2. 山东省海洋资源与环境研究院 山东 烟台 264006
2. Shandong Marine Resource and Environment Research Institute, Yantai, 264006, China
近年来,我国海水鱼养殖产业发展较为迅速,养殖产量逐年上升,至2020年已达174.98万t (农业农村部渔业渔政管理局等, 2021)。但养殖规模的扩大、集约化程度的提高、养殖管理水平的粗放以及病害防控技术手段的限制导致各种细菌性疾病暴发(Hamed et al, 2021; Cao et al, 2021; Diao et al, 2020),给养殖产业造成了巨大的经济损失(Fazio, 2019),制约了产业的健康发展。因此,降低养殖鱼类细菌性疾病感染率、提高养殖成活率显得尤为重要。
基于肠道功能微生物开发的益生菌能抑制病原菌生长(Kong et al, 2020)、提高机体酶活性(El-Saadony et al, 2021)、调节微生态平衡(杜瑞等, 2019)、改善水质(Kim et al, 2021),受到广大科研工作者的青睐。目前,芽孢杆菌(Bacillus)、乳酸菌(Lactic acid bacteria)等益生菌已被开发应用到海水鱼养殖中(Chen et al, 2020; Alonso et al, 2019; 李彬等, 2021),但部分菌株的非鱼源性使得它们很难在养殖中高效地发挥作用,限制了其在海水鱼养殖中的应用(张碧云等, 2021)。张在等(2020)研究发现,理想的益生菌应该来自动物自身的胃肠道。因此,为筛选兼具产酶活性和抑制病原菌特性的海水鱼源益生菌,本研究从海捕野生许氏平鲉(Sebastes schlegelii)和大泷六线鱼(Hexagrammos otakii)的消化道内壁黏膜分离纯化的80株细菌中筛选出2株产酶能力强、抑菌特性好的潜在益生菌TS2和TH8。菌株鉴定后对其生长特性和安全性进行了研究,以期为海水鱼类益生菌的开发与应用提供候选菌株。
1 材料与方法 1.1 实验动物野生许氏平鲉[全长为(14.1±0.86) cm,体质量为(66.4±1.3) g]和大泷六线鱼[全长为(21.6±0.88) cm,体质量为(98.8±1.7) g]捕获于山东大钦岛、砣矶岛海域,用于菌株的分离。
健康许氏平鲉[全长为(7.42±0.34) cm,体质量为(10.72±1.04) g]、大泷六线鱼[全长为(8.5±0.54) cm,体质量为(9.69±2.31) g]由威海圣航海洋科技有限公司提供,用于安全性检测实验。
1.2 培养基脱脂奶粉培养基:牛肉膏3 g,蛋白胨10 g,2%脱脂奶粉,NaCl 5 g,琼脂16 g,H2O 1 000 mL;淀粉酶培养基:牛肉膏3 g,蛋白胨10 g,可溶性淀粉10 g,NaCl 5 g,琼脂16 g,H2O 1 000 mL;脂肪酶筛选培养基:蛋白胨10 g,CaCl2⋅H2O 0.1 g,Tween-80 10 mL,琼脂16 g,H2O 1 000 mL。培养基在121 ℃,15 min灭菌。
1.3 菌株的分离纯化75%酒精擦拭鱼体腹部表面,无菌器具剪下鱼体胃、幽门盲囊及前中肠段,去除内容物,无菌生理盐水冲洗2次,刮取各器官内壁的黏液,加入到无菌生理盐水中,涡旋振荡摇匀后,一部分涂布于MRS固体培养基并置于厌氧袋中,一部分放入80 ℃水浴孵育20 min后涂布于LB固体培养基,30 ℃培养至出现菌落,重复平板划线分离出单菌落。挑取单菌落,30 ℃扩大培养后,于甘油中保种。
1.4 产酶菌的筛选将上述分离得到的菌株活化24 h后分别点种于脱脂奶粉培养基、淀粉培养基和脂肪酶筛选培养基上,30 ℃培养24 h,根据菌株能否产生水解圈筛选能分泌蛋白酶、淀粉酶和脂肪酶的菌株,并测量水解圈与菌落的直径。
1.5 TS2和TH8产物的抑菌能力分析选取海水鱼主要致病菌鳗弧菌(Vibrio anguillarum)、溶藻弧菌(Vibrio alginolyticus)、副溶血弧菌(Vibrio parahaemolyticus)、哈维氏弧菌(Vibrio harvey)、假交替单胞菌(Pseudoalteromonas nigrifaciens)、嗜水气单胞菌(Aeromonas hydrophila)、金黄色葡萄球菌(Staphyloccocus aureus)和大肠杆菌(Escherichia coli)为指示菌,采用平板扩散法检测分离菌株的抑菌能力。其中,鳗弧菌、哈维氏弧菌、假交替单胞菌和嗜水气单胞菌用2216E液体培养基在28 ℃、150 r/min条件下振荡培养。溶藻弧菌和副溶血弧菌用TCBS液体培养基在30 ℃、150 r/min条件下振荡培养。金黄色葡萄球菌、大肠杆菌用LB液体培养基在37 ℃、150 r/min条件下振荡培养。指示菌培养至指数生长期后,加入到相应的未冷凝的固体培养基中,待培养基凝固后,使用牛津杯制作直径为5 mm的加样孔。TS2和TH8分别置于LB和MRS液体培养基中培养24 h后,10 000 r/min离心5 min,吸取200 μL上清液置于加样孔内,对照组加入等量无菌液体培养基,在30 ℃培养24 h后,观察加样孔周围是否出现明显的抑菌圈,并测量抑菌圈的直径。
1.6 TS2和TH8的形态学观察及生理生化检测观察菌株纯化后的菌落形态,并进行革兰氏染色实验。分别采用梅里埃API 50CHB试剂盒与API 50CHL试剂盒对菌株TS2和TH8进行生理生化检测,参考《常见细菌系统鉴定手册》(东秀珠等, 2001)进行归类判定。
1.7 TS2和TH8的分子生物学鉴定提取细菌DNA,采用通用引物扩增其16S rDNA序列,正向引物为8F (5′-AGAGTTTGATCCTGGCTC AG-3′)、反向引物为1492R (5′-GGCTACCTTGTTACG ACTT-3′)。循环条件为94 ℃ 10 min;94 ℃ 30 s,55 ℃ 30 s,72 ℃ 2 min,30个循环;72 ℃ 10 min,4 ℃保存。扩增产物经1%琼脂糖凝胶电泳检测后,送至生工生物工程(上海)股份有限公司测序,测序结果采用BLAST检索系统与GenBank数据库中所搜集的相关种属的16S rDNA序列进行同源性分析,并利用MEGA7.0软件构建系统发育树。
1.8 TS2和TH8的生长特性分析 1.8.1 温度对TS2和TH8生长的影响各取100 μL培养至指数生长期的TS2和TH8,分别接种于LB和MRS液体培养基,于5、10、15、20、25、30、35、40、45和50 ℃条件下培养24 h,检测OD600 nm,每个实验组设3个平行。
1.8.2 NaCl浓度对TS2和TH8生长的影响各取100 μL培养至指数生长期的TS2和TH8,分别接种于LB和MRS液体培养基,于NaCl浓度(g/L)为0、0.01、0.02、0.03、0.04、0.05、0.06、0.07、0.08、0.09和0.10 g/L条件下,30 ℃培养24 h后检测OD600 nm,每个实验组设3个平行。
1.8.3 pH对TS2和TH8生长的影响取100 μL培养至指数生长期的TS2,接种于pH为3、4、5、6、7、8、9、10和11的LB液体培养基中;取100 μL培养至指数生长期的TH8,接种于pH分别为3、4、5、6、7、8、9、9.5、10、10.5、11、11.5、12、12.5、13、13.5、14的MRS液体培养基中,30 ℃培养24 h后检测OD600 nm,每个实验组设3个平行。
1.8.4 TS2和TH8生长曲线的测定各取50 μL培养至指数期的TS2和TH8,分别接种于1 000 μL LB和MRS液体培养基,在30 ℃、150 r/min条件下培养,每隔2 h取样,检测OD600 nm,每个实验组设3个平行。
1.9 安全性检测选取健康的许氏平鲉和大泷六线鱼各60尾,分别分为2组(实验组与对照组),每组30尾,分为3个平行,暂养于60 L水槽,水温为(23±0.5) ℃。日换水2次,每次换水4/5,连续充气,不投喂食物,及时清理粪便,暂养1周后用于实验。菌株在30 ℃,150 r/min条件下振荡培养至指数生长期后,6 000 r/min离心5 min,用PBS重悬至108 CFU/mL。
采用腹腔注射法进行安全性检测,实验组许氏平鲉和大泷六线鱼分别每尾注射100 μL 108 CFU/mL的TS2和TH8菌悬液,许氏平鲉和大泷六线鱼对照组的每尾鱼注射100 μL无菌PBS。注射后连续观察10 d,统计各组许氏平鲉、大泷六线鱼的生长及死亡状况。
1.10 数据统计与分析采用SPSS 22.0软件中的单因素方差分析(one-way ANOVA)比较数据差异显著水平,P < 0.05时表示差异显著;采用GraphPad Prism 8.0软件绘图,实验数据均用平均值±标准差(
对消化道内壁黏膜样品进行细菌分离纯化,获得80株可培养细菌(表 1)。通过细菌产酶能力分析筛选出产酶能力较好的菌株TS2和TH8。TS2产蛋白酶、淀粉酶和脂肪酶,其产酶水解圈直径与菌落直径比分别为3.37±0.32、3.24±0.12和2.27±0.13;TH8产蛋白酶和脂肪酶,其产酶水解圈直径与菌落直径比为2.32±0.09和2.06±0.19 (表 2)。
|
|
表 1 菌株来源及编号 Tab.1 Strain source and number |
|
|
表 2 分离菌株的产酶情况 Tab.2 Enzyme production of isolated strains |
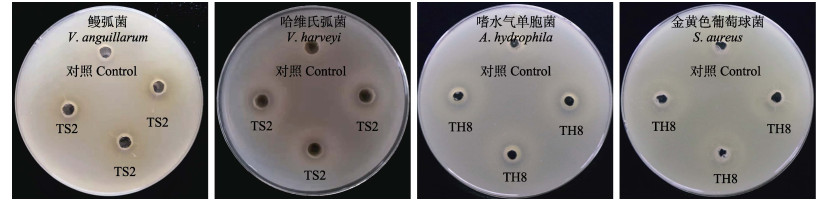
菌株产物的抑菌活性实验发现,TS2产物抑制鳗弧菌、副溶血弧菌、哈维氏弧菌和假交替单胞菌的生长,TH8产物抑制鳗弧菌、溶藻弧菌、副溶血弧菌、假交替单胞菌、嗜水气单胞菌、金黄色葡萄球菌和大肠杆菌的生长(表 3)。部分抑菌效果如图 1所示,TS2、TH8的产物具有广谱抑菌性。
|
|
表 3 菌株产物对指示菌的抑制作用 Tab.3 Inhibition of strain products on indicator bacteria |
|
图 1 菌株对部分病原菌的抑制效果 Fig.1 Inhibitory effect of strains on some pathogenic bacteria |
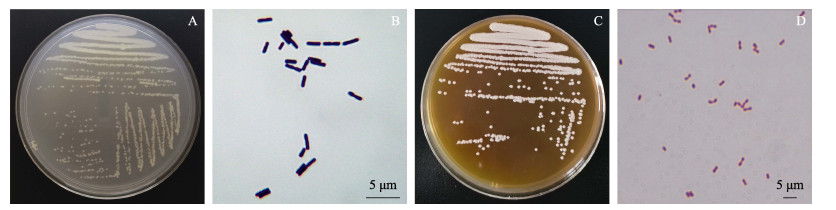
TS2在LB固体培养基中形成表面粗糙、不透明、污白色或微黄色菌落,在显微镜下呈杆状,革兰氏染色呈阳性;TH8在MRS固体培养基中形成表面光滑、边缘整齐、乳白色菌落,在显微镜下呈圆形或椭圆形,革兰氏染色呈阳性(图 2)。TS2和TH8的生理生化特性检测结果如表 4所示。初步判断TS2为芽孢杆菌科,TH8为肠球菌科。
|
图 2 菌落的形态特性及革兰氏染色镜检图 Fig.2 The morphological characteristics of the strain and the microscopic examination of gram stain A:TS2形态特征;B:TS2革兰氏染色;C:TH8形态特征;D:TH8革兰氏染色 A: TS2 morphological characteristics; B: TS2 Gram stain; C: TH8 morphological characteristics; D: TH8 Gram stain |
|
|
表 4 生理生化检测结果 Tab.4 Physiological and biochemical identification results |
TS2和TH8的16S rDNA序列扩增大小分别为1459和1466 bp,通过NCBI数据库Blast比对发现,TS2与枯草芽孢杆菌Y37 (KF641801)和0-2 (FJ959367)相似度分别达到99.79%和99.66%,TH8与河流漫游球菌(Vagococcus fluvialis) TRG15 (MH329632)和SS1339 (GQ337040)相似度分别达到98.97%和98.97%。
基于16S rDNA序列的TS2和TH8系统发育树(图 3)结果显示,TS2与枯草芽孢杆菌KF641801和FJ959367聚为一支,TH8与河流漫游球菌MH329632和GQ337040聚为一支。结合形态学、生理生化检测及16S rDNA序列分析结果,将TS2鉴定为枯草芽孢杆菌,TH8鉴定为河流漫游球菌。

|
图 3 基于16S rDNA序列的菌株TS2和TH8系统发育树 Fig.3 The clustering results of strain TS2 and TH8 based on 16S rDNA gene sequence |
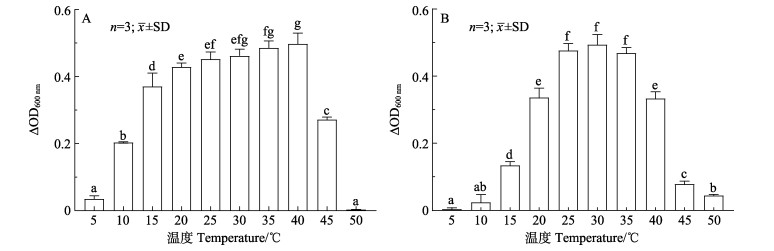
TS2在5~45 ℃均能生长,最适生长温度为40 ℃,在5~40 ℃时,随着培养温度的升高,TS2培养液的ΔOD600 nm (终止时OD600 nm–初始时OD600 nm)逐渐增大;随着培养温度继续升高,TS2培养液的ΔOD600 nm逐渐降低。
TH8在10~50 ℃均能生长,最适生长温度为30 ℃,在10~30 ℃时,随着培养温度的升高,TH8培养液的ΔOD600 nm逐渐增大;随着培养温度继续升高,TH8培养液的ΔOD600 nm逐渐降低(图 4)。
|
图 4 不同温度条件下TS2 (A)和TH8 (B)的生长情况 Fig.4 Growth of TS2 (A) and TH8 (B) at different temperatures 柱形图上方字母的不同表示差异显著(P < 0.05),下同。 Different letters on the column indicate significant differences (P < 0.05), the same as below. |
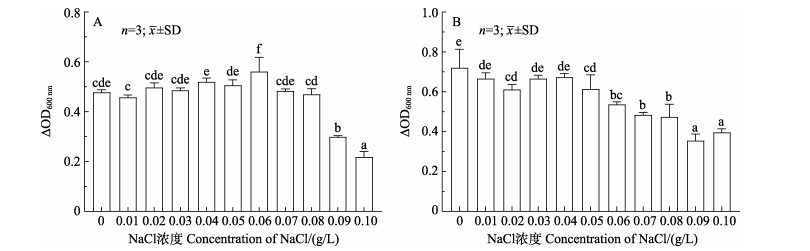
TS2在NaCl浓度为0~0.10 g/L的LB液体培养基中均能生长,其中,0~0.08 g/L时,随着NaCl浓度的升高,TS2培养液的ΔOD600 nm基本不变;随着NaCl浓度继续升高,TS2培养液的ΔOD600 nm逐渐降低。TH8在NaCl浓度为0~0.10 g/L的MRS液体培养基中均能生长,其中0~0.05 g/L时,随着NaCl浓度的升高,TH8培养液的ΔOD600 nm基本不变;随着NaCl浓度继续升高,TH8培养液的ΔOD600 nm逐渐降低(图 5)。
|
图 5 不同NaCl浓度条件下TS2 (A)和TH8 (B)的生长情况 Fig.5 The growth of TS2 (A) and TH8 (B) with different concentrations of NaCl |
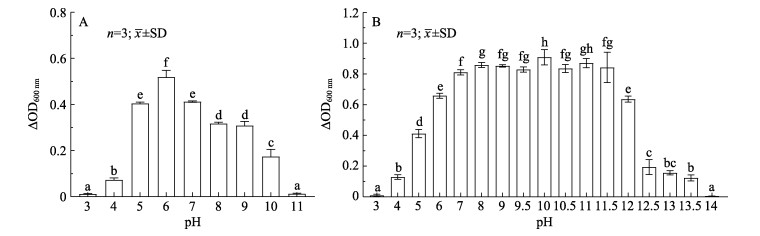
TS2在pH为3~11的LB液体培养基中均能生长,在pH为3~6时,随着pH的升高,TS2培养液的ΔOD600 nm逐渐升高;在pH为7~11时,随着pH的升高,TS2培养液的ΔOD600 nm逐渐降低。TH8在pH为3~14的MRS液体培养基中均能生长,在pH为3~8时,随着pH的升高,TH8培养液的ΔOD600 nm逐渐升高;在pH为9~11.5时,随着pH的升高,TH8培养液的ΔOD600 nm基本不变;在pH为12~14时,随着pH的升高,TH8培养液的ΔOD600 nm逐渐降低(图 6)。
|
图 6 不同pH条件下TS2(A)与TH8(B)的生长情况 Fig.6 The growth of TS2 (A) and TH8 (B) at different pH |
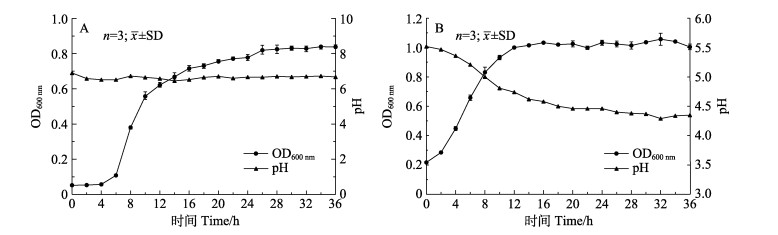
TS2和TH8的生长曲线呈典型的“S”型,可以观察到延滞期、对数生长期和稳定期。TS2有明显的延滞期,约为6 h,该时期菌株数量变化不大,随后进入对数生长期,此时菌株数量迅速增长,26 h后,菌株生长进入稳定期。TH8延滞期并不明显,进入对数生长期后生长旺盛,14 h后,菌株生长进入稳定期。在TS2培养过程中培养液pH基本不变,在TH8培养过程中培养液pH逐渐降低(图 7)。
|
图 7 菌株TS2(A)与TH8(B)的生长曲线与产酸曲线 Fig.7 Curves of growth and acid producing of the strain TS2 (A) and TH8 (B) |
以108 CFU/mL浓度向许氏平鲉和大泷六线鱼腹腔分别注射100 μL枯草芽孢杆菌和100 μL河流漫游球菌,整个实验过程中,许氏平鲉和大泷六线鱼活力良好,无发病和死亡现象(表 5)。
|
|
表 5 菌株的安全性实验 Tab.5 Safety test of strains |
本研究从海捕野生许氏平鲉和大泷六线鱼消化道内分离筛选出2株特征明显的菌株TS2和TH8,基于形态学观察、生理生化检测及16S rDNA序列分析,将TS2和TH8分别鉴定为枯草芽孢杆菌和河流漫游球菌。枯草芽孢杆菌具有产酶、抑菌、提高群体免疫力等特点(Liu Q et al, 2021; 罗璋等, 2021),已被广泛应用于水产养殖(Shah et al, 2021)、植物抗病(Zhang et al, 2021)、水质净化(Guo et al, 2021)等领域,并取得了良好的效果。河流漫游球菌于1989年首次被发现(Collins et al, 1989),已有文献记载其具有抑菌、刺激免疫基因体外表达等特性(Feliatra et al, 2021; Giannattasio-Ferraz et al, 2021),但目前为止,尚未检索到有关河流漫游球菌在水产养殖中应用的报道,本研究将进一步开发该菌,应用于养殖生产中。
益生菌可在动物肠道中分泌胞外酶进而参与宿主的消化和免疫过程(Li et al, 2021; 樊英等, 2021)。Xu等(2021)研究发现,解淀粉芽孢杆菌(Bacillus amyloliquefaciens)能产生胞外酶,增强克氏原螯虾(Procambarus clarkii)肠道消化酶活性。Tamilarasu等(2021)研究发现,在饲料中添加枯草芽孢杆菌可显著提高凡纳滨对虾(Penaeus vannamei)肠道蛋白酶、淀粉酶和脂肪酶活性,其生长率和存活率随之显著提高。Ashouri等(2020)研究发现,在饲料中添加乳酸片球菌(Pediococcus acidlatictici),鲈鱼(Lates calcarifer)肠道内消化酶活性显著增加。本研究所筛选的菌株TS2具有产蛋白酶、淀粉酶和脂肪酶能力,TH8具有产蛋白酶和脂肪酶能力,表明TS2具有提高许氏平鲉肠道酶活性的潜力,TH8具有提高大泷六线鱼肠道酶活性的潜力,在肠道定植后能够促进鱼体对营养物质的消化吸收,进而促进生长。
动物消化道中的有益微生物可以抑制或杀死病原微生物(Galdeano et al, 2019; 练小军等, 2020)。研究发现,乳酸杆菌(Lactobacillus)和双歧杆菌(Bifidobacterium)可以抑制大肠杆菌MG1655的生长(Darvishi et al, 2021),植物乳杆菌(Lactobacillus plantarum)可减少沙门氏菌(Salmonella)的定植,抑制病原菌对HCT-116细胞的黏附和渗透(Wang et al, 2018)。短乳杆菌(Lactobacillus brevis) gp104对金黄色葡萄球菌具有抑制作用,能够竞争、抑制和取代金黄色葡萄球菌对Caco-2细胞的黏附(Hojjati et al, 2020)。本研究中,TS2可抑制鳗弧菌、副溶血弧菌、哈维氏弧菌和假交替单胞菌的生长,TH8可抑制鳗弧菌、溶藻弧菌、副溶血弧菌、假交替单胞菌、嗜水气单胞菌、金黄色葡萄球菌和大肠杆菌的生长。筛选的菌株对多种病原菌具有较好的抑制作用,暗示其能够在海水鱼抵御病原菌感染的过程中发挥作用,作为益生菌添加到鱼体内有助于降低养殖鱼类疾病的发生,在水产养殖疾病防控方面具有较大的应用潜力。乳酸菌可以产生有机酸,如乙酸、乳酸、碳原子数较多的脂肪酸和苯乳酸等来抑制有害微生物生长,也可以异型发酵产生CO2抑制微生物生长(Liu Z et al, 2021)。本研究中,通过培养菌株TH8 24 h后发现,TH8可显著降低培养液的pH,且有气体产生。说明菌株在生长的同时会释放酸性物质和气体,产生的酸性物质可能通过竞争作用、增加细菌外膜通透性、改变胞内渗透压及抑制大分子的合成等方式来抑制有害微生物生长。其次,产生的气体可以形成厌氧环境,从而使得需氧型微生物受到抑制(吕懿超等, 2021)。本研究将进一步分析TH8菌株产生酸性物质和气体的成分,进而探究该菌株的抑菌机制。
益生菌的生长受温度、NaCl浓度和pH等因素的影响,明确细菌适宜生长的温度、NaCl浓度和pH等条件对其生产应用具有重要的意义。本研究中,TS2在温度为15~40 ℃、NaCl浓度为0~0.08 g/L、pH为5~9时生长较快,6 h进入对数生长期,26 h后进入稳定期;TH8在温度为20~40 ℃、NaCl浓度为0~0.08 g/L、pH为5~12时生长较快,2 h进入对数生长期,14 h后进入稳定期。2株分离菌株均表现出耐高温、耐酸碱、耐盐、生长速度快等优点,对生长条件具有广泛的适应性,适合进一步开发,应用于海水鱼类养殖生产中。
开发益生菌应用于养殖生产前应确保其对机体无致病性,因此,安全性检测是必不可少的重要环节。Das等(2021)研究发现,向淡水鲶鱼(Heteropneustes fossilis)腹腔分别注射抗生素链霉菌(Streptomyces antibioticus)和蜡样芽孢杆菌(Bacillus cereus),10 d后并未发现鲶鱼有病变、出血、水肿、鳞片脱落或死亡现象。Amenyogbed等(2021)研究发现,向军曹鱼(Rachycentron canadum)腹腔分别注射芽孢杆菌、成团泛菌(Pantoea agglomerans)和腊样芽孢杆菌,10 d后,实验组和对照组中军曹鱼均无发病、死亡现象。本研究发现,菌株TS2和TH8在不高于108 CFU/mL的浓度下对同源宿主是相对安全的。研究结果为下一步探究TS2和TH8对同源宿主生长和免疫的影响提供了理论依据,为其应用于生产提供了可能性。
综上所述,本研究从许氏平鲉和大泷六线鱼消化道内分离筛选了2株潜在益生菌TS2和TH8,建立了系统发育树,明确了最适生长条件,验证了其产酶特性、抑菌特性和动物安全性,显示了菌株作为有益菌应用于水产养殖的潜力,为其开发为益生菌制剂及其在海水鱼养殖业中的应用提供数据支撑。
ALONSO S, CASTRO M C, BERDASCO M, et al. Isolation and partial characterization of lactic acid bacteria from the gut microbiota of marine fishes for potential application as probiotics in aquaculture. Probiotics and Antimicrobial Proteins, 2019, 11(2): 569-579 DOI:10.1007/s12602-018-9439-2 |
AMENYOGBE E, HUANG J S, CHEN G, et al. Probiotic potential of indigenous (Bacillus sp. RCS1, Pantoea agglomerans RCS2, and Bacillus cereus strain RCS3) isolated from cobia fish (Rachycentron canadum) and their antagonistic effects on the growth of pathogenic Vibrio alginolyticus, Vibrio harveyi, Streptococcus iniae, and Streptococcus agalactiae. Frontiers in Marine Science, 2021, 8: 672213 DOI:10.3389/fmars.2021.672213 |
ASHOURI G, SOOFIANI N M, HOSEINIFAR S H, et al. Influence of dietary sodium alginate and Pediococcus acidilactici on liver antioxidant status, intestinal lysozyme gene expression, histomorphology, microbiota, and digestive enzymes activity, in Asian sea bass (Lates calcarifer) juveniles. Aquaculture, 2020, 518: 734638 DOI:10.1016/j.aquaculture.2019.734638 |
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2021. Beijing: China Agriculture Press, 2021 [农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 中国渔业统计年鉴2021. 北京: 中国农业出版社, 2021]
|
CAO M, YAN X, LI Q, et al. Genome-wide identification and analysis of NOD-like receptors and their potential roles in response to Edwardsiella tarda infection in black rockfish (Sebastes schlegelii). Aquaculture, 2021, 541: 736803 DOI:10.1016/j.aquaculture.2021.736803 |
CHEN B, PENG M, TONG W, et al. The quorum quenching bacterium Bacillus licheniformis T-1 protects zebrafish against Aeromonas hydrophila infection. Probiotics and Antimicrobial Proteins, 2020, 12(1): 160-171 DOI:10.1007/s12602-018-9495-7 |
COLLINS M D, ASH C, FARROW J A E, et al. 16S ribosomal ribonucleic acid sequence analyses of lactococci and related taxa. Description of Vagococcus fluvialis gen. nov., sp. nov.. Journal of Applied Bacteriology, 1989, 67(4): 453-460 |
DARVISHI N, FARD N A, SADRNIA M. Genomic and proteomic comparisons of bacteriocins in probiotic species Lactobacillus and Bifidobacterium and inhibitory ability of Escherichia coli MG 1655. Biotechnology Reports, 2021, 31: e00654 DOI:10.1016/j.btre.2021.e00654 |
DAS S, MONDAL K, PAL A K. Evaluation of the probiotic potential of Streptomyces antibioticus and Bacillus cereus on growth performance of freshwater catfish Heteropneustes fossilis. Aquaculture Reports, 2021, 20: 100752 DOI:10.1016/j.aqrep.2021.100752 |
DIAO J, YU X Q, WANG X L, et al. Full-length transcriptome sequencing combined with RNA-seq analysis revealed the immune response of fat greenling (Hexagrammos otakii) to Vibrio harveyi in early infection. Microbial Pathogenesis, 2020, 149: 104527 DOI:10.1016/j.micpath.2020.104527 |
DONG X Z, CAI M Y. Common bacteria system identification manual. Beijing: Science Press, 2001: 9-294 [东秀珠, 蔡妙英. 常见细菌系统鉴定手册. 北京: 科学出版社, 2001: 9-294]
|
DU R, WANG B H, LUO Y L, et al. Advance in studying the effect of probiotics on gastrointestinal tract microorganism to improve meat quality. Microbiology China, 2019, 46(9): 2378-2385 [杜瑞, 王柏辉, 罗玉龙, 等. 益生菌调控胃肠道菌群改善肉品质的研究进展. 微生物学通报, 2019, 46(9): 2378-2385 DOI:10.13344/j.microbiol.china.180692] |
EL-SAADONY M T, ALAGAWANY M, PATRA A K, et al. The functionality of probiotics in aquaculture: An overview. Fish and Shellfish Immunology, 2021, 117: 36-52 DOI:10.1016/j.fsi.2021.07.007 |
FAN Y, WANG X L, YU X Q, et al. Effect of Bacillus licheniformis on growth, intestinal digestive enzymes, serum non-special immune and resistance against Aeromonas salraonicida in fat greenling, Hexagrammos otakii. Progress in Fishery Sciences, 2021, 42(1): 63-73 [樊英, 王晓璐, 于晓清, 等. 地衣芽孢杆菌对大泷六线鱼生长、肠道消化酶、血清非特异性免疫及抗病力的影响. 渔业科学进展, 2021, 42(1): 63-73 DOI:10.19663/j.issn2095-9869.20191111001] |
FAZIO F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture, 2019, 500: 237-242 DOI:10.1016/j.aquaculture.2018.10.030 |
FELIATRA F, BATUBARA U M, NURULITA Y, et al. The potentials of secondary metabolites from Bacillus cereus SN7 and Vagococcus fluvialis CT21 against fish pathogenic bacteria. Microbial Pathogenesis, 2021, 158: 105062 DOI:10.1016/j.micpath.2021.105062 |
GALDEANO C M, CAZORLA S I, DUMIT J M L, et al. Beneficial effects of probiotic consumption on the immune system. Annals of Nutrition and Metabolism, 2019, 74(2): 115-124 DOI:10.1159/000496426 |
GIANNATTASIO-FERRAZ S, ENE A, MASKERI L, et al. Vagococcus fluvialis isolation and sequencing from urine of healthy cattle. G3, 2021, 11(1): jkaa034 DOI:10.1093/g3journal/jkaa034 |
GUO J, CHEN C, CHEN W, et al. Effective immobilization of Bacillus subtilis in chitosan-sodium alginate composite carrier for ammonia removal from anaerobically digested swine wastewater. Chemosphere, 2021, 284: 131266 DOI:10.1016/j.chemosphere.2021.131266 |
HAMED S B, TAPIA-PANIAGUA S T, MORIÑIGO M Á, et al. Advances in vaccines developed for bacterial fish diseases, performance and limits. Aquaculture Research, 2021, 52(6): 2377-2390 DOI:10.1111/are.15114 |
HOJJATI M, BEHABAHANI B A, FALAH F. Aggregation, adherence, anti-adhesion and antagonistic activity properties relating to surface charge of probiotic Lactobacillus brevis gp104 against Staphylococcus aureus. Microbial Pathogenesis, 2020, 147: 104420 DOI:10.1016/j.micpath.2020.104420 |
KIM S, JEON H, HAN H S, et al. Evaluation of Bacillus albus SMG-1 and B. safensis SMG-2 isolated from Saemangeum Lake as probiotics for aquaculture of white shrimp (Litopenaeus vannamei). Aquaculture Reports, 2021, 20: 100743 DOI:10.1016/j.aqrep.2021.100743 |
KONG Y, LI M, LI R, et al. Evaluation of cholesterol lowering property and antibacterial activity of two potential lactic acid bacteria isolated from the intestine of snakehead fish (Channa argus). Aquaculture Reports, 2020, 17: 100342 DOI:10.1016/j.aqrep.2020.100342 |
LI B, WANG Y G, LIAO M J, et al. Isolation and biological characterization of Lactobacillus paracasei isolated from a sea cucumber pond culture system. Progress in Fishery Sciences, 2021, 42(3): 100-107 [李彬, 王印庚, 廖梅杰, 等. 刺参池塘养殖系统中1株副干酪乳杆菌的分离及其生物学特性研究. 渔业科学进展, 2021, 42(3): 100-107] |
LI Y, YANG Y, SONG L, et al. Effects of dietary supplementation of Lactobacillus plantarum and Bacillus subtilis on growth performance, survival, immune response, antioxidant capacity and digestive enzyme activity in olive flounder (Paralichthys olivaceus). Aquaculture and Fisheries, 2021, 6(3): 283-288 DOI:10.1016/j.aaf.2020.10.006 |
LIAN X J, ZHU K L, ZHANG Q Q, et al. Effects of probiotics-supplemented diets on the growth and survival of Litopenaeus vannamei carrying multiple pathogens. Progress in Fishery Sciences, 2020, 41(2): 121-130 [练小军, 朱开玲, 张庆起, 等. 饲料添加益生菌对多病原阳性的凡纳滨对虾生长与存活的影响. 渔业科学进展, 2020, 41(2): 121-130] |
LIU Q, WEN L, PAN X, et al. Dietary supplementation of Bacillus subtilis and Enterococcus faecalis can effectively improve the growth performance, immunity, and resistance of tilapia against Streptococcus agalactiae. Aquaculture Nutrition, 2021, 27(4): 1160-1172 DOI:10.1111/anu.13256 |
LIU Z, XU C, TIAN R, et al. Screening beneficial bacteriostatic lactic acid bacteria in the intestine and studies of bacteriostatic substances. Journal of Zhejiang University- Science B, 2021, 22(7): 533-547 DOI:10.1631/jzus.B2000602 |
LÜ Y C, LI X A, WANG K B, et al. Antimicrobial mechanism of lactic acid bacteria as biopreservative organisms and their application in food industry: A review. Food Science, 2021, 42(19): 281-290 [吕懿超, 李香澳, 王凯博, 等. 乳酸菌作为生物保护菌的抑菌机理及其在食品中应用的研究进展. 食品科学, 2021, 42(19): 281-290 DOI:10.7506/spkx1002-6630-20200620-279] |
LUO Z, CHEN H L, ZHANG Z G, et al. Oral vaccination for Ctenopharyngodon idella against Flavobacterium columnare using Bacillus subtilis expressing lip gene. Progress in Fishery Sciences, 2021, 42(6): 151-157 [罗璋, 陈红莲, 张振国, 等. 枯草芽孢杆菌表达柱状黄杆菌lip基因口服疫苗对草鱼免疫保护效果的研究. 渔业科学进展, 2021, 42(6): 151-157] |
SHAH S, CHESTI A, RATHER M, et al. Effect of probiotics (Bacillus subtilis) on the growth and survival of fingerlings of grass carp, Ctenopharyngodon idella. Current Journal of Applied Science and Technology, 2021, 40(15): 31-37 |
TAMILARASU A, AHILAN B, GOPALAKANNAN A, et al. Evaluation of probiotic potential of Bacillus strains on growth performance and physiological responses in Penaeus vannamei. Aquaculture Research, 2021, 52(7): 3124-3136 DOI:10.1111/are.15159 |
WANG L, LI L, LV Y, et al. Lactobacillus plantarum restores intestinal permeability disrupted by Salmonella infection in newly-hatched chicks. Scientific Reports, 2018, 8(1): 2229 DOI:10.1038/s41598-018-20752-z |
XU L, YUAN J, CHEN X, et al. Screening of intestinal probiotics and the effects of feeding probiotics on the digestive enzyme activity, immune, intestinal flora and WSSV resistance of Procambarus clarkii. Aquaculture, 2021, 540: 736748 DOI:10.1016/j.aquaculture.2021.736748 |
ZHANG B Y, YANG H L, WANG P, et al. Advances in the interactions between intestinal microorganisms and host immune system in fish. Acta Microbiologica Sinica, 2021, 61(10): 3046-3058 [张碧云, 杨红玲, 汪攀, 等. 鱼类肠道微生物与宿主免疫系统相互作用研究进展. 微生物学报, 2021, 61(10): 3046-3058] |
ZHANG Q, STUMMER B E, GUO Q, et al. Quantification of Pseudomonas protegens FD6 and Bacillus subtilis NCD-2 in soil and the wheat rhizosphere and suppression of root pathogenic Rhizoctonia solani AG-8. Biological Control, 2021, 154: 104504 |
ZHANG Z, ZHENG R C, FANG Q L, et al. Isolation, identification and biological characteristics of probiotics from swine origin in Guangxi. China Animal Husbandry and Veterinary Medicine, 2020, 47(6): 1921-1933 [张在, 郑瑞程, 方庆励, 等. 广西猪源益生菌的分离鉴定与生物学特性研究. 中国畜牧兽医, 2020, 47(6): 1921-1933] |



