2. 中国水产科学研究院黄海水产研究所 山东 青岛 266071
 (Lophius litulon)、鲬(Platycephalus indicus)、长绵鳚(Zoarces viviparu)、角木叶鲽(Pleuronichthys cornutus)和带纹条鳎(Zebrias zebrinus)的形体指标、肌肉和肝脏的粗成分,以及肌肉、肝脏、脑、眼、皮肤、肠道等组织的脂肪酸组成,以全面评估底栖型代表鱼类的脂肪和脂肪酸组成特征。实验鱼每种鱼9尾,3尾一组混样作为一个重复。结果显示,在5种鱼中,肌肉脂肪含量整体偏低,黄
(Lophius litulon)、鲬(Platycephalus indicus)、长绵鳚(Zoarces viviparu)、角木叶鲽(Pleuronichthys cornutus)和带纹条鳎(Zebrias zebrinus)的形体指标、肌肉和肝脏的粗成分,以及肌肉、肝脏、脑、眼、皮肤、肠道等组织的脂肪酸组成,以全面评估底栖型代表鱼类的脂肪和脂肪酸组成特征。实验鱼每种鱼9尾,3尾一组混样作为一个重复。结果显示,在5种鱼中,肌肉脂肪含量整体偏低,黄 和带纹条鳎肌肉脂肪含量尤其低(0.3%~0.4%),但黄
和带纹条鳎肌肉脂肪含量尤其低(0.3%~0.4%),但黄 、鲬和角木叶鲽具有较高的肝脏脂肪含量(19%~29%),而带纹条鳎和长绵鳚肝脏脂肪含量偏低(4%~5%)。脂肪酸组成方面,黄
、鲬和角木叶鲽具有较高的肝脏脂肪含量(19%~29%),而带纹条鳎和长绵鳚肝脏脂肪含量偏低(4%~5%)。脂肪酸组成方面,黄 肌肉中n-3系列长链多不饱和脂肪酸(long chain-polyunsaturated fatty acid, LC-PUFA)含量显著高于其他鱼,尤其以DHA最为明显。黄
肌肉中n-3系列长链多不饱和脂肪酸(long chain-polyunsaturated fatty acid, LC-PUFA)含量显著高于其他鱼,尤其以DHA最为明显。黄 背肌中还具有最高含量的18:1n-9。长绵鳚背肌脂肪酸组成最明显的特征是较低的16:0含量和较高的20:4n-6及EPA含量。肝脏脂肪酸中,鲬和角木叶鲽具有显著高的18:1n-9等单不饱和脂肪酸含量,但其DHA含量较低。在黄
背肌中还具有最高含量的18:1n-9。长绵鳚背肌脂肪酸组成最明显的特征是较低的16:0含量和较高的20:4n-6及EPA含量。肝脏脂肪酸中,鲬和角木叶鲽具有显著高的18:1n-9等单不饱和脂肪酸含量,但其DHA含量较低。在黄 、带纹条鳎和长绵鳚的大多组织中均有较高的DHA、20:4n-6和22:5n-3,但EPA含量较低。研究表明,即使同为底栖型鱼类,不同种类间脂肪和脂肪酸组成差异也非常显著。黄
、带纹条鳎和长绵鳚的大多组织中均有较高的DHA、20:4n-6和22:5n-3,但EPA含量较低。研究表明,即使同为底栖型鱼类,不同种类间脂肪和脂肪酸组成差异也非常显著。黄 具有显著高的n-3 LC-PUFA含量,脂肪酸营养价值较高。
具有显著高的n-3 LC-PUFA含量,脂肪酸营养价值较高。 鲬 角木叶鲽 带纹条鳎 长绵鳚
鲬 角木叶鲽 带纹条鳎 长绵鳚 2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
鱼类是人类食用长链多不饱和脂肪酸(LC-PUFA),尤其是二十二碳六烯酸(DHA, 22:6n-3)和二十碳五烯酸(EPA, 20:5n-3)的主要来源。LC-PUFA在脂质代谢调节和预防心脑血管疾病方面具有重要的生理功能,它们在神经、视觉发育以及免疫调节等生理过程中也发挥着重要作用(Maillard et al, 2018; Xu et al, 2021; Yamagata, 2021; Yu et al, 2021)。
鱼类中脂质和脂肪酸的组织分布在不同的物种中,具有高度多样性(Takama et al, 1994)。组织间的脂质分布模式决定了鱼的许多脂质相关特征,包括一些与鱼肉品质直接相关的特征,如脂肪酸分布、脂质代谢物、胆固醇含量和鱼肉质地等(Katikou et al, 2001)。然而,尽管已经有一些研究比较了不同鱼种(包括淡水和海水鱼种)肌肉的营养成分,但鱼体内脂质分布和脂肪酸组成的多样性还没有被完全了解。目前,已在一些物种之间进行脂质分布模式的比较,主要集中于鲑鳟鱼类,如大西洋鲑鱼(Salmo salar)和虹鳟鱼(Oncorhynchus mykiss)以及常见的淡水物种,如罗非鱼(Oreochromis mossambicus)、鲤鱼(Cyprinus carpio)、鲶鱼(Silurus asotus)和印尼拟松鲷(Datnioides pulcher) (Katikou et al, 2001; Nanton et al, 2007; Wu et al, 2015; Ren et al, 2018; Khieokhajonkhet et al, 2019; 宋红梅等, 2020)。本实验室最近的一项研究也揭示了3种具有不同脂质储存模式的瘦肌型海水鱼类即大菱鲆(Scophthalmus maximus)、红鳍东方鲀(Takifugu rubripes)和花鲈(Lateolabrax japonicus)的不同脂质分布情况。
本研究旨在研究青岛地区(黄海) 5种底栖海水鱼类的脂肪和脂肪酸组成以及形体指标和粗成分等。本研究以黄
本实验用鱼在山东省青岛市十五大街农贸市场购买,分别为黄
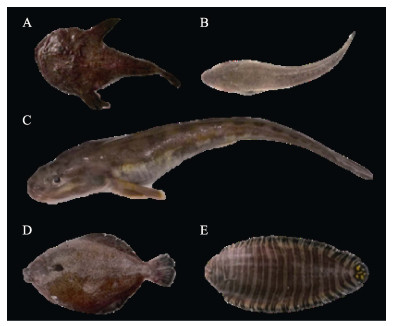
|
图 1 本研究的5种底栖型海水鱼类
Fig.1 The five benthic marine fish species investigated in this study
A: 黄 ; B: 鲬; C: 长绵鳚; D: 角木叶鲽; E: 带纹条鳎。
A: Yellow anglerfish; B: Flathead; C: Eelpout; D: East Asian flatfish; E: Zebra sole. ; B: 鲬; C: 长绵鳚; D: 角木叶鲽; E: 带纹条鳎。
A: Yellow anglerfish; B: Flathead; C: Eelpout; D: East Asian flatfish; E: Zebra sole.
|
随后,取肌肉、肝脏、脑、眼、皮肤、肠道、鳍条周围皮下组织(仅角木叶鲽和带纹条鳎)和腹腔内脂肪组织(仅鲬)的样品进行脂肪和脂肪酸分析。其中,背部肌肉取样位置为将鱼头朝向右,取背部右下方肌肉作为背部肌肉,取腹部相应肌肉作为腹部肌肉。其中,黄
使用AOAC的标准方法,对背肌、腹肌和肝脏(每3尾混一组)的粗成分(%湿重)进行了分析。水分测定为105 ℃烘干至恒重;粗蛋白测定采用凯氏定氮法;粗脂肪测定采用索氏抽提法;粗灰分测定为马弗炉550 ℃灼烧法。肌肉脂肪含量采用氯仿甲醇法进行测定。
所有组织的脂肪酸用气相色谱仪(GC-2010,岛津,日本)进行测定。使用冷冻干燥机将肌肉冷冻干燥24 h,每尾鱼一份组织样品,每种鱼三尾鱼的组织进行混样,然后在72 ℃水浴条件下依次用氢氧化钾–甲醇和氯化氢–甲醇进行甲酯化,用正己烷萃取。取上清液上机测定,采用二氧化硅毛细管柱(SH-RT-2560, 100.00 m×0.25 mm×0.20 μm)和火焰离子检测器。色谱柱升温程序:从150 ℃到200 ℃,升温速率为15 ℃/min;然后,以2 ℃/min的速度从200 ℃升到250 ℃。进样器和检测器的温度均为250 ℃。结果表示为每种脂肪酸相对于总脂肪酸的百分比(%TFA)。
1.3 计算和统计分析脏体比(VSI)=内脏湿重/鱼体重×100;
肝体比(HSI)=肝脏湿重/鱼体重×100;
肥满度(CF)=鱼体重/(鱼体长3)×100。
所有数据均在SPSS 16.0中进行单因素方差(one-way ANOVA)分析。符合方差齐性检验后,采用Duncan′s检验进行多重比较,计算结果以平均值±标准误(Mean±SE)的形式表示。P < 0.05为显著差异。
2 结果 2.1 形态指标HSI的差异(P < 0.05)为黄


|
|
表 1 5种鱼的形体指标(平均值±标准误) Tab.1 Somatic parameters of five fish species (Mean±SE) |
通过粗脂肪结果可知(图 2),鲬的背肌粗脂肪显著高于其他组(P < 0.05),且其他组间无显著差异(P > 0.05)。黄
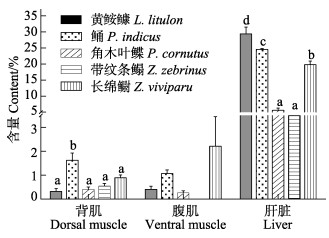
|
图 2 5种鱼的背肌、腹肌和肝脏的粗脂肪含量(湿重%, 平均值±标准误) Fig.2 Crude lipid content in dorsal muscle, ventral muscle and liver of five fish species (% wet weight, Mean±SE) 不同上标字母的同一组织的数据有显著差异(P < 0.05),下同。 Data bars for a same tissue not sharing a same superscript letter were significantly different (P < 0.05), the same as below. |
粗蛋白结果如图 3所示,其中,鲬的背肌和腹肌粗蛋白含量显著高于(P < 0.05)其他组,黄

|
图 3 5种鱼的背肌、腹肌和肝脏的粗蛋白含量(湿重%, 平均值±标准误) Fig.3 Crude protein content in dorsal muscle, ventral muscle and liver of five fish species (% wet weight, Mean±SE) |
水分含量如图 4所示,黄


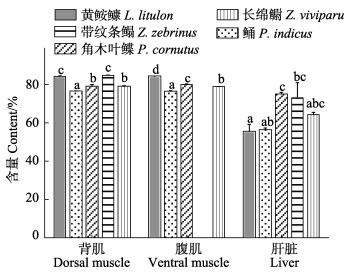
|
图 4 5种鱼的背肌、腹肌和肝脏的水分含量(湿重%, 平均值±标准误) Fig.4 Moisture content in dorsal muscle, ventral muscle and liver of five fish species (% wet weight, Mean±SE) |
背肌的脂肪酸组成如表 2 (图 5和图 6)所示。对于饱和脂肪酸(SFA),鲬背肌中总饱和脂肪酸(ΣSFA)含量最高,特别是16:0和14:0。相比之下,长绵鳚的16:0含量最低。
|
|
表 2 5种鱼背肌脂肪酸组成(%TFA, 平均值±标准误) Tab.2 The fatty acid composition in dorsal muscle of five fish species (% TFA, Mean±SE) |
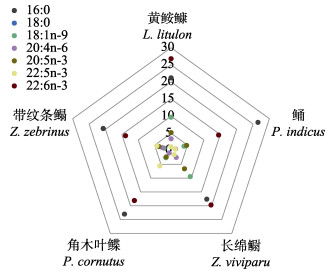
|
图 5 5种鱼类背肌中一些重要的脂肪酸组成 Fig.5 Some important fatty acid composition in dorsal muscle of five fish species |
|
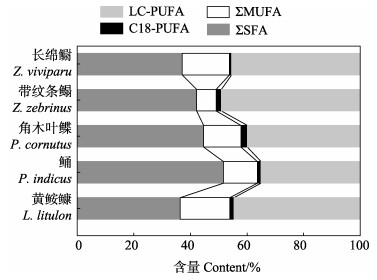
图 6 5种鱼的ΣSFA、ΣMUFA、ΣPUFA和ΣHUFA的相对含量 Fig.6 Relative content of ΣSFA, ΣMUFA, ΣPUFA, and ΣHUFA of five fish species SFA: 14:0, 16:0, 18:0 and 20:0; MUFA: 16:1n-7, 18:1n-9, 20:1n-9; PUFA: 18:2n-6, 18:3n-6, 18:3n-3; HUFA: 20:2n-6, 20:4n-6, 20:5n-3, 22:5n-3, 22:6n-3. |
对于单不饱和脂肪酸(MUFA),黄
黄

腹肌的ΣSFA无明显差异,但角木叶鲽的16:0含量明显高于黄


|
|
表 3 5种鱼腹肌脂肪酸(%TFA, 平均值±标准误) Tab.3 The fatty acid composition in ventral muscle of five fish species (% TFA, Mean±SE) |
不同鱼种之间的ΣSFA没有显著差异(表 4)。鲬的MUFA含量最高,特别是18:1n-9和16:1n-7,但这些脂肪酸的含量在带纹条鳎和长绵鳚中低得多。长绵鳚的ARA含量是黄

|
|
表 4 5种鱼肝脏脂肪酸(%TFA, 平均值±标准误) Tab.4 The fatty acid composition in liver of five fish species (% TFA, Mean±SE) |
各物种间脑部脂肪酸组成与肝脏非常相似,带纹条鳎的16:0含量较低,鲬的16:1n-7含量较高,长绵鳚的16:1n-7含量较低,长绵鳚的ARA含量远高于鲬和角木叶鲽,黄
|
|
表 5 5种鱼脑脂肪酸(%TFA, 平均值±标准误) Tab.5 The fatty acid composition in brain of five fish species (% TFA, Mean±SE) |
带纹条鳎的16:0含量较低,长绵鳚的ARA含量较高,以及角木叶鲽的22:5n-3含量高,但EPA含量低,这些在其他组织中的差异在眼中也同样被发现(表 6)。然而,对于16:1n-7,角木叶鲽中的含量比黄

|
|
表 6 5种鱼眼脂肪酸(%TFA, 平均值±标准误) Tab.6 The fatty acid composition in eye of five fish species (% TFA, Mean±SE) |
在皮肤中,黄

|
|
表 7 5种鱼皮肤脂肪酸(%TFA, 平均值±标准误) Tab.7 The fatty acid composition in skin of five fish species (% TFA, Mean±SE) |
角木叶鲽和带纹条鳎的皮下脂肪组织分别以高16:0和低DHA含量为特征。鲬腹腔内脂肪组织的特点是18:1n-9和EPA含量高(表 8)。
|
|
表 8 角木叶鲽和带纹条鳎鳍条附近皮下脂肪组织、鲬腹腔内脂肪组织的脂肪酸组成(%TFA, 平均值±标准误) Tab.8 The fatty acid composition in the subcutaneous adipose tissue around the fin of P. cornutus and Z. zebrinus, intraperitoneal adipose tissue of P. indicus (% TFA, Mean±SE) |
脂质在各组织中的含量可以反映在形体指标上。与其他鲆鲽鱼类一样,角木叶鲽和带纹条鳎的HSI和VSI较低。鲆鲽类的扁平体型决定了这类鱼的肝脏及腹腔内的脂肪组织不能储存较多的脂质,正如在被广泛研究的大菱鲆中观察到的一样(Wu et al, 2015; Xu et al, 2021)。相反,它们在鳍条周围的皮下脂肪组织中具有相对较高的脂肪含量(Wu et al, 2015; Xu et al, 2021)。这就是只对角木叶鲽和带纹条鳎进行鳍条周围皮下脂肪组织采样的原因。相比之下,黄

一般来说,硬骨鱼有4种典型的脂质储存模式。在这4种模式中,脂质主要分别储存在腹腔内脂肪组织(鲤鱼)、腹腔内脂肪组织+肌肉(大西洋鲑)、肝脏[红鳍东方鲀和大西洋鳕鱼(Gadus morhua)]和皮下脂肪组织(大菱鲆)(Katikou et al, 2001; Kaneko et al, 2016; 毕清竹等, 2020; Xu et al, 2021)。本实验室对红鳍东方鲀进行多年研究,发现其在脂肪酸积累和利用方面具有特殊之处(Xu et al, 2019a、b; Yu et al, 2019)。然而,这些特点似乎很少与生态位有关。红鳍东方鲀和黄
无论脂质分布模式如何,虽然肝脏和眼可以加工成鱼油产品并被人类间接食用。肌肉仍是人类食用的主要部分。在研究的5种底栖型鱼类中,肌肉脂质含量都很低(˂2%)。这与中上层鱼类不同,中上层鱼类在游泳过程中会在肌肉中储存大量的脂质,如大西洋鲑鱼(7.4%~18.3%)、太平洋蓝鳍金枪鱼(Thunnus orientalis) (7.5%~20.1%)和玉筋鱼(Ammodytes personatus) (4.13%~6.99%) (Tsukamasa et al, 2007; Ornholt-Johansson et al, 2017; 刘胜男等, 2022)。相反,它们的蛋白(鲬、角木叶鲽和带纹条鳎)或水分(黄


黄




关于其他LC-PUFA,研究发现角木叶鲽的22:5n-3含量较高,但EPA含量较低,这是值得探究的。我们最近的一项研究显示,海水鱼类中具有高含量的22:5n-3,可能是来源于EPA的延长(Xu et al, 2020a)。考虑到大多数海洋鱼类缺乏有效表达Elovl2和Δ6去饱和酶的能力,22:5n-3进一步生物转化为DHA是有限的(Tocher et al, 2006; Geay et al, 2010; Castro et al, 2016)。本研究表明,角木叶鲽可能有相对较强的将EPA延长为22:5n-3的能力。此外,上述研究显示,鱼类肌肉中的22:5n-3与盐度呈正相关(R2=0.324; P<0.001)。显然5种鱼之间没有盐度等级。因此,需要进一步研究角木叶鲽的C22:5n-3含量高的具体原因。
在5种鱼中,长绵鳚在几乎所有组织中的ARA含量都比其他鱼种高很多。与DHA和EPA相比,食物中ARA的重要性相对被忽视。然而,特别是在过去的20年中,人们越来越关注ARA在鱼类营养和人类健康中的需求和功能(Bell et al, 2003; Astudillo et al, 2012; Stenson, 2014; Xu et al, 2017; Turchini et al, 2022)。在海水草食性鱼类如褐篮子鱼(Siganus fuscescens)中观察到了非常高的ARA水平(Osakoa et al, 2006; Jiarpinijnun et al, 2017),但Shanab等(2018)研究发现,这可能是由于食物来源形成的,即藻类中的高ARA水平。在所研究的5种鱼类中,长绵鳚的营养级是最低的(3.2)。其体内高ARA含量可能是其摄食富含ARA的藻类。此外,ARA的抗逆调节作用也可能解释了一些鱼体中的高ARA含量的原因。例如,在河口环境的地中海沿岸泻湖中,观察到鱼类的高ARA含量(Koussoroplis et al, 2011),高ARA需求可能与环境抗逆需求相关。当花鲈养殖在波动大的温度、氧气和浑浊度下,研究发现,在饲料中添加ARA具有显著的必要性(Xu et al, 2010)。Norambuena等(2012)也有同样发现,在水温发生变化时,塞内加尔鳎鱼(Solea senegalensis)将优先选择富含ARA的饲料,均表明鱼体可能增加对ARA的需求以应对环境胁迫。长绵鳚经常生活在海浪较大的近岸岩石环境中,这一环节胁迫,这可能是这种鱼中ARA含量高的部分原因。
与LC-PUFA相比,主要由18:2n-6和18:3n-3组成的18C的多不饱和脂肪酸(18C-PUFA),在5种鱼中含量不高。18C-PUFA主要来自陆生植物(Griel et al, 2008),因此在海洋鱼类中并不丰富,当然,这些脂肪酸也不是鱼产品消费者的关注点。
关于MUFA和SFA,鲬在腹肌、肝脏、脑、眼和皮肤中的16:1n-7含量高得多,肝脏中18:1n-9含量高,背肌中16:0含量也比其他鱼高。黄
研究表明,鲆鲽鱼类鳍周围的皮下脂肪组织和鲬的腹腔脂肪组织是主要的脂质储存场所。鲬的腹腔脂肪组织具有较高的18:1n-9含量,该脂肪酸是已知的三酰甘油合成的主要底物,通常用于能量储存(Szabo et al, 2011)。然而,角木叶鲽和带纹条鳎的皮下脂肪组织中16:0含量比18:1n-9高,表明在这一部位的脂质储存中可能更优先选择16:0。16:0是鱼类β-氧化的首选底物之一(Stubhaug et al, 2006)。此外,16:0在磷脂中也很丰富,特别是在磷脂酰胆碱中,其次是在磷脂酰乙醇胺中(Trushenski et al, 2008),因此,16:0可能在某种程度上被选择性地保留在富含磷脂的组织。虽然没有进行测定,但角木叶鲽和带纹条鳎的皮下脂肪组织中磷脂含量可能很高。
不同组织的脂肪酸差异在不同底栖型鱼类中存在大致相同的趋势。大体上,肌肉中16:0、18:0、EPA和DHA含量较高(胡文静等, 2020; 王腾等, 2021);眼和脑等组织中富含DHA和EPA等长链多不饱和脂肪酸含量;而肝脏、皮下脂肪和腹腔脂肪组织作为脂质储存的场所,其18:1n-9含量较高。不同组织间的脂肪酸组成差别与特定脂肪酸在各组织中的功能密切相关,如肌肉需为游泳运动提供能量,作为氧化供能的重要底物16:0含量则高于其他组织(Stubhaug et al, 2006),此外,肌肉含有大量的磷脂,磷脂中富含16:0和18:0 (Trushenski et al, 2008),因此,16:0和18:0相对高于其他组织;而作为能量储存的组织,肝脏、皮下脂肪和腹腔脂肪组织中脂质以三酰甘油的形式存在,18:1n-9是三酰甘油的主要底物(Szabo et al, 2011),因此,这些组织中18:1n-9较多于其他组织。此外,DHA、EPA和ARA对于神经和视觉发育起着重要作用,由此这些脂肪酸在眼和脑组织中含量较高。
4 结论本研究中,5种鱼中肌肉脂肪含量都很低,特别是黄






ASTUDILLO A M, BALGOMA D, BALBOA M A, et al. Dynamics of arachidonic acid mobilization by inflammatory cells. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2012, 1821(2): 249-256 |
BAGTHASINGH C, ARAN S S, VETRI V, et al. Seasonal variation in the proximate composition of sardine (Sardinella gibbosa) from Thoothukudi coast. Indian Journal of Geo-Marine Sciences, 2016, 45: 800-806 |
BELL G J, SARGENT, J R. Arachidonic acid in aquaculture feeds: Current status and future opportunities. Aquaculture, 2003, 218(1/2/3/4): 491-499 |
BI Q Z, LIANG M Q, LIAO Z B, et al. Effect of dietary bile acid supplementation on fatty acid composition and anti-oxidative capacity of tiger puffer Takifugu rubripes. Journal of Shanghai Ocean University, 2020, 29(6): 829-839 [毕清竹, 梁萌青, 廖章斌, 等. 饲料中胆汁酸对红鳍东方鲀脂肪酸组成及抗氧化能力的影响. 上海海洋大学学报, 2020, 29(6): 829-839] |
CASTRO L F C, TOCHER D R, MONROIG O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Progress in Lipid Research, 2016, 62: 25-40 DOI:10.1016/j.plipres.2016.01.001 |
FROESE R, PAULY D. FishBase [EB/OL]. (2019-8) [2022-2-13]. http://www.fishbase.de/search.php
|
GEAY F, CULI E S I, CORPOREAU C, et al. Regulation of FADS2 expression and activity in European sea bass (Dicentrarchus labrax, L.) fed a vegetable diet. Comparative Biochemistry and Physiology, Part B: Biochemistry and Molecular Biology, 2010, 156(4): 237-243 DOI:10.1016/j.cbpb.2010.03.008 |
GRIEL A E, CAO Y, BAGSHAW D D, et al. A macadamia nut-rich diet reduces total and LDL-Cholesterol in mildly hypercholesterolemic men and women. Journal of Nutrition, 2008, 138(4): 761-767 DOI:10.1093/jn/138.4.761 |
HENDERSON R J. Fatty acid metabolism in freshwater fish with particular reference to polyunsaturated fatty acids. Archives of Animal Nutrition, 1996, 49(1): 5-22 |
HU W J, SU C Q, LIU T, et al. Analysis of muscle nutrient composition of Cyprinus carpio var color in Qingtian and Jinhua. Journal of Shanghai Ocean University, 2020, 29(4): 552-558 [胡文静, 苏超群, 刘韬, 等. 青田田鱼和金华田鱼肌肉营养成分的比较. 上海海洋大学学报, 2020, 29(4): 552-558] |
JIARPINIJNUN A, BENJAKUL S, PORNPHATDETAUDOM A, et al. High arachidonic acid levels in the tissues of herbivorous fish species (Siganus fuscescens, Calotomus japonicus and Kyphosus bigibbus). Lipids, 2017, 52(4): 363-373 DOI:10.1007/s11745-017-4244-3 |
KANEKO G, SHIRAKAMI H, HIRANO Y, et al. Diversity of lipid distribution in fish skeletal muscle. Zoological Science, 2016, 33(2): 170-178 DOI:10.2108/zs150096 |
KATIKOU P, HUGHES S I, ROBB D H F. Lipid distribution within Atlantic salmon (Salmo salar) fillets. Aquaculture, 2001, 202(1/2): 89-99 |
KAYA Y, TURAN H. Comparison of protein, lipid and fatty acids composition of anchovy (Engraulis encrasicolus L. 1758) during the commercial catching season. Journal of Muscle Foods, 2010, 21(3): 474-483 |
KHIEOKHAJONKHET A, KLONGCHAI S, MAPHUM O, et al. Lipid distribution patterns of nine commercial fish in Thailand. Aquaculture Research, 2019, 50(4): 1348-1360 DOI:10.1111/are.14011 |
KIM S M, KIM H, LEE W C, et al. Monthly variation in the proximate composition of jack mackerel (Trachurus japonicus) from Geumo Island, Korea. Fisheries Research, 2016, 183: 371-378 DOI:10.1016/j.fishres.2016.07.009 |
KOUSSOROPLIS A M, BEC A, PERGA M E, et al. Fatty acid transfer in the food web of a coastal Mediterranean lagoon: Evidence for high arachidonic acid retention in fish. Estuarine, Coastal and Shelf Science, 2011, 91(3): 450-461 DOI:10.1016/j.ecss.2010.11.010 |
LIAO Z, SUN Z, BI Q, et al. Application of the fish oil-finishing strategy in a lean marine teleost, tiger puffer (Takifugu rubripes). Aquaculture, 2021, 534: 736306 DOI:10.1016/j.aquaculture.2020.736306 |
LIU S N, WANG S Y, CAO R, et al. Nutritional composition analysis and quality evaluation of different sizes of Ammodytes personatus. Progress in Fishery Sciences, 2022, 43(1): 188-194 [刘胜男, 王善宇, 曹荣, 等. 不同规格玉筋鱼的营养分析与评价. 渔业科学进展, 2022, 43(1): 188-194] |
MAILLARD V, DESMARCHAIS A, DURCIN M, et al. Docosahexaenoic acid (DHA) effects on proliferation and steroidogenesis of bovine granulosa cells. Reproductive Biology and Endocrinology, 2018, 16(1): 1-18 DOI:10.1186/s12958-017-0318-6 |
NANTON D, VEGUSDAL A, RØRÅ A M B, et al. Muscle lipid storage pattern, composition, and adipocyte distribution in different parts of Atlantic salmon (Salmo salar) fed fish oil and vegetable oil. Aquaculture, 2007, 265: 230-243 DOI:10.1016/j.aquaculture.2006.03.053 |
NORAMBUENA F, ESTÉVEZ A, SÁNCHEZ-VÁZQUEZ F J, et al. Self-selection of diets with different contents of arachidonic acid by Senegalese sole (Solea senegalensis) broodstock. Aquaculture, 2012, 364: 198-205 |
ORNHOLT-JOHANSSON G, FROSCH S, JORGENSEN B M. Variation in some quality attributes of Atlantic salmon fillets from aquaculture related to geographic origin and water temperature. Aquaculture, 2017, 479: 378-383 DOI:10.1016/j.aquaculture.2017.06.016 |
OSAKOA K, SAITOB H, KUWAHARAA K, et al. Year-round high arachidonic acid levels in herbivorous rabbit fish Siganus fuscescens tissues. Lipids, 2006, 41(5): 473-489 DOI:10.1007/s11745-006-5121-7 |
REN W, LI J, TAN P, et al. Lipid deposition patterns among different sizes of three commercial fish species. Aquaculture Research, 2018, 49(2): 1046-1052 DOI:10.1111/are.13553 |
SARGENT J R, TOCHER D R, BELL J G. Fish nutrition (third edition). San Diego: Academic Press, 2002
|
SHANAB S M M, HAFEZ R M, FOUAD A S. A review on algae and plants as potential source of arachidonic acid. Journal of Advanced Research, 2018, 11: 3-13 DOI:10.1016/j.jare.2018.03.004 |
SONG H M, QU Z W, WANG X J, et al. Analysis and assessment for nutritional components of the muscle of Datnioides pulcher. Progress in Fishery Sciences, 2020, 41(5): 177-184 [宋红梅, 屈政委, 汪学杰, 等. 印尼拟松鲷肌肉营养成分分析与评价. 渔业科学进展, 2020, 41(5): 177-184] |
STENSON W. The universe of arachidonic acid metabolites in inflammatory bowel disease: Can we tell the good from the bad?. Current Opinion in Gastroenterology, 2014, 30(4): 347-351 DOI:10.1097/MOG.0000000000000075 |
STUBHAUG I, FROYLAND L, TORSTENSEN B E. β-oxidation capacity of red and white muscle and liver in Atlantic salmon (Salmo salar L.)—Effects of increasing dietary rapeseed oil and olive oil to replace capelin oil. Lipids, 2005, a, 40(1): 39-47 |
STUBHAUG I, LIE O, TORSTENSEN B E. Fatty acid productive value and β-oxidation capacity in Atlantic salmon (Salmo salar L.) fed on different lipid sources along the whole growth period. Aquaculture Nutrition, 2007, 13(2): 145-155 DOI:10.1111/j.1365-2095.2007.00462.x |
STUBHAUG I, LIE O, TORSTENSEN B E. β-oxidation capacity in liver increases during parr-smolt transformation of Atlantic salmon (Salmo salar L.) fed vegetable and fish oil. Journal of Fish Biology, 2006, 69(2): 504-517 DOI:10.1111/j.1095-8649.2006.01122.x |
STUBHAUG I, TOCHER D R, BELL J G, et al. Fatty acid metabolism in Atlantic salmon (Salmo salar L.) hepatocytes and influence of dietary vegetable oil. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2005, b, 1734(3): 277-288 |
SU C C, SHAN X J, YANG T, et al. Interdecadal changes in keystone species of fish community during autumn in the Yellow Sea. Journal of Shanghai Ocean University, 2021, 42(6): 1-14 [苏程程, 单秀娟, 杨涛, 等. 黄海秋季鱼类群落关键种的年代际变化. 渔业科学进展, 2021, 42(6): 1-14] |
SZABO A, MEZES M, HANCZ C, et al. Incorporation dynamics of dietary vegetable oil fatty acids into the triacylglycerols and phospholipids of tilapia (Oreochromis niloticus) tissues (fillet, liver, visceral fat and gonads). Aquaculture Nutrition, 2011, 17(2): e132-e147 DOI:10.1111/j.1365-2095.2009.00743.x |
TAKAMA K, SUZUKI T, YOSHIDA K, et al. Lipid content and fatty acid composition of phospholipids in white-flesh fish species. Fisheries Science, 1994, 60(2): 177-184 DOI:10.2331/fishsci.60.177 |
TEOH C Y, TURCHINI G M, NG W K. Genetically improved farmed Nile tilapia and red hybrid tilapia showed differences in fatty acid metabolism when fed diets with added fish oil or a vegetable oil blend. Aquaculture, 2011, 312: 126-136 DOI:10.1016/j.aquaculture.2010.12.018 |
TOCHER D R, BETANCOR M B, SPRAGUE M, et al. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients, 2019, 11(1): 89 DOI:10.3390/nu11010089 |
TOCHER D R, FONSECA-MADRIGAL J, BELL J G, et al. Effects of diets containing linseed oil on fatty acid desaturation and oxidation in hepatocytes and intestinal enterocytes in Atlantic salmon (Salmo salar). Fish Physiology and Biochemistry, 2002, 26(2): 157-170 DOI:10.1023/A:1025416731014 |
TOCHER D R, ZHENG X, SCHLECHTRIEM C, et al. Highly unsaturated fatty acid synthesis in marine fish: Cloning, functional characterization, and nutritional regulation of fatty acyl Δ6 desaturase of Atlantic cod (Gadus morhua L.). Lipids, 2006, 41(11): 1003-1016 DOI:10.1007/s11745-006-5051-4 |
TOCHER D R. Metabolism and functions of lipids and fatty acids in teleost fish. Reviews in Fisheries Science, 2003, 11(2): 107-184 DOI:10.1080/713610925 |
TORSTENSEN B E, FROYLAND L, LIE O. Replacing dietary fish oil with increasing levels of rapeseed oil and olive oil—Effects on Atlantic salmon (Salmo salar L.) tissue and lipoprotein lipid composition and lipogenic enzyme activities. Aquaculture Nutrition, 2004, 10(3): 175-192 DOI:10.1111/j.1365-2095.2004.00289.x |
TRUSHENSKI J T, LEWIS H A, KOHLER C C. Fatty acid profile of sunshine bass: Ⅱ Profile change differs among fillet lipid classes. Lipids, 2008, 43(7): 643-653 |
TSUKAMASA Y, NAKAMURA Y N, ANDO M, et al. Changes in myoglobin content and proximate compositions of the dorsal ordinary muscles of full-cycle cultured Pacific bluefin tuna Thunnus orientalis (Temminck et Schlegel) with body size. Aquaculture Research, 2007, 38(2): 201-205 DOI:10.1111/j.1365-2109.2007.01656.x |
TURCHINI G M, FRANCIS D S, DU Z, et al. Fish nutrition. San Diego: Academic Press, 2022
|
TURCHINI G M, FRANCIS D S. Fatty acid metabolism (desaturation, elongation and β-oxidation) in rainbow trout fed fish oil-or linseed oil-based diets. British Journal of Nutrition, 2009, 102(1): 69-81 DOI:10.1017/S0007114508137874 |
WANG T, GAO C X, WANG S Q, et al. Characteristics of fatty acid composition and dietary indication of small yellow croaker in the offshore waters of southern Zhejiang. Journal of Shanghai Ocean University, 2021, 30(6): 992-1001 [王腾, 高春霞, 王少琴, 等. 浙江南部近海小黄鱼肌肉脂肪酸组成及食源指示分析. 上海海洋大学学报, 2021, 30(6): 992-1001] |
WU J L, ZHANG J L, DU X X, et al. Evaluation of the distribution of adipose tissues in fish using magnetic resonance imaging (MRI). Aquaculture, 2015, 448: 112-122 DOI:10.1016/j.aquaculture.2015.06.002 |
XU H G, AI Q H, MAI K S, et al. Effects of dietary arachidonic acid on growth performance, survival, immune response and tissue fatty acid composition of juvenile Japanese seabass, Lateolabrax japonicus. Aquaculture, 2010, 307(1/2): 75-82 |
XU H G, CAO L, ZHANG Y Q, et al. Dietary arachidonic acid differentially regulates the gonadal steroidogenesis in the marine teleost, tongue sole (Cynoglossus semilaevis), depending on fish gender and maturation stage. Aquaculture, 2017, 468(1): 378-385 |
XU H G, LIAO Z B, ZHANG Q G, et al. A moderately high level of dietary lipid inhibited the protein secretion function of liver in juvenile tiger puffer Takifugu rubripes. Aquaculture, 2019, a, 498: 17-27 |
XU H G, LIAO Z B, ZHANG Q G, et al. Effects of dietary n-6 polyunsaturated fatty acids on growth performance, body composition, haematolog ameters and hepatic physiology of juvenile tiger puffer (Takifugu rubripes). Aquaculture Nutrition, 2019, b, 25(5): 1073-1086 |
XU H G, TURCHINI G M, FRANCIS D S, et al. Are fish what they eat? A fatty acid's perspective. Progress in Lipid Research, 2020, a, 80: 101064 |
XU H G, ZHANG Q G, KIM S K, et al. Dietary taurine stimulates the hepatic biosynthesis of both bile acids and cholesterol in the marine teleost, tiger puffer (Takifugu rubripes). British Journal of Nutrition, 2020, b, 123(12): 1345-1356 |
XU J J, GUO Y J, HUANG X C, et al. Effects of DHA dietary intervention on hepatic lipid metabolism in apolipoprotein E-deficient and C57BL/6J wild-type mice. Biomed. Biomedicine and Pharmacotherapy, 2021, 144: 112329 |
YAMAGATA K. Dietary docosahexaenoic acid inhibits neurodegeneration and prevents stroke. Journal of Neuroscience Research, 2021, 99: 561-572 |
YANG J M. A study on food and trophic levels of Bohai Sea intertebrates. Modern Fisheries Information, 2001, 16(10): 10-19 [杨纪明. 渤海鱼类的食性和营养级研究. 现代渔业信息, 2001, 16(10): 10-19] |
YU G J, OU W H, LIAO Z B, et al. Intestinal homeostasis of juvenile tiger puffer Takifugu rubripes was sensitive to dietary arachidonic acid in terms of mucosal barrier and microbiota. Aquaculture, 2019, 502: 97-106 |
YU R, JIN L B, FUJIMOTO M. Dihydroartemisinin: A potential drug for the treatment of malignancies and inflammatory diseases. Frontiers in Oncology, 2021, 99(3): 193-202 |



