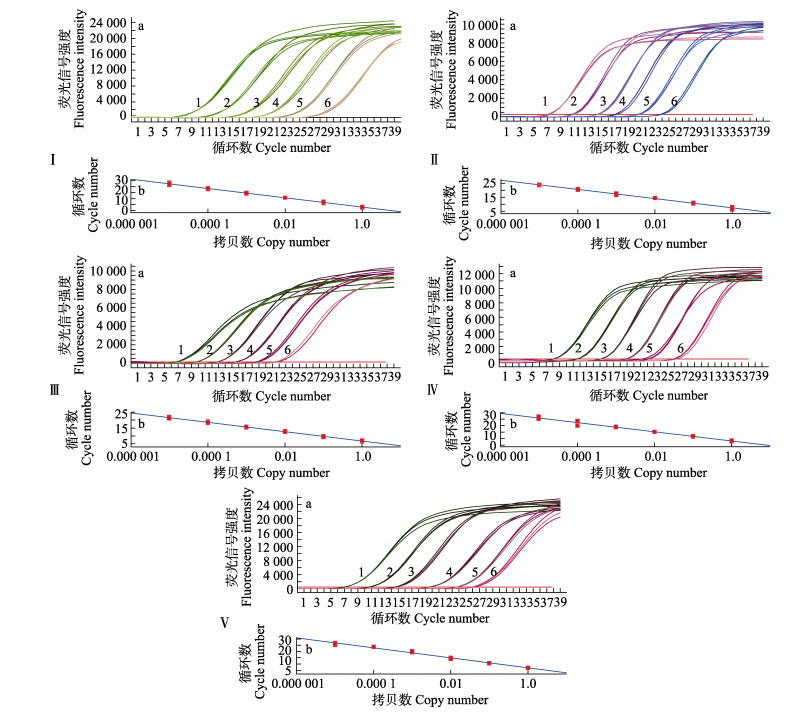
|
图 4 质粒标准品的扩增曲线和标准曲线 Fig.4 Amplification curve and standard curve of plasmid standard a:扩增曲线;b:标准曲线;1~6分别为109、108、107、106、105和104 copies /μL;Ⅰ:哈维氏弧菌;Ⅱ:副溶血弧菌;Ⅲ:大菱鲆弧菌;Ⅳ:鳗弧菌;Ⅴ:美人鱼发光杆菌美人鱼亚种 a: Amplification curve; b: Standard curve; 1~6 indicate 109, 108, 107, 106, 105 and 104 copies /μL, respectively; Ⅰ: V. harveyi; Ⅱ: V. parahaemolyticus; Ⅲ: V. scophthalmi; Ⅳ: V. anguillarum; Ⅴ: P. damselae subsp. damselae |
将5对引物集成到同一芯片上,进行引物间交叉反应分析。结果显示,5对引物在同一芯片上交叉反应影响较小,在25个反应循环数内不影响结果判读(图 5)。
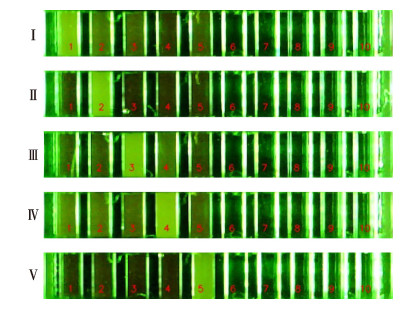
|
图 5 引物交叉反应的微流控荧光定量PCR分析结果 Fig.5 Results of cross reaction analysis through microfluidic fluorescence quantitative PCR 荧光信号强证明发生阳性反应。Ⅰ:哈维氏弧菌;Ⅱ:鳗弧菌;Ⅲ:美人鱼发光杆菌美人鱼亚种;Ⅳ:副溶血弧菌;Ⅴ:大菱鲆弧菌;1、6:哈维氏弧菌引物;2、7:鳗弧菌引物;3、8:美人鱼发光杆菌美人鱼亚种引物;4、9:副溶血弧菌引物;5、10:大菱鲆弧菌引物;6~10:空白对照。 The apparent turbidity of the channel was positive reaction. Ⅰ: V. harveyi; Ⅱ: V. anguillarum; Ⅲ: P. damselae subsp. damselae; Ⅳ: V. parahaemolyticus; Ⅴ: V. scophthalmi; 1, 6: Primers of V. harveyi; 2, 7: Primers of V. anguillarum; 3, 8: Primers of P. damselae subsp. damselae; 4, 9: Primers of V. parahaemolyticus; 5, 10: Primers of V. scophthalmi; 6~10: Negative control. |
将5种病原菌的特异性引物集成到同一个微流控芯片上,分别用哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种DNA作为模板,在优化好的体系和反应条件下,同时进行微流控荧光定量PCR扩增,验证该方法的灵敏度。实验结果如图 6,集成后的微流控芯片对上述5种病原菌的最低检测限分别为40 CFU/mL (2.0×10–3 ng/μL)、20 CFU/mL (2.0×10–3 ng/μL)、200 CFU/mL (2.0× 10–2 ng/μL)、500 CFU/mL (1.0×10–2 ng/μL)、20 CFU/mL (1.0×10–3 ng/μL)。因此,判定集成后的芯片对5种病原菌的最低可检限为100 CFU/mL (0.01 ng/μL),具有较高的灵敏度。

|
图 6 微流控荧光定量PCR集成芯片对5种病原菌同步检测的灵敏度验证结果 Fig.6 Sensitivity of multiplex microfluidic quantitative PCR on the 5 pathogenic bacteria Ⅰ~Ⅵ分别为105、104、103、102、101和100 CFU/mL的菌液;1:哈维氏弧菌;2:副溶血弧菌;3:美人鱼发光杆菌美人鱼亚种;4:鳗弧菌;5:大菱鲆弧菌;6~10:空白对照。 Ⅰ~Ⅵ indicated 105, 104, 103, 102, 101 and 100 CFU/mL bacteria suspension, respectively; 1: V. harveyi; 2: V. parahaemolyticus; 3: P. damselae subsp. damselae; 4: V. anguillarum; 5: V. scophthalmi; 6~10: Negative control. |
选取大菱鲆、凡纳滨对虾、仿刺参为实验对象,验证5种病原菌在大菱鲆(肌肉、鳃、肝)、凡纳滨对虾(肌肉、鳃)、仿刺参(呼吸树、肠道)组织中的检出效果,筛选出最佳检测部位。应用已建立的多重微流控荧光定量PCR检测方法对含有5种病原菌的上述组织进行样品检测,同时应用实验室常规实时荧光定量PCR进行同步检测作对比。结果如表 3所示,普通实时荧光定量PCR检测样品结果分别为100% (21/21)、100% (21/21)、100% (21/21)、100% (21/21)、95.2% (20/21)。所建立的多重微流控荧光定量PCR检测方法的准确率达到普通实时荧光定量PCR检测方法的96.2%以上,实验中未加菌液的组织检测结果均为阴性。样品平均检测时间仅为26 min,检测时长较普通实时荧光定量PCR反应时间(1 h 40 min)明显缩短。
|
|
表 3 微流控荧光定量PCR检测不同组织中5种病原菌的应用效果 Tab.3 Results of microfluidic fluorescence quantitative PCR in different tissues of 5 pathogens |
近年来,病害频发导致了水产养殖业严重的经济损失,2020年我国水产病害损失约为589亿元,达到了历史最高值(崔利锋, 2021)。哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种是目前文献报道相对频繁的几种海水养殖主要致病菌,可感染鱼类(Qi et al, 2021; 兰欣等, 2020; 靳晓敏等, 2015; Mei et al, 2010; Kim et al, 2013)、凡纳滨对虾(Paria et al, 2021; 郑国兴等, 1990)和贝类(刘小莉等, 2017; Hidalgo et al, 2008)等多种海水经济动物。此外,美人鱼发光杆菌美人鱼亚种对鲸鱼和海豚等海洋哺乳动物(Rivas et al, 2013)以及人类也有致病性(Alvarez et al, 2006)。加强对上述病原菌的快速检测技术研究,可为防控这些病原菌的感染和传播提供重要的技术支撑。
目前,对病原菌的检测技术研究通常选用管家基因和毒力基因作为靶基因。本研究分别选取了哈维氏弧菌的vhhA基因、副溶血弧菌的toxR基因、大菱鲆弧菌的luxR基因、鳗弧菌的empA基因和美人鱼发光杆菌美人鱼亚种的Mcp基因作为目标基因开展快速检测技术研究。vhhA基因是哈维氏弧菌的溶血素编码基因,最早在高致病性毒株VIB 645中被发现(Zhang et al, 2005),在NCBI上Blast比对发现该基因序列与哈维氏弧菌簇下其他菌种的溶血素基因相似度在87%以下。毒力基因toxR最先在霍乱弧菌(Vibrio cholerae)中被报道,后来陆续在副溶血弧菌、创伤弧菌(Vibrio vulnificus)、鳗弧菌、拟态弧菌(Vibrio mimicus)、溶藻弧菌(Vibrio alginolyticus)等多种弧菌中证实了该基因的存在(Lin et al, 1993)。有研究发现,异种细菌之间的toxR基因序列相似性较低,如副溶血弧菌和溶藻弧菌的toxR基因同源性仅为61.7%,可在种的水平上用于检测副溶血弧菌(Kim et al, 1999)。luxR基因是家族蛋白调控基因,广泛存在于革兰氏阴性菌中(Chaparian et al, 2016)。高志鑫等(2015)以大菱鲆弧菌的luxR基因为靶点设计LAMP扩增引物,建立了大菱鲆弧菌LAMP检测方法。鳗弧菌的empA是金属蛋白酶的编码基因,金属蛋白酶被认为是鳗弧菌侵入宿主体内不可缺少的重要因子(刘小莉等, 2017)。将病原菌特有的金属蛋白酶基因作为靶基因来检测病原菌,具有灵敏度高、特异性强等优势(余俊红等, 2002)。Mcp基因是美人鱼发光杆菌美人鱼亚种特有基因,可作为美人鱼发光杆菌美人鱼亚种检测的靶基因(Zhang et al, 2021)。本研究分别选用这5个基因作为检测目标菌的靶基因,均具有良好的种间特异性,为5种病原菌多重微流控荧光定量PCR检测方法的建立奠定了基础,保障所建立的检测技术具有较高的特异性和灵敏度。
微流控芯片最早由Manz等(1990)提出,是通过细微加工技术将微型功能元件组建成具有微流体控制的集成分析系统,具有自动化、集成化、便携化、成本低和快速化等特点(朱婧旸等, 2021)。本实验室在前期的研究中,已经针对单一的病原菌开展了微流控快速检测技术的研究(陈京等, 2020; Zhang et al, 2021)。而将不同病原菌的反应体系和反应条件在同一芯片进行集成化,可以满足多样品和多病原的实时、快速检测需求,显著提高检测效率,进而可开发应用于养殖现场的多病原微流控同步快速检测技术。余东阳等(2020)结合微流控与荧光定量PCR技术建立了寨卡病毒(ZIKV)、登革病毒(DENV)、黄热病毒(YFV)和基孔肯雅病毒(CHIKV)的快速核酸检测方法,25 min内即可完成1次上述4种病毒的同步快速检测,适用于现场检测与早期诊断。El-tholoth等(2021)基于微流控装置建立了环介导等温扩增(RT-LAMP)技术,实现了猪流行性腹泻病毒(PEDV)、传染性胃肠炎病毒(TGEV)和猪三角洲冠状病毒(PDCoV)的同步检测,耗时仅需30 min。但在水产领域,微流控检测技术研究相对较少,也未开展实践应用。本研究基于UF-150微流控荧光定量PCR仪平台,通过对微流控芯片的集成应用,建立了针对水产动物5种常见病原菌的多重微流控荧光定量PCR检测方法,实现了在同一检测芯片特异性扩增哈维氏弧菌、副溶血弧菌、大菱鲆弧菌、鳗弧菌和美人鱼发光杆菌美人鱼亚种5种病原菌,并且具有较高的灵敏度。通过应用所建立的多重微流控荧光定量PCR检测方法对水产动物样品进行临床检测,以及与实验室普通荧光定量PCR检测准确率进行对比,发现该方法检测动物组织样品的准确率可达到实验室普通实时荧光定量PCR检测方法准确率的96.2%以上,但检测时间只需26 min左右,较实验室普通实时荧光定量PCR的检测时间明显缩短,为进一步开发实用化的水产多病原现场快速检测技术奠定了工作基础。
ALVAREZ J R, LAMBA S, DYER K Y, et al. An unusual case of urinary tract infection in a pregnant woman with Photobacterium damsel. Infectious Diseases in Obstetrics and Gynecology, 2006(2): 80682 |
CHAPARIAN R R, OLNEY S G, HUSTMYER C M, et al. Integration host factor and LuxR synergistically bind DNA to coactivate quorum-sensing genes in Vibrio harveyi. Molecular Microbiology, 2016, 101(5): 823-840 DOI:10.1111/mmi.13425 |
CHEN J X, YANG H, YAN X H, et al. Cloning of hemolysin gene from a pathogenic Vibrio harveyi SF1 and its detection in diseased marine animals and marine environments. Journal of Fishery Sciences of China, 2005, 12(5): 580-587 [陈吉祥, 杨慧, 颜显辉, 等. 致病性哈维氏弧菌溶血素基因克隆及其检测. 中国水产科学, 2005, 12(5): 580-587 DOI:10.3321/j.issn:1005-8737.2005.05.009] |
CHEN J, YU Y X, ZHANG Z, et al. Establishment of a fluorescence quantitative microfluidic rapid detection technique for Vibrio rotiferianus based on toxR gene. Journal of Fisheries of China, 2020, 44(12): 2066-2075 [陈京, 于永翔, 张正, 等. 基于toxR基因的轮虫弧菌荧光定量微流控快速检测技术的建立. 水产学报, 2020, 44(12): 2066-2075] |
CHEN Y Z, ZHONG S L, ZHOU C. Prevention and treatment of luminous disease of adult prawns. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2000, 39(S1): 218-223 [陈月忠, 钟硕良, 周宸. 成虾发光病病原体的分离鉴定及防治技术研究. 中山大学学报(自然科学版), 2000, 39(S1): 218-223] |
CUI L F. China aquatic animal health report 2021. Beijing: China Agriculture Press, 2021 [崔利锋. 中国水生动物卫生状况报告2021. 北京: 中国农业出版社, 2021]
|
El-THOLOTH M, BAI H, MAUK M G, et al. A portable, 3-D printed, microfluidic device for multiplexed, real time, molecular detection of porcine epidemic diarrhea virus, transmissible gastroenteritis virus, and porcine deltacoronavirus at the point of need. Lab on a Chip, 2021, 21(6): 1118-1130 DOI:10.1039/D0LC01229G |
FIDALGO B, RUBIO E, PASTOR V, et al. Improved diagnosis of gastrointestinal infections using a semi-automated multiplex real-time PCR for detection of enteropathogens. Journal of Medical Microbiology, 2021, 70(9): 001367 |
GAO Z X, ZHANG M, WANG X X, et al. The development of a loop-mediated isothermal amplification (LAMP) method for detection of Vibrio scophthalmi and its initial application. Journal of Qingdao Agricultural University (Natural Science), 2015, 32(4): 284-288 [高志鑫, 张敏, 王西西, 等. 大菱鲆弧菌LAMP快速检测方法的建立与初步应用. 青岛农业大学学报(自然科学版), 2015, 32(4): 284-288 DOI:10.3969/J.ISSN.1674-148X.2015.04.010] |
HE J X, TIAN X H. Application of PCR in detection of foodborne pathogens. China Food Safety Magazine, 2021(6): 183 [何军霞, 田香红. 食源性致病菌检测中PCR技术的应用. 食品安全导刊, 2021(6): 183] |
HIDALGO R B, CLEENWERCK I, BALBOA S, et al. Diversity of Vibrios associated with reared clams in Galicia (NW Spain). Systematic and Applied Microbiology, 2008, 31(3): 215-222 DOI:10.1016/j.syapm.2008.04.001 |
JIN X M, GE M X, ZHANG Y Y, et al. Detection of drug resistance phenotypes and genes in Vibrio anguillarum from turbot Scophthalmus maximus. Fisheries Science, 2015, 34(8): 510-514 [靳晓敏, 葛慕湘, 张艳英, 等. 大菱鲆源鳗弧菌耐药表型及耐药基因检测. 水产科学, 2015, 34(8): 510-514] |
KIM S H, WOO S H, LEE S J, et al. The infection characteristics of Vibrio scophthalmi isolated from olive flounder, Paralichthys olivaceus. Journal of Fish Pathology, 2013, 26(3): 207-217 DOI:10.7847/jfp.2013.26.3.207 |
KIM Y B, OKUDA J, MATSUMOTO C, et al. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. Journal of Clinical Microbiology, 1999, 37(4): 1173-1177 DOI:10.1128/JCM.37.4.1173-1177.1999 |
LAN X, LI J, Li G Y, et al. Sequencing and phylogenetic analysis of the 16S rRNA genes of bacterial strains isolated from diseased flatfish. Progress in Fishery Sciences, 2020, 41(3): 142-150 [兰欣, 李杰, 李贵阳, 等. 发病鲆鲽类分离菌株的16S rRNA基因测序分析. 渔业科学进展, 2020, 41(3): 142-150] |
LEI Q, ZHAO Z K. Simultaneous quantitative detection of four mixed cooked meat provenances by multiple fluorescence quantitative PCR. China Food Safety Magazine, 2021(21): 83-87 [雷庆, 赵中开. 多重荧光定量PCR法同时定量检测4种混合熟肉种源. 食品安全导刊, 2021(21): 83-87] |
LI C P, ZHAI Q Q, WANG X, et al. Isolation and identification of Vibrio parahaemolyticus from shrimp culture ponds and analysis of its drug resistance and virulence genes. Progress in Fishery Sciences, 2020, 41(6): 174-180 [李翠苹, 翟倩倩, 王想, 等. 对虾养殖池副溶血弧菌的分离鉴定及其耐药特征、毒力基因分析. 渔业科学进展, 2020, 41(6): 174-180] |
LIN Z, KUMAGAI K, BABA K, et al. Vibrio parahaemolyticus has a homolog of the Vibrio cholerae toxRS operon that mediates environmentally induced regulation of the thermostable direct hemolysin gene. Journal of Bacteriology, 1993, 175(12): 3844-3855 DOI:10.1128/jb.175.12.3844-3855.1993 |
LIU X L, HOU C Z, LAI Y K, et al. Metabolic responses of Manila clam Ruditapes philippinarum to Vibrio anguillarum and Vibrio splendidus challenges. Fisheries Science, 2017, 36(2): 143-147 [刘小莉, 侯承宗, 来涌凯, 等. 两种弧菌对菲律宾蛤仔代谢途径影响的比较研究. 水产科学, 2017, 36(2): 143-147] |
LIU Z C. Developmentof multiplex PCR and real-time PCR for the detection of 4 bacterial pathogensin cultured turbot (Scophthalmus maximus). Masterxs Thesis of Ocean University of China, 2012 [刘智超. 养殖大菱鲆4种致病菌多重PCR和荧光定量PCR检测方法的建立. 中国海洋大硕士研究生学位论文, 2012]
|
MANZ A, GRABER N, WIDMER H M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sensors and Actuators B: Chemical, 1990, 1(1/2/3/4/5/6): 244-248 |
MEI B, ZHOU Y C, XU X D, et al. Isolation and identification of bacteria pathogens from Epinephelus coioides with tail- rotted disease. Journal of Tropical Oceanography, 2010, 29(6): 118-124 |
PARIA P, BEHERA B K, MOHAPATRA P D, et al. Virulence factor genes and comparative pathogenicity study of tdh, trh and tlh positive Vibrio parahaemolyticus strains isolated from Whiteleg shrimp, Litopenaeus vannamei (Boone, 1931) in India. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases, 2021, 95: 105083 |
QI L J, CHEN Y D, SHI K P, et al. Combining of transcriptomic and proteomic data to mine immune-related genes and proteins in the liver of Cynoglossus semilaevis challenged with Vibrio anguillarum. Comparative Biochemistry and Physiology- Part D: Genomics and Proteomics, 2021, 39: 100846 |
RIVAS A J, LEMOS M L, OSORIO C R. Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Frontiers in Microbiology, 2013, 4: 283 |
WANG K C, SUN R N, JIANG N, et al. Application of microfluidic chip technology in microbial detection. China Animal Health Inspection, 2021, 38(1): 87-93 [王楷宬, 孙瑞妮, 姜楠, 等. 微流控芯片技术在微生物检测中的应用. 中国动物检疫, 2021, 38(1): 87-93] |
WANG K K, YANG K, ZHAO J, et al. Rapid detection of hepatitis B virus nucleic acid based on microfluidic chip using fluorescence quantitative PCR. Journal of Analytical Science, 2018, 34(4): 450-454 [王可可, 杨柯, 赵俊, 等. 基于微流控芯片的荧光定量PCR法快速检测乙肝病毒核酸. 分析科学学报, 2018, 34(4): 450-454] |
WU S, WU Z Y, ZHANG X F, et al. Comparison on sensitivities of PCR, real-time PCR and microfluidic chip for detection of foreign gene in soybean products. Journal of Chinese Institute of Food Science and Technology, 2009, 9(2): 176-186 [吴姗, 吴志毅, 张晓峰, 等. 普通PCR法、荧光定量PCR法及微流控芯片法检测大豆产品中外源基因灵敏度的比较. 中国食品学报, 2009, 9(2): 176-186] |
YU D Y, LI A Q, LIU Y, et al. Development and evaluation of the detection method of microfluidic real-time RT-PCR assays for Zika, Dengue, Yellow fever and Chikungunya viruses. Chinese Journal of Experimental and Clinical Virology, 2020(2): 186-190 [余东阳, 李阿茜, 刘洋, 等. 寨卡病毒、登革病毒、黄热病毒、基孔肯雅病毒微流控荧光定量RT-PCR检测方法的建立及评价. 中华实验和临床病毒学杂志, 2020(2): 186-190] |
YU J H, CHEN J X, LI Y, et al. Detection of Vibrio anguillarum from Lateolabrax japonicus by using polymerase chain reaction (PCR). Journal of Oceanography of Huanghai and Bohai Seas, 2002, 20(2): 60-64 [余俊红, 陈吉祥, 厉云, 等. 聚合酶链反应(PCR)检测花鲈鳗弧菌感染. 黄渤海海洋, 2002, 20(2): 60-64] |
ZHANG F, SU Y Q, WANG J, et al. Studies on the isolation, identification and virulence of Photobacterium damselae isolated from Pseudosciaena crocea. Oceanologia et Limnologia Sinica, 2012, 43(6): 1202-1208 [张飞, 苏永全, 王军, 等. 大黄鱼(Pseudosciaena crocea)源美人鱼发光杆菌(Photobacterium damselae)的分离鉴定及致病性研究. 海洋与湖沼, 2012, 43(6): 1202-1208] |
ZHANG R N, YU L, WANG Z, et al. Application of multiple fluorescence quantitative PCR in animal detection. Shandong Journal of Animal Science and Veterinary Medicine, 2020, 41(4): 75-78 [张若楠, 于雷, 王钊, 等. 多重荧光定量PCR在动物检测中的应用. 山东畜牧兽医, 2020, 41(4): 75-78] |
ZHANG X H, AUSTIN B. Haemolysins in Vibrio species. Journal of Applied Microbiology, 2005, 98(5): 1011-1019 |
ZHANG Z, YU Y X, CHEN J, et al. Development of a microfluidics-based quantitative real-time PCR to rapidly identify Photobacterium damselae subsp. damselae with different pathogenicity by detecting the presence of Mcp or Dly gene. Journal of Ocean University of China, 2021, 20(2): 445-453 |
ZHENG G X, SHEN Y L, LI H. Vibrio anguillarum as a cause of disease in Penaeus orientalis Kishinouye. Journal of Fisheries of China, 1990, 14(1): 1-7 [郑国兴, 沈亚林, 李何. 中国对虾病原菌(鳗弧菌)的研究. 水产学报, 1990, 14(1): 1-7] |
ZHU J Y, DONG X H, ZHANG W Y, et al. Application of microfluidic technology in food safety rapid detection. Chemical Reagents, 2021, 43(5): 632-639 [朱婧旸, 董旭华, 张维宜, 等. 微流控技术在食品安全快速检测中的应用. 化学试剂, 2021, 43(5): 632-639] |



