2. 山东省海洋预报减灾中心 山东 青岛 266104;
3. 山东省海水养殖病害防治重点实验室 山东 青岛 266104
2. Shandong Marine Forecast and Hazard Migitation Service, Qingdao 266104, China;
3. Key Laboratory of Mariculture Disease Control of Shandong Province, Qingdao 266104, China
凡纳滨对虾(Litopenaeus vannamei)又称南美白对虾,属对虾科(Penaeidae)、滨对虾属(Penaeus),是甲壳类的重要养殖品种之一,在水产养殖中一直占据重要地位。近年来,随着对虾养殖规模的扩大,对虾养殖业逐渐向集约化、高密度的模式发展,容易导致环境恶化和病害的大面积暴发。抗生素的广泛使用又造成了病原菌耐药性增加、环境污染和生态失衡(王春迪, 2016),对水体造成二次污染。此外,抗生素残留引发人们对水产品安全的担忧,影响了对虾养殖业的健康稳定发展。因此,生物防控在水产养殖中越来越受到人们的重视。
益生菌为活体微生物制剂,可以促进养殖动物的健康和生长(练小军等, 2020),具有健康、绿色、安全的特点,常被用作抗生素的重要替代品。迄今为止,枯草芽孢杆菌(Bacillus subtilis)(Abdollahi-Arpanahi et al, 2018; Fan et al, 2018; Interaminense et al, 2018)、蜡样芽孢杆菌(Bacillus cereus)(Jiang et al, 2019; 刘文亮等, 2017; Navinchandran et al, 2014)和短小芽孢杆菌(Bacillus pumilus)(Liu et al, 2020)等抑菌型芽孢杆菌被广泛应用于对虾养殖过程中。此类益生菌分泌如有机酸、过氧化氢、溶菌酶和细菌素等具有杀菌或抑菌作用的物质,能够改变肠道和养殖环境,影响有害菌的定植和生长。侧孢短芽孢杆菌(Brevibacillus laterosporu)作为一种生物防治益生菌,常见其应用于农作物和兽禽类的疾病防治。Khadija (2020)研究发现,侧孢短芽孢杆菌蛋白激发子PeBL1具有诱导番茄和黄瓜抗桃蚜(Myzus persicae)的作用;厉彦芳(2020)报道,侧孢短芽孢杆菌B8能够抗击植物病毒和促进植物生长;Purba (2020)的研究表明,德克萨斯侧孢短芽孢杆菌(Brevibacillus laterosporus)培养物可以保护鸡免受沙门氏菌(Salmonella pullorum)感染,而侧孢短芽孢杆菌在水产养殖中的应用鲜见报道。本研究通过向饲料中添加不同水平的侧孢短芽孢杆菌FAS05,以评估其对凡纳滨对虾生长、抗病、免疫力以及养殖环境的影响,为该菌株作为饲料添加剂的应用提供参考依据。
1 材料与方法 1.1 菌株来源与计数侧孢短芽孢杆菌(BL FAS05)为实验室保藏菌株,从夏季弧菌数低的养殖池水中分离得到,已保藏于中国微生物菌种保藏管理委员会普通微生物中心,保藏编号为CGMCC No.20038,体外实验证实其可抑制副溶血弧菌(Vibrio parahemolyticus)、哈维氏弧菌(Vibrio harveyi)等致病菌的生长。将侧孢短芽孢杆菌FAS05按1%的比例接种到LB营养肉汤培养基中,30 ℃、200 r/min过夜培养进行活化,随后逐级扩大培养。3 000 r/min离心20 min,弃掉上清液,收集菌体。用适量无菌生理盐水调整菌株浓度(OD600 nm),逐级稀释添加到饲料制作过程中。
菌株浓度采用平板菌落计数法进行统计。用无菌生理盐水将菌液梯度稀释后进行涂布,每个梯度涂布3个平板作为平行,挑选合适的稀释梯度进行计数,计数结果取3个平行的平均值。
1.2 饲料制作鱼粉、豆粕和玉米粕为主要蛋白源,鱼油和磷脂油为脂肪源,小麦面粉为主要糖源,并补充无机盐、维生素等配制出基础饲料,具体饲料配方见表 1。饲料原料经粉碎、80目过筛、混合均匀后,添加不同体积固定浓度的侧孢短芽孢杆菌FAS05配制终浓度为105 CFU/g (BL1)、107 CFU/g (BL2)、109 CFU/g (BL3)的实验饲料,对照组(C)用等体积的生理盐水补齐。原料全部混合均匀后,喷水制备面团,用饲料颗粒机挤压膨化成型,常温阴至半干,剪切成1.5 mm×3~5 mm的颗粒,随即阴干,于阴暗干燥处保存。
|
|
表 1 基础饲料配方 Tab.1 Composition of basal diets |
实验用凡纳滨对虾购自山东省威海市乳山市蜊子嘴村对虾养殖场,实验开始前放于温度为25~28 ℃、盐度为27~30的海水循环系统中暂养2周以适应环境。暂养期间饲喂基础饲料。随后挑选体重(1.00±0.08) g的对虾随机投放到12个100 L的玻璃缸中进行实验。
实验共设置1个对照组和3个不同浓度的处理组,每个处理设3重复,每个重复50尾虾。养殖过程中,每天分4次投喂(06:00、12:00、17:00和22:00),并清除残饵和粪便,记录摄食量和虾健康状况。每天换水1次,换水量为总水体的的2/3。每周测定一次对虾体长和体重。养殖全过程不间断充气,溶解氧含量不低于5 mg/L,氨氮含量低于0.03 mg/L。
1.4 样品采集养殖实验结束后停食24 h,捞出对虾放于冰袋上麻醉。收集对虾血淋巴细胞、血清和肝胰腺,进行后续实验的测定。
使用含有抗凝剂的1 mL无菌注射器从对虾的围心腔内抽取血淋巴。抗凝剂与血淋巴的体积比为1∶1,注入无菌的1.5 mL离心管中,3只对虾的血淋巴合一管。血淋巴离心,用PBS调整至血细胞达到106 cell/mL,进行吞噬活性和呼吸爆发实验,每个处理6个平行。
收集对虾血清(不含抗凝剂的对虾血淋巴4℃放置过夜,3 500 r/min离心10 min,收集上清液)和肝胰腺组织置于液氮速冻,储存于–80 ℃,用于免疫相关酶活性的分析,每个处理8个平行。临近测定时,将适量对虾肝胰腺样品加入PBS匀浆缓冲液中制成20%的组织匀浆样品,4 ℃、2 500 g离心10 min,收集上层澄清匀浆液分装备用;血清样品直接化冻备用。
1.5 侵染实验饲养实验结束后,进行为期1周的副溶血弧菌侵染实验,所用副溶血弧菌为中国科学院烟台海岸带研究所杨顶珑副研究员惠赠,从发病凡纳滨对虾中分离获得。每个玻璃缸中留50 L海水和25只对虾,用浓度为107 CFU/mL的副溶血弧菌浸浴。侵染期间不换水,投喂添加菌株的饲料,每12 h统计一次死亡情况,及时捞出死虾和粪便。
1.6 指标测定 1.6.1 生长表现测定在养殖实验过程中,每2周每缸随机选取8尾对虾测定其体长体重,并计算其肥满度。实验结束后,对各缸对虾进行计数,计算成活率(survival rate, SR)、肥满度(condition factor, CF)和特定生长率(specific growth rate, SGR)。
| $\begin{array}{c} \mathrm{SR}(\%)=\text { 成活尾数/总尾数 } \times 100\\ \mathrm{CF}=W_t / L_t^3 \times 100\\ \operatorname{SGR}(\% / \mathrm{d})=\left(\ln W_t-\ln W_\mathit{0}\right) / t \times 100 \end{array}$ |
式中,W0为对虾初始平均体重,Wt为实验结束时对虾平均体重,Lt为实验结束时对虾平均体长,t为养殖实验时间。
1.6.2 养殖水体中弧菌数的检测采用平板计数法测定养殖水体中的弧菌数量,方法简述如下:取1 mL养殖水样,分别用灭菌生理盐水进行10、102、103和104梯度稀释,移取取0.1 mL稀释液涂布于TCBS培养基上,每个稀释梯度设置3个平行,28 ℃培养2 d,选取平均菌落数在30~300之间的平板计数,计数菌落形成单位数(CFU)。
1.6.3 血细胞免疫反应测定吞噬活性:实验方法在Delaporte等(2003)的方法基础上稍作修改,简述如下:用PBS调整血细胞浓度为106 cell/mL;取4 μL,25%的荧光微球加入400 μL血细胞中,混合均匀,18 ℃下避光孵育1 h;加入26 μL多聚甲醛终止反应;4 ℃,800 g离心10 min去除多余的荧光微球;加入400 μL PBS重悬血细胞,采用流式细胞仪FL-1通道检测分析血细胞的绿色荧光。采用吞噬微球的血细胞数目占血细胞总数的百分比来表示吞噬活性。
呼吸爆发:血细胞呼吸爆发产生的ROS可以将二氢罗丹明123 (DHR)氧化为发荧光的罗丹明123 (RHO) (Kalgraff et al, 2011),经流式细胞仪检测,观察峰的偏移程度以比较血细胞的呼吸爆发程度。将对虾血细胞调整至浓度为106 cell/mL,于25 ℃、黑暗条件下孵育10 min,添加丙二醇甲醚醋酸酯(PMA)使其终浓度达到0.1 μg/mL,继续孵育10 min后添加DHR,使DHR终浓度为2 μg/mL,继续孵育30 min。采用流式细胞仪FL-1通道检测分析呼吸爆发的峰的偏移量。
1.6.4 酶活力测定肝胰腺中免疫相关酶活力,如超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)、酸性磷酸酶(acid phosphatase, ACP)、碱性磷酸酶(alkaline phosphatase, ALP)和血清中免疫相关酶活力,如酚氧化酶(phenol oxidase, PO)、溶菌酶(lysozyme, LZM)均采用南京建成试剂盒测定。肝胰腺和血清中的蛋白含量采用碧云天BCA蛋白浓度测定试剂盒测定。
1.7 数据处理与分析实验结果以平均值±标准差(Mean±SD)表示,采用统计软件SPSS 16.0对所有数据进行单因素方差分析(one-way ANOVA),显著性水平为P < 0.05;采用LSD方差齐性检验方法比较各实验处理组与对照组间的差异。
2 实验结果 2.1 饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾生长性能的影响饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾生长性能的影响如图 1所示。各组对虾的成活率无显著差异(图 1A, P > 0.05)。在养殖第14天时,BL1和BL2组的对虾的体长和体重显著高于对照组(C组) (P < 0.05),各实验组之间的体长和体重无显著差异(图 1B、C, P > 0.05);BL1和BL2组的对虾肥满度与对照组无明显差异(P > 0.05),BL3组的对虾肥满度显著低于对照组(P < 0.05) (图 1D)。在养殖28 d时,BL1和BL2组的体长显著高于BL3组(P < 0.05),体重在各实验组中无显著差异(P > 0.05),BL1组的肥满度显著高于对照组(P < 0.05)。养殖结束后,BL1和BL2组的特定生长率显著高于对照组(图 1E, P < 0.05),各实验组之间特定生长率差异不显著(P > 0.05)。综合实验结果显示,饲料中添加侧孢短芽孢杆菌FAS05可以促进凡纳滨对虾的生长,且对其存活率没有显著影响。
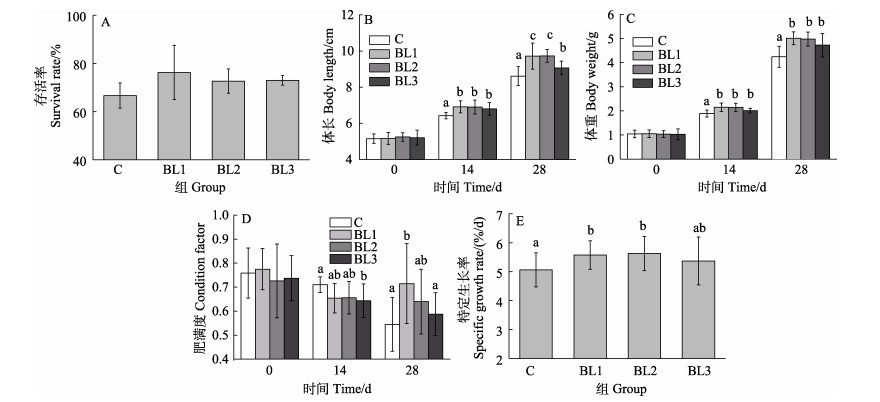
|
图 1 饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾生长性能的影响 Fig.1 Effects of adding B. laterosporu FAS05 to feed on growth performance of L. vannamei C:对照组;BL1:105 CFU/g添加组;BL2:107 CFU/g添加组;BL3:109 CFU/g添加组。不同字母表示差异显著(P < 0.05)。下同。 C: Control groups; BL1: Add 105 CFU/g B. laterosporu groups; BL2: Add 107 CFU/g B. laterosporu groups; BL3: Add 109 CFU/g B. laterosporu groups. Different superscript letters are significantly different from each other at P < 0.05. The same as below. |
凡纳滨对虾养殖水体中弧菌数变化如图 2示。自养殖第7天始,与对照组相比,各实验组的弧菌数显著降低(P < 0.05),且一直维持在较低水平。BL2和BL3组的弧菌数略低于BL1组,BL2与BL3组之间的弧菌数几乎没有差异。
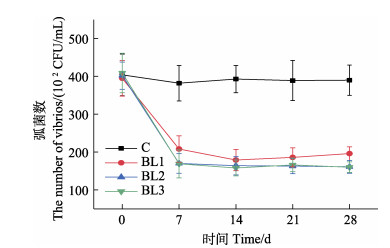
|
图 2 饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾养殖水体弧菌数的影响 Fig.2 Effects of adding B. laterosporu FAS05 to feed on vibrio quantity in cultured water of L. vannamei |
侵染副溶血弧菌后,凡纳滨对虾的存活曲线如图 3所示。侵染持续至46 h时,各实验组的侵染存活率显著高于对照组。至侵染结束时,对照组的侵染存活率为45%左右,而实验组的存活率达到80%以上,各实验组之间的差异不显著。
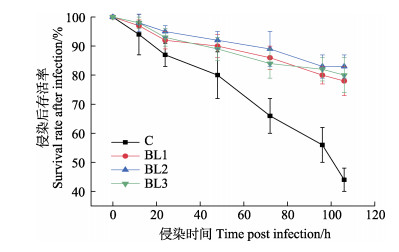
|
图 3 饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾抗病力的影响 Fig.3 Effects of adding B. laterosporu FAS05 to feed on disease resistance of L. vannamei |
饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾血细胞免疫活性的影响如图 4所示。与对照组相比,饲喂28 d后,饲料中补充了侧孢短芽孢杆菌FAS05的对虾血细胞吞噬率显著增加(P < 0.05),BL2组的吞噬率显著高于BL1和BL3组(P < 0.05),BL1和BL3组之间差异不显著(图 4A, P > 0.05)。饲料中补充了侧孢短芽孢杆菌FAS05的对虾血细胞呼吸爆发活性即血细胞ROS产量显著降低(P < 0.05),BL2和BL3组的呼吸爆发活性显著低于BL1组(P < 0.05),BL2和BL3组之间差异不显著(图 4B, P > 0.05)。
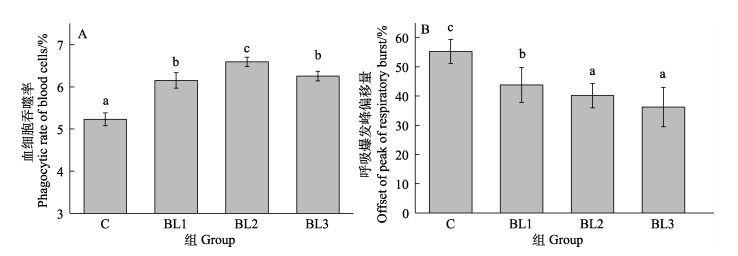
|
图 4 饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾血细胞免疫活性的影响 Fig.4 Effects of adding B. laterosporu FAS05 to feed on blood cell immune activity of L. vannamei |
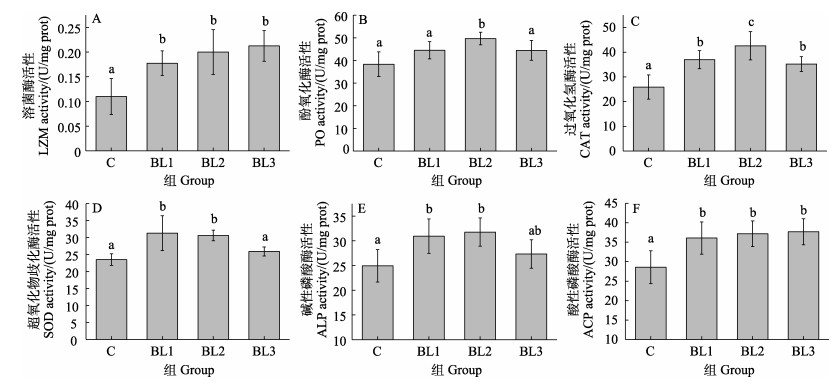
饲料中补充侧孢短芽孢杆菌FAS05能够不同程度地提高对虾血清和肝胰腺中的免疫相关酶活性(图 5)。各实验组对虾的溶菌酶、过氧化氢酶和酸性磷酸酶均显著高于C组(P < 0.05),实验组之间的溶菌酶和酸性磷酸酶活性没有显著差异(P > 0.05)。BL2组中的酚氧化酶显著高于其他各组,BL1和BL2组中的碱性磷酸酶和超氧化物歧化酶显著高于C组,BL1和BL2组之间显著不差异(P > 0.05),C组与BL3组之间也不存在显著差异(P > 0.05)。
|
图 5 饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾免疫相关酶的影响 Fig.5 Effects of adding B. laterosporu FAS05 to feed on immune related enzymes of L. vannamei |
在本研究中,饲料中添加适宜浓度的侧孢短芽孢杆菌FAS05对凡纳滨对虾生存没有显著影响,说明此浓度范围内侧孢短芽孢杆菌FAS05是相对安全的。一般芽孢杆菌均具有丰富的产酶系统,可促进饲料中大分子营养物质的利用,促进水产动物的健康生长(李军亮, 2018; 练小军, 2017)。相似地,饲料中添加侧孢短芽孢杆菌FAS05也促进了凡纳滨对虾的生长。
本研究中,105和107 CFU/g的添加量均可显著促进对虾的体长、体重以及特定生长率,这与李军亮(2018)和刘龙镇等(2018)的研究结果相近,具体的添加量的差异可能与菌株特性有关。
3.2 饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾养殖水环境弧菌数的影响生物拮抗是微生物群落内普遍存在的自然现象,在同一生态位中多种环境微生物之间通过营养或空间竞争、分泌抗生素或者细菌素等抑制其他微生物的生长(王春迪, 2016)。本研究结果显示,投喂含有侧孢短芽孢杆菌FAS05的饲料可以降低水体中的弧菌数量,这可能是由于饲料中的侧孢短芽孢杆菌FAS05未全部定植于对虾肠道,同粪便一起排到养殖环境中,起到了抑制弧菌生长作用。侧孢短芽孢杆菌FAS05的弧菌抑制作用与添加量没有剂量–效应关系,说明低浓度的侧孢短芽孢杆菌FAS05即具有抑制弧菌生长的作用。
3.3 饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾抗病力的影响病害暴发是阻碍对虾养殖产业发展的重要因素,如对虾急性肝胰腺坏死综合征即由携带PirA和PirB毒力基因的副溶血弧菌所致(王春迪, 2016; 刘龙镇等, 2018)。Pieters等(2010)将添加益生菌的饲料投喂凡纳滨对虾,使其抗病能力得到提高。Vaseeharan等(2010)研究发现,枯草芽孢杆菌可以使斑节对虾(Penaeus monodon)感染哈维氏弧菌后的死亡率减少90%。本研究中的结果同样显示,饲料中添加侧孢短芽孢杆菌FAS05时,凡纳滨对虾的副溶血弧菌侵染存活率从45%提高到了80%以上,充分发挥了免疫防御作用。但对虾侵染存活率与侧孢短芽孢杆菌FAS05的添加浓度没有相关性,这说明低浓度的侧孢短芽孢杆菌FAS05依旧具有抗病效果。
3.4 饲料中添加侧孢短芽孢杆菌FAS05对凡纳滨对虾非特异性免疫力的影响凡纳滨对虾抗病力的提高是细胞免疫和体液免疫互相协同作用的结果。酚氧化酶原非活化状态存在于血细胞的颗粒内,极微量微生物多糖可引起血细胞的胞吐作用,释放、激活酚氧化酶原系统(陈国福等, 2007)。Rengpipat等(2000)研究表明,芽孢杆菌S11可以激活细胞免疫防御功能。本研究中,凡纳滨对虾血细胞的吞噬作用随着侧孢短芽孢杆菌FAS05的添加先上升后下降,原因可能是一定量的侧孢短芽孢杆菌FAS05被摄入后利用表面抗原或其产生的代谢物刺激了对虾的免疫防御系统,使对虾血细胞吞噬率显著提高。但过多的侧孢短芽孢杆菌FAS05可能会损伤细胞免疫状态,造成吞噬率降低。在吞噬作用期间,通过呼吸爆发产生大量的ROS来杀死病原体,过量的ROS会产生氧化损伤和氧化应激。107和109 CFU/g的侧孢短芽孢杆菌FAS05可以抑制血细胞呼吸爆发活性,以降低过量的ROS对机体的破坏。
本研究中,107 CFU/g组的凡纳滨对虾血清中酚氧化酶和溶菌酶活性显著上升,这是激活了酚氧化酶原系统的必然结果。类似地,谢佳磊等(2007)的研究表明,饲料中添加枯草芽孢杆菌(5×109 CFU/kg)可以提高克氏原螯虾(Procambarus clarkii)血清溶菌酶和酚氧化酶活性。超氧化物歧化酶催化过氧阴离子发生歧化生成过氧化氢和氧,过氧化氢酶能够有效清除过量的过氧化氢,以消除细胞损害,避免生物体损伤(王春迪, 2016)。本研究中,添加107 CFU/g的侧孢短芽孢杆菌FAS05使肝胰腺超氧化物歧化酶和过氧化氢酶活性均显著高于对照组,109 CFU/g组的酶活力显著低于107 CFU/g组,表明适量的侧孢短芽孢杆菌FAS05可增强二者活性,提高免疫反应。这与王苓等(2017)以枯草芽孢杆菌饲喂凡纳滨对虾后所得结果相似。酸性磷酸酶是吞噬溶酶体的重要组成部分,本研究中添加105和107 CFU/g的侧孢短芽孢杆菌FAS05可以显著提高肝胰腺中的酸性磷酸酶活力和碱性磷酸酶活力,这与谷舞(2020)和刘强强(2017)的研究结果相一致。
4 结论侧孢短芽孢杆菌FAS05对凡纳滨对虾的益生作用具体表现在促进对虾生长、抑制周围环境弧菌生长、激活免疫体系、提高抗病力四个方面。105和107 CFU/g的侧孢短芽孢杆菌FAS05可以显著促进对虾生长,107 CFU/g的侧孢短芽孢杆菌FAS05可以使凡纳滨对虾的非特异性免疫指标得到有效提高。综上所述,益生菌侧孢短芽孢杆菌FAS05可以作为饲料添加剂在对虾养殖过程中持续使用,参考使用量为105 CFU/g即可显著促进对虾生长,提高其免疫和抗病能力;在病害严重时,可以加大用量至107 CFU/g,以进一步提高对虾的非特异性免疫力。
ABDOLLAHI-ARPANAHI D, SOLTANI E, JAFARYAN H, et al. Efficacy of two commercial and indigenous probiotics, Bacillus subtilis and Bacillus licheniformis on growth performance, immunophysiology and resistance response of juvenile white shrimp (Litopenaeus vannamei). Aquaculture, 2018, 496: 43-49 DOI:10.1016/j.aquaculture.2018.06.082 |
CHEN G F, SONG X L, HUANG J, et al. Effects of A3α-peptidoglycan on activities of phosphatase and intracellular phenoloxidase of Litopenaeus vannamei. Marine Fisheries Research, 2007, 28(1): 59-64 [陈国福, 宋晓玲, 黄倢, 等. A3α-肽聚糖对凡纳滨对虾磷酸酶及细胞内酚氧化酶活性的影响. 海洋水产研究, 2007, 28(1): 59-64] |
DELAPORTE M, SOUDANT P, MOAL J, et al. Effect of a mono-specific algal diet on immune functions in two bivalve species - Crassostrea gigas and Ruditapes philippinarum. Journal of Experimental Biology, 2003, 206: 3053-3064 DOI:10.1242/jeb.00518 |
FAN Y, LIU L, ZHAO L, et al. Influence of Bacillus subtilis ANSB060 on growth, digestive enzyme and aflatoxin residue in Yellow River carp fed diets contaminated with aflatoxin B1. Food and Chemical Toxicology, 2018, 113: 108-114 DOI:10.1016/j.fct.2018.01.033 |
GU W. Probiotics Bacillus screening from the intestine of Litopenaeus vannamei and its disease resistance mechanism. Master′s Thesis of Shenyang Agricultural University, 2020, 25–37 [谷舞. 凡纳滨对虾肠道益生芽孢杆菌的筛选及抗病机理研究. 沈阳农业大学硕士研究生学位论文, 2020, 25–37]
|
INTERAMINENSE J, VOGELEY J, GOUVEIA C, et al. In vitro and in vivo potential probiotic activity of Bacillus subtilis and Shewanella algae for use in Litopenaeus vannamei rearing. Aquaculture, 2018, 488: 114-122 DOI:10.1016/j.aquaculture.2018.01.027 |
JIANG Y H, ZHOU S X, CHU W H. The effects of dietary Bacillus cereus QSI-1 on skin mucus proteins profile and immune response in crucian carp (Carassius auratus gibelio). Fish and Shellfish Immunology, 2019, 89: 319-325 DOI:10.1016/j.fsi.2019.04.014 |
KALGRAFF C A K, WERGELAND H I, PETTERSEN E F. Flow cytometry assays of respiratory burst in Atlantic salmon (Salmo salar L.) and in Atlantic cod (Gadus morhua L.) leucocytes. Fish and Shellfish Immunology, 2011, 31(3): 381-388 DOI:10.1016/j.fsi.2011.05.028 |
KHADIJA J. Evaluation of protein PeBL1 from Brevibacillus laterosporus to induce resistance against Myzus persicae on tomato and cucumber. Doctoral Dissertation of Chinese Academy of Agricultural Sciences, 2020, 17–57 [Khadija Javed. 侧孢短芽孢杆菌蛋白激发子PeBL1诱导番茄和黄瓜抗桃蚜的作用. 中国农业科学院博士研究生学位论文, 2020, 17–57]
|
LI J L. Effects of two probiotics on growth performance, disease resistance for juvenile Litopenaeus vannamei and juvenile Epinephelus sp. Master´s Thesis of Guangdong Ocean University, 2018, 1–3 [李军亮. 两种益生菌对凡纳滨对虾幼虾和石斑鱼幼鱼生长和抗病力的影响. 广东海洋大学硕士研究生学位论文, 2018, 1–3]
|
LI Y F. Analysis of antiviral substance of Brevibacillus laterosporus strain B8 and its mechanism against tobacco mosaic vius. Master′s Thesis of Shenyang Agricultural University, 2020, 17–24 [厉彦芳. 侧孢短芽孢杆菌B8抗病毒活性物质分析及其作用机理研究. 沈阳农业大学硕士研究生学位论文, 2020, 17–24]
|
LIAN X J, ZHU K L, ZHANG Q Q, et al. Effects of probiotics-supplemented diets on the growth and survival of Litopenaeus vannamei carrying multiple pathogens. Progress in Fishery Sciences, 2020, 41(2): 121-130 [练小军, 朱开玲, 张庆起, 等. 饲料添加益生菌对多病原阳性的凡纳滨对虾生长与存活的影响. 渔业科学进展, 2020, 41(2): 121-130 DOI:10.19663/j.issn2095-9869.20190316001] |
LIAN X J. Effects of two probiotics on the growth and immune indexes of Litopenaeus vannamei. Master′s Thesis of Shanghai Ocean University, 2017, 2–4 [练小军. 两株益生菌发酵颗粒饲料对凡纳滨对虾生长及免疫指标的影响. 上海海洋大学硕士研究生学位论文, 2017, 2–4]
|
LIU L Z, TIAN X L, WANG M Y, et al. Effects of additive patterns of omnibiotics on the growth performance, non-specific immunity and disease resistance of Litopenaeus vannamei. Periodical of Ocean University of China (Natural Science), 2018, 48: 23-31 [刘龙镇, 田相利, 王明阳, 等. 不同复合微生态制剂添加方式对凡纳滨对虾生长、非特异性免疫及抗病力的影响. 中国海洋大学学报(自然科学版), 2018, 48: 23-31] |
LIU Q Q. Effects of adding dietary probiotics and probiotics (yeast cell wall) on growth and immunity of Litopenaeus vannamei. Master′s Thesis of Tianjin Agricultural University, 2017, 14–24 [刘强强. 饲料中添加几种益生菌和益生元(酵母细胞壁)对凡纳滨对虾生长及免疫的影响. 天津农学院硕士研究生学位论文, 2017, 14–24]
|
LIU S, WANG S, CAI Y, et al. Beneficial effects of a host gut-derived probiotic, Bacillus pumilus, on the growth, non-specific immune response and disease resistance of juvenile golden pompano, Trachinotus ovatus. Aquaculture, 2020, 514: 734446 DOI:10.1016/j.aquaculture.2019.734446 |
LIU W L, XU H, TANG Y, et al. The Effect of diet with Bacillus cereus biofilm on the growth rate, disease resistance and intestinal microflora of Litopenaeus vannamei. Progress in Fishery Sciences, 2017, 38(4): 87-95 [刘文亮, 许华, 唐杨, 等. 饲料中补充蜡样芽孢杆菌(Bacillus cereus) 生物膜对凡纳滨对虾(Litopenaeus vannamei)生长、病力及其肠道微生物组成的影响. 渔业科学进展, 2017, 38(4): 87-95] |
NAVINCHANDRAN M, IYAPPARAJ P, MOOVENDHAN S, et al. Influence of probiotic bacterium Bacillus cereus isolated from the gut of wild shrimp Penaeus monodon in turn as a potent growth promoter and immune enhancer in P. monodon. Fish and Shellfish Immunology, 2014, 36(1): 38-45 DOI:10.1016/j.fsi.2013.10.004 |
PIETERS N, BRUNT J, AUSTIN B, et al. Efficacy of in-feed probiotics against Aeromonas bestiarum and Ichthyophthirius multifiliis skin infections in rainbow trout (Oncorhynchus mykiss, Walbaum). Journal of Applied Microbiology, 2010, 105(3): 723-32 |
PURBA M A. A study about protective effect of Brevibacillus laterosporus texasporus culture on broiler chickens infected with Salmonella pullorum. Master′s Thesis of Chinese Academy of Agricultural Sciences, 2020, 10–17 [MHD ADANAN PURBA. 德克萨斯侧孢短芽孢杆菌培养物对沙门氏菌感染肉鸡的保护作用研究. 中国农业科学院硕士研究生学位论文, 2020, 10–17]
|
RENGPIPAT S, RUKPRATANPORN S, PIYATIRATITIVORAKUL S, et al. Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture, 2000, 191(4): 271-288 DOI:10.1016/S0044-8486(00)00440-3 |
VASEEHARAN B, RAMASAMY P. Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. Letters in Applied Microbiology, 2010, 36(2): 83-87 |
WANG C D. Screening and evalution of potential probiotics against disease in Litopenaenaeus vannamei. Master´s Thesis of Shanghai Ocean University, 2016, 1–3 [王春迪. 凡纳滨对虾防病益生菌的筛选和效果评价. 上海海洋大学硕士研究生学位论文, 2016, 1–3]
|
WANG L, TIAN X L, DONG S L, et al. Effects of two Bacillus on growth performance and serum non-specific immunity of Litopenaeus vannamei. Periodical of Ocean University of China (Natural Science), 2017, 47(4): 14-21 [王苓, 田相利, 董双林, 等. 两株芽孢杆菌对凡纳滨对虾生长和血清非特异性免疫指标的影响研究. 中国海洋大学学报(自然科学版), 2017, 47(4): 14-21] |
XIE F, ZENG W, ZHOU Q, et al. Dietary lysine requirement of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 2012, 358/359: 116-121 DOI:10.1016/j.aquaculture.2012.06.027 |
XIE J L, XIAO D, YIN D, et al. Effects of Bacillus subtilis on immune function of Procambarus clarkii. Freshwater Fisheries, 2007(6): 24-27 [谢佳磊, 肖丹, 殷蝶, 等. 枯草芽孢杆菌对克氏原螯虾免疫机能的影响. 淡水渔业, 2007(6): 24-27] |



