2. 青岛大学基础医学院 山东 青岛 266071;
3. 大连海洋大学水产与生命学院 辽宁 大连 116023
2. Basic Medicine College, Qingdao University, Qingdao 266071, China;
3. College of Fisheries and Life Science, Dalian Ocean University, Dalian 116023, China
抗菌肽(antimicrobial peptides, AMPs)主要通过参与先天免疫系统来保护生物体免受病原体的侵害(Fjell et al, 2011; Nguyen et al, 2011)。不同种类的AMP对细菌、病毒和原生动物等均表现出一定杀伤作用(Boas et al, 2016; Hong et al, 2019; Mohammadi et al, 2020; Travkova et al, 2017; Shaykhiev et al, 2005; Valero et al, 2020)。肝脏表达抗菌肽2 (liver-expressed antimicrobial peptide 2, LEAP-2)属于抗菌肽家族,是脊椎动物先天免疫系统抵御病原体入侵的关键成分。LEAP-2在进化过程中高度保守,含有4个高度保守的半胱氨酸残基和2个二硫键,能破坏细菌细胞膜的结构完整性,具有抗菌活性(Li et al, 2015)。
LEAP-2在脊椎动物中得到了广泛研究,Krause等(2003)从血液中首次分离出人类LEAP-2,并对其进行了鉴定。研究表明,哺乳动物LEAP-2不仅具有抗菌、抗病毒功能,还具有调节生长发育的作用(Omar et al, 2018; Ge et al, 2018)。鱼类LEAP-2的研究主要集中于分子特征和抗菌活性分析上,最早关于LEAP-2的研究是克隆了虹鳟(Oncorhynchus mykiss)的LEAP-2A和LEAP-2B基因(Zhang et al, 2004)。进一步研究发现,鲶鱼(Silurus asotus)、团头鲂(Megalobrama amblycephala)、草鱼(Ctenopharynodon idellus)、卵形鲳鲹(Trachinotus ovatus) LEAP-2均包含一个信号肽和一个成熟肽,且成熟肽中包含4个高度保守的半胱氨酸残基(Bao et al, 2006; Liang et al, 2013; Liu et al, 2010; Liu et al, 2020)。
俄罗斯鲟(Acipenser gueldenstaedti)在我国具有一定的养殖规模,嗜水气单胞菌(Aeromonas hydrophila)是鲟鱼的主要病原菌,感染后会出现腹水和组织出血等症状,导致鲟鱼大量死亡,从而造成巨大经济损失(Di et al, 2018)。水产养殖业中化学药物和抗生素的禁用,使得重组抗菌肽研究引起重视。目前,俄罗斯鲟LEAP-2 (AgLEAP-2)基因的研究未见报道。本研究克隆并鉴定AgLEAP-2基因,并对其组织分布情况、病原菌感染后的表达模式以及抗菌活性等进行研究,旨在为进一步研究鲟鱼LEAP-2的病原防御机制提供理论基础。
1 材料与方法 1.1 实验材料实验鱼为体长(47.5±2.5) cm、体重(450±10) g、1.5龄的健康俄罗斯鲟(杭州千岛湖鲟鱼集团有限公司提供)。在直径2 m、水深0.5 m、水温17~20 ℃的圆形玻璃钢养殖箱中暂养15 d,以备后续实验使用。
实验菌包括用于感染俄罗斯鲟的嗜水气单胞菌,以及用于体外抗菌活性检测的大肠杆菌(Escherichia coli)、金黄色葡萄球菌(Staphylococcus aureus)、链球菌(Streptococcus)、鳗弧菌(Vbrio anguillarum)和希瓦氏菌(Shewanella)。实验过程中所用到的菌种均为本实验室长期保存菌种,冻存于–80 ℃。
1.2 样品采集 1.2.1 健康俄罗斯鲟组织样品采集选取3尾健康俄罗斯鲟个体,分别收集肝脏、脾脏、肠道、鳃、血液、头肾、后肾、胃、皮肤、脑、心脏、肌肉和卵巢共13个组织,立即置于液氮中冷冻,然后转入–80 ℃保存。
1.2.2 嗜水气单胞菌感染及样品采集采用腹腔注射的方式,注射体积为100 µL半致死浓度为2.07× 104 CFU/g的嗜水气单胞菌悬液。对照组注射等体积的PBS溶液。设定注射前0 h和注射后6、12、24、48和72 h共6个时间点,每个时间点分别采集3尾俄罗斯鲟的肝、脾、鳃、血液、头肾和肠6种组织,立即投入液氮中,并转入–80 ℃保存,用于RNA提取。
1.3 RNA提取和cDNA合成取出冷冻组织,根据TransZol Up试剂盒(全式金)的方法提取组织总RNA。RNA浓度和纯度通过微量分光光度计测定,RNA完整性通过1%琼脂糖凝胶电泳检测。确定RNA质量后,用PrimeScriptTM第一链cDNA合成试剂盒(TaKaRa,日本)合成cDNA,于–20 ℃保存。
1.4 AgLEAP-2基因全长cDNA的克隆以俄罗斯鲟性腺转录组数据中筛选出的AgLEAP-2基因的部分cDNA序列为靶序列(Chen et al, 2016),通过Primer Premier 5.0软件设计特异性引物P1和P2 (表 1)。以健康俄罗斯鲟13个组织的混合cDNA为模板进行PCR扩增,并验证其ORF。普通PCR反应体系:10 µL 2×Taq Master Mix (诺唯赞,中国),引物P1和P2各1 µL,cDNA模板2 µL,ddH2O补至50 µL。反应条件:94 ℃ 5 min;30个循环(94 ℃ 30 s,60 ℃ 30 s,72 ℃ 1 min);72 ℃ 10 min。将PCR产物进行1%琼脂糖凝胶电泳检测,并使用胶回收试剂盒(天根,中国)进行纯化回收。将胶回收产物与pEASY-T1载体(全式金,中国)连接,42 ℃热激转入Trans-T1感受态细胞,过夜培养并选择阳性克隆进行测序,获得AgLEAP-2的ORF序列。
按照获得的AgLEAP-2基因的ORF序列,设计2条特异引物(GSP5′和GSP3′),通过RACE方法扩增未知cDNA序列。RACE扩增的通用引物为short primer和long primer (表 1)。使用SmartTM RACE cDNA扩增试剂盒(BD,美国)进行5′-RACE和3′-RACE扩增。Touchdown PCR条件:5个循环(94 ℃ 2 min;94 ℃ 5 s,72 ℃ 3 min);10个循环(94 ℃ 5 s,70 ℃ 10 s,72 ℃ 3 min);20个循环(94 ℃ 5 s,64 ℃ 10 s,72 ℃ 3 min);最后72 ℃ 10 min。扩增得到的片段使用QiaexⅡ凝胶提取试剂盒(Qiagen,德国)进行分离纯化,纯化产物与载体连接,转入感受态细胞,过夜培养并选择阳性克隆进行测序,获得AgLEAP-2全长cDNA序列。
|
|
表 1 本研究中使用的引物及序列 Tab.1 The primers used in this study |
使用BLAST (http://www.ncbi.nlm.nih.gov/BLAST/)将AgLEAP-2和其他物种LEAP-2的氨基酸序列进行同源性比对。采用在线软件SMART (http://SMART.embl-heidelberg.de/)预测蛋白质结构域。使用在线软件ExPASy (http://au.expasy.org/tools/protparam.html)计算AgLEAP-2的相对分子质量。通过ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder/)查找AgLEAP-2的开放阅读框。通过DNAMAN(http://www.Lynnon.com/)进行氨基酸序列比对。通过MEGA-X软件,基于AgLEAP-2及其他鱼类LEAP-2A、LEAP-2B和LEAP-2C的氨基酸序列,使用NJ法构建系统发育树。使用软件SWISS-MODEL (https://swissmodel.expasy.org/)预测AgLEAP-2和人类LEAP-2蛋白的三维结构。
1.6 AgLEAP-2基因mRNA表达水平分析基于测序获得的AgLEAP-2部分cDNA序列,将qRT-PCR引物为P5和P6 (表 1),并将俄罗斯鲟的18S rRNA作为内参基因,引物为P7和P8 (表 1) (Livak et al, 2001; Lu et al, 2015)。根据Talent qPCR PreMix (SYBR Green) (天根,中国)的说明书,在ABI 7500快速实时定量扩增仪(ABI,美国)进行目的基因qRT-PCR检测。每个样本设置3个生物重复,用2–ΔΔCt法计算目的基因在不同组织中的相对表达量。
1.7 rAgLEAP-2蛋白的表达和纯化根据CDS (coding domain sequence)区序列,设计特异性引物P3和P4 (表 1),用健康俄罗斯鲟13种组织的混合cDNA作为模板进行PCR扩增反应。将阳性克隆产物连接到pEASY-Blunt E1 (全式金,中国)上。然后将连接产物转入BL21 (DE3)感受态细胞过夜培养,挑取阳性克隆并测序。
将测序正确的阳性克隆在含有氨苄青霉素的LB培养基中培养,当OD600 nm达到0.6~0.8时,使用终浓度为1 mmol/L的IPTG,在28 ℃条件下,转速200 r/min过夜诱导12 h。4 ℃条件下,转速12 000 r/min离心5 min,收集细菌沉淀。向收集的菌体沉淀中加入15 mL裂解液、终浓度为1 mmol/L的PMSF和0.3 mg/mL的溶菌酶,充分混合并通过离心收集沉淀。将预冷的溶解液加入到沉淀物中,4 ℃条件下,磁力搅拌2 h,直到溶液透明。离心收集上清液,用His Trap FF 1 mL纯化柱纯化rAgLEAP-2蛋白。
1.8 体外抗菌活性实验大肠杆菌、鳗弧菌、希瓦氏菌、链球菌和金黄色葡萄球菌的培养条件见表 2。当菌液吸光度OD600 nm为0.6~0.8时,将菌液稀释至102~103 CFU/mL。1×PBS为对照组,将rAgLEAP-2蛋白以100、200、300、400和500 μg/mL浓度梯度混合溶解于琼脂培养基中,然后将不同细菌的菌液分别涂布于混合不同浓度rAgLEAP-2蛋白的琼脂培养基表面。根据不同菌种的适宜温度培养过夜,第2天进行菌落计数并统计分析rAgLEAP-2蛋白对不同细菌的抗菌效果。
|
|
表 2 实验菌的培养条件 Tab.2 Culture conditions of experimental bacteria |
qRT-PCR结果均以平均值±标准误(Mean±SE)表示,为确保实验过程中数据的准确性,设置3个生物学重复,每个生物学重复设置3个技术重复。采用SPSS 26.0统计软件对嗜水气单胞菌感染组和PBS组之间AgLEAP-2基因的相对表达量结果进行单因素方差分析(one-way ANOVA)和Duncan多重比较,并认为P < 0.05时,具有显著性差异。
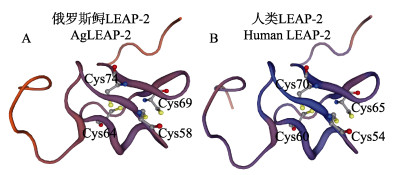
2 结果 2.1 AgLEAP-2基因克隆通过RACE获得AgLEAP-2基因全长,其cDNA全长为644 bp,5′-UTR为184 bp和3′-UTR为192 bp。AgLEAP-2的ORF长度为246 bp,编码81个氨基酸。AgLEAP-2蛋白的分子量为11.2 kDa,理论等电点为9.15。信号肽和结构域分析结果显示,该氨基酸序列包含由25个氨基酸组成的信号肽和由43个氨基酸组成的成熟肽(图 1)。蛋白质结构预测分析表明(图 2A、B),AgLEAP-2和人类(Homo sapiens) LEAP-2蛋白质均含有4个保守的半胱氨酸残基,AgLEAP-2在Cys58-Cys69和Cys64-Cys74的相对位置之间分别形成1对二硫键,人类LEAP-2在Cys54-Cys65和Cys60-Cys70的相对位置之间分别形成1对二硫键,AgLEAP-2符合LEAP-2家族的结构特征。

|
图 1 AgLEAP-2基因的核苷酸与氨基酸序列 Fig.1 Nucleotide and deduced amino acid sequence of AgLEAP-2 大写字母:开放阅读框;小写字母:非编码区;下划线:信号肽;蓝色框:成熟肽;黑色框:起始密码子(ATG)和终止密码子(TAG);灰色阴影:半胱氨酸残基(C)。 Uppercase letters: ORF; Lowercase letters: UTR; Underline: Signal peptide; Blue box: Mature peptide; Black box: Start codon (ATG) and stop codon (TAG); Gray shadow: Cysteine residue (C). |
|
图 2 俄罗斯鲟(A)和人类(B) LEAP-2蛋白质的空间结构 Fig.2 Spatial structure of LEAP-2 protein of A. gueldenstaedti (A) and human (B) |
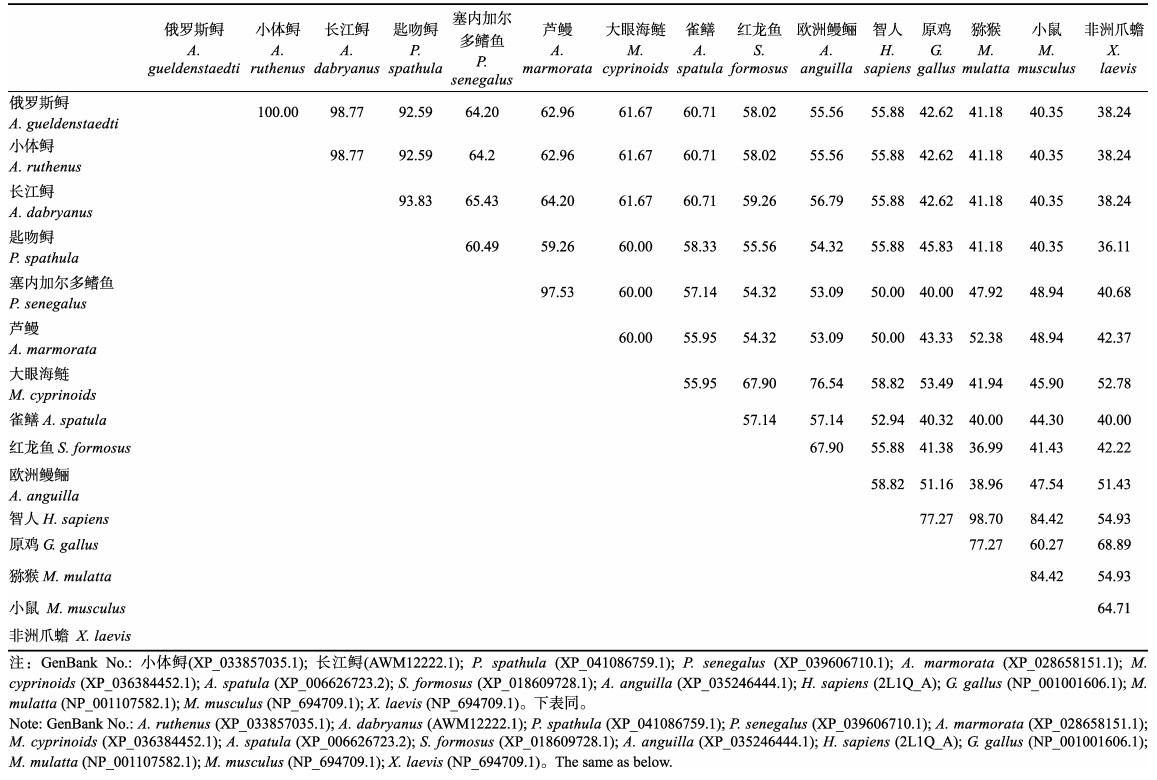
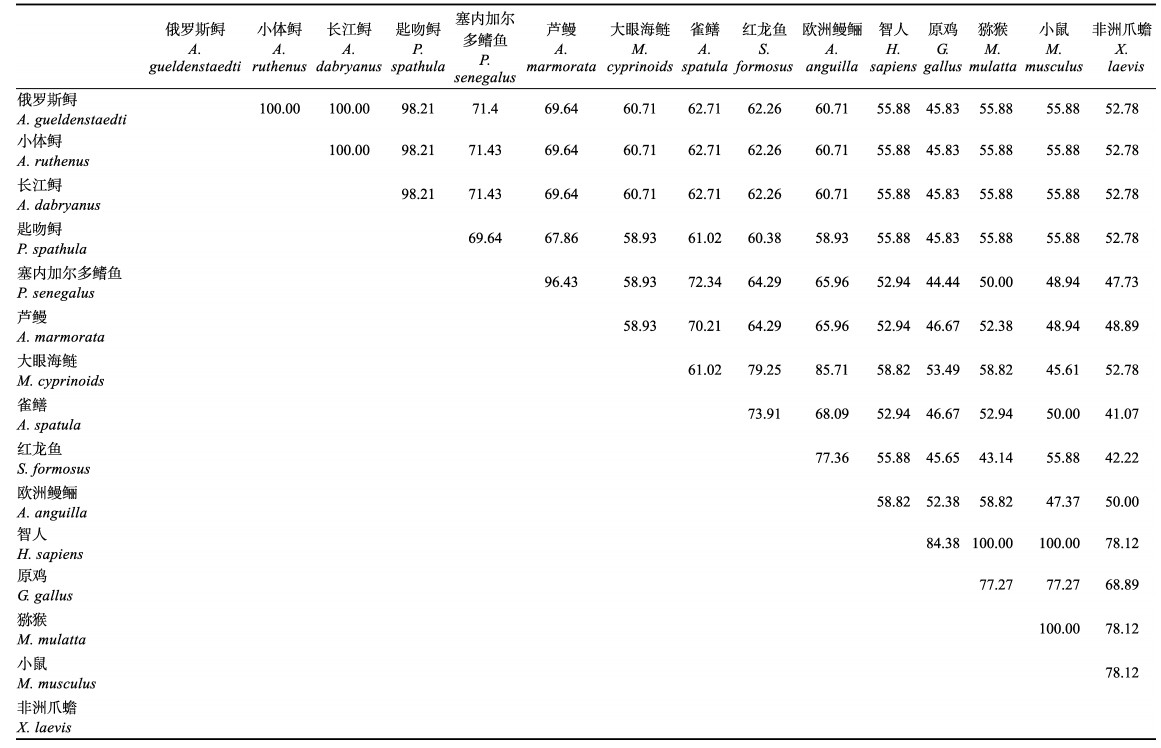
利用BLAST和DNAMAN软件比较了包含信号肽和去除信号肽的AgLEAP-2与哺乳动物、两栖动物、鸟类和鱼类等14种动物的氨基酸序列。氨基酸多重序列比对结果显示,包含信号肽的AgLEAP-2与其他物种LEAP-2的序列相似性在38.24%和100%之间(表 3)。AgLEAP-2与鱼类的亲缘关系更近,其中与小体鲟(Acipenser ruthenus)相似性最高(100%),与欧洲鳗鲡(Anguilla anguilla)相似性最低(55.56%);与哺乳动物、两栖动物和鸟类相比,与人类相似性最高,与热带爪蟾(Xenopus tropicalis)相似性最低(38.24%) (图 3A)。去除信号肽的AgLEAP-2与其他物种LEAP-2序列的相似性为45.83%~100% (表 4)。其中,与小体鲟和长江鲟(Acipenser dabryanus)的相似性最高(100%),与大眼海鲢(Megalops cyprinoides)的相似性最低(60.71%);与人类的相似性最高(55.88%),与原鸡(Gallus gallus)的相似性最低(45.83%) (图 3B)。
|
|
表 3 含有信号肽的LEAP-2蛋白的两两相似性比较/% Tab.3 Pairwise similarity of selected LEAP-2 proteins with signal peptide/% |

|
图 3 包含信号肽(A)和去除信号肽(B)的AgLEAP-2与其他物种LEAP-2多重序列比对 Fig.3 Multiple sequence alignment of AgLEAP-2 with (A) and without (B) signal peptides with LEAP-2 from other species 100%相同的残基用黑色阴影表示,≥75%相同的残基用粉色阴影表示。信号肽用黑框表示。用红框标记的c代表半胱氨酸残基,2个相邻残基之间形成的二硫键被标记。分析中使用的序列列于表 3和表 4中。 100% identical residues are indicated by black shading, ≥75% identical residues are indicated by pink shading. Signal peptides are indicated by black boxes. c marked with red box represents conserved cysteine residue, and disulfide bonds formed between two opposite residues are marked. The sequences used in the analysis are listed in Tab.3 and Tab.4. |
|
|
表 4 不含信号肽的LEAP-2蛋白的两两相似性比较/% Tab.4 Pairwise similarity of LEAP-2 protein without signal peptide/% |
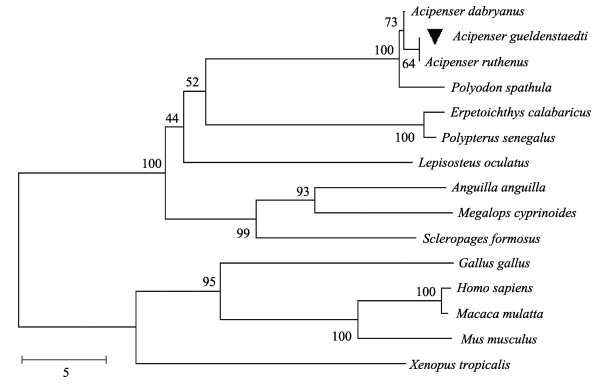
基于上述物种LEAP-2的氨基酸序列,通过NJ法构建系统进化树。结果显示,系统发育树分成2个分支,AgLEAP-2和鱼类为一个分支,而哺乳动物、两栖动物和鸟类聚集在另一个分支。与AgLEAP-2关系最密切的是小体鲟LEAP-2 (图 4)。基于AgLEAP-2和其他鱼类LEAP-2A、LEAP-2B和LEAP-2C的氨基酸序列构建系统发育树。结果显示,AgLEAP-2和LEAP-2C聚为一个分支。LEAP-2A和LEAP-2B被集中到另一个分支。AgLEAP-2与中华鲟和小体鲟LEAP-2C的亲缘关系最近。
|
图 4 基于AgLEAP-2和其他物种LEAP-2氨基酸序列构建的系统发育树 Fig.4 Phylogenetic tree based on the amino acid sequences of AgLEAP-2 and LEAP-2 from other species 利用MEGA X软件,采用邻接法重复1 000次构建系统发育树。“▼”表示AgLEAP-2。每个节点代表一个分类单元。各物种的GenBank登录号见表 3。 Using MEGA X software, the phylogenetic tree was constructed by repeating the NJ method for 1 000 times. AgLEAP-2 marked with "▼". Each node represents a classification unit. The GenBank No. of species are same in Tab.3. |

|
图 5 基于AgLEAP-2与其他鱼类LEAP-2A、LEAP-2B和LEAP-2C氨基酸序列构建的系统发育树 Fig.5 Phylogenetic tree based on amino acid sequences of AgLEAP-2 and LEAP-2A, LEAP-2B and LEAP-2C from other fishes 利用MEGA X软件,采用邻接法重复1 000次构建系统发育树。红色三角形表示AgLEAP-2。每个节点代表一个分类单元。LEAP-2A、LEAP-2B和LEAP-2C来源及GenBank登录号如下,LEAP-2A:大弹涂鱼(ANO39624.1)、锦鲤(AGK89728.1)、滨岸虹鳟(NP_001117936.1)、大黄鱼(AHY01375.1)、青鳉(XP_004080006.1)、剑尾鱼(XP_005806470.1)、红鳍东方鲀(XP_003966912.1)、斑马鱼(AAI62807.1)、大西洋鲑鱼(XP_014062864.1)、尼罗罗非鱼(XP_003457771.1)、半滑舌鳎(XP_008318762.1);LEAP-2B:锦鲤(AGK89729.1)、滨岸虹鳟(NP_001117937.1)、香鱼(AIZ00779.1)、大西洋鲑鱼(XP_013985003.1)、长江鲟(AWO14378.1)、中华鲟(AWO14379.1);LEAP-2C:滨岸虹鳟(ADN34603.1)、大黄鱼(AHY01377.1)、青鳉(XP_004074868.1)、斑马拟丽鱼(XP_004538259.1)、剑尾鱼(XP_005810076.1)、红鳍东方鲀(XP_011603261.1)、斑马鱼(NP_001373333.1)、大西洋鲑鱼(ADN34604.1)、尼罗罗非鱼(XP_013126243.1)、半滑舌鳎(XP_008322564.1)、中华鲟(AWO14380.1)。 Using MEGA X software, the phylogenetic tree was constructed by repeating the NJ method for 1 000 times. The red triangle indicates AgLEAP-2. Each node represents a classification unit. The sources and GenBank No. of LEAP-2A, LEAP-2B and LEAP-2C are as follows, LEAP-2A: Boleophthalmus pectinirostris (ANO39624.1), Cyprinus carpio (AGK89728.1), Oncorhynchus mykiss (NP_001117936.1), Larimichthys crocea (AHY01375.1), Oryzias latipes (XP_004080006.1), Xiphophorus maculatus (XP_005806470.1), Takifugu rubripes (XP_003966912.1), Danio rerio (AAI62807.1), Salmo salar (XP_014062864.1), Oreochromis niloticus (XP_003457771.1), Cynoglossus semilaevis (XP_008318762.1); LEAP-2B: Cyprinus carpio (AGK89729.1), Oncorhynchus mykiss (NP_001117937.1), Plecoglossus altivelis (AIZ00779.1), Salmo salar (XP_013985003.1), Acipenser dabryanus (AWO14378.1), Acipenser sinensis (AWO14379.1); LEAP-2C: Oncorhynchus mykiss (ADN34603.1), Larimichthys crocea (AHY01377.1), Oryzias latipes (XP_004074868.1), Maylandia zebra (XP_004538259.1), Xiphophorus maculatus (XP_005810076.1), Takifugu rubripes (XP_011603261.1), Danio rerio (NP_001373333.1), Salmo salar (ADN34604.1), Oreochromis niloticus (XP_013126243.1), Cynoglossus semilaevis (XP_008322564.1), Acipenser sinensis (AWO14380.1). |
利用qRT-PCR分析健康俄罗斯鲟13个组织的总RNA分布情况(表 1)。结果显示,AgLEAP-2在不同组织中广泛表达。在鳃中的表达量最低,其表达量设定为标准值1.0。表达水平最高的组织是肝脏(80倍),其次是肠(55倍)、肌肉(24倍)和卵巢(20倍)。在脾脏、血液、皮肤和其他组织中的表达水平较低(图 6)。

|
图 6 不同组织中AgLEAP-2的相对表达量(平均值±标准差, n=3) Fig.6 Relative expression levels of AgLEAP-2 in different tissues (Mean±SD, n=3) Li:肝脏;In:肠道;Sp:脾脏;Hk:头肾;Bl:血液;Gi:鳃;Sk:皮肤;Ov:卵巢;Br:脑;He:心脏;St:胃;Me:后肾;Mu:肌肉。柱上不同字母表示差异显著(P < 0.05)。下同。 Li: Liver; In: Intestine; Sp: Spleen; Hk: Head kidney; Bl: Blood; Gi: Gill; Sk: Skin; Ov: Ovary; Br: Brain; He: Heart; St: Stomach; Me: Metanephros; Mu: Muscle. The different letters indicate significant difference (P < 0.05). The same as below. |
利用qRT-PCR技术对俄罗斯鲟6种免疫组织中AgLEAP-2在不同时间点的mRNA表达模式结果进行分析(图 7)。AgLEAP-2在6种免疫组织中的表达在72 h内整体呈上调表达趋势(P<0.05),表达量达到峰值的时间点集中在48 h和72 h。肝脏、脾脏、头肾、鳃和血液中的表达量在72 h达到峰值。鳃中72 h的表达量最高,是0 h的5 000倍;脾脏中的表达量在72 h达到峰值,是0 h的1 500倍;头肾中的表达量在72 h达到峰值,是0 h的1 100倍;血液中的表达量在72 h达到峰值,是0 h的950倍;肝脏的表达量同样在72 h达到峰值,为0 h的150倍。而肠道中的表达量在48 h达到峰值,是0 h的900倍,并且在72 h仍保持较高水平,为0 h的600倍。

|
图 7 嗜水气单胞菌感染后6种免疫组织中AgLEAP-2的相对表达量(平均值±标准差, n=3) Fig.7 Relative expression level of AgLEAP-2 in hemocytes after A. hydrophila challenge (Mean±SD, n=3) |
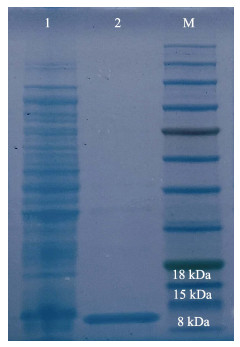
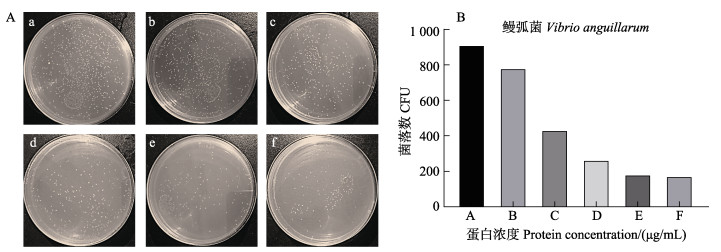
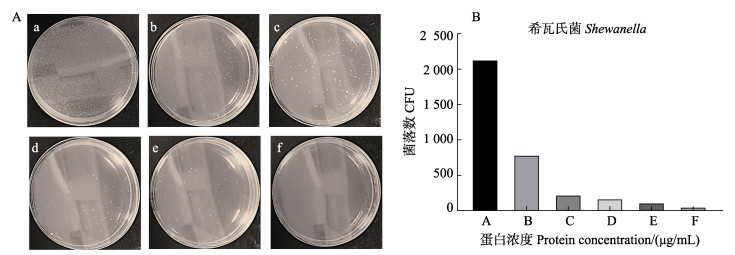
采用IPTG诱导AgLEAP-2重组蛋白表达,SDS-PAGE电泳显示,位于8~15 kDa处出现一条与预期重组蛋白大小一致的条带。经过His Trap FF纯化柱纯化得到rAgLEAP-2蛋白条带单一,其分子量约为11.2 kDa,与蛋白质的预测分子量一致(图 8)。rAgLEAP-2蛋白对革兰氏阳性菌(链球菌和金黄色葡萄球菌)和革兰氏阴性菌(大肠杆菌、鳗弧菌和希瓦氏菌)都表现出良好的抗菌效果,随着蛋白浓度的增加,抑菌效果出现剂量依赖性(图 9、10、11、12和13)。rAgLEAP-2蛋白的浓度达到500 μg/mL时,平板上只有少量细菌生长,菌落数目明显少于空白对照组。
|
图 8 AgLEAP-2的SDS-PAGE分析 Fig.8 SDS-PAGE analysis of AgLEAP-2 泳道M:标准蛋白分子量标记物;泳道1:纯化前的rAgLEAP-2;泳道2:纯化后的rAgLEAP-2 Lane M: Protein marker; Lane 1: rAgLEAP-2 before purification; Lane 2: Purified rAgLEAP-2 |

|
图 9 不同浓度rAgLEAP-2蛋白抑制大肠杆菌生长情况(A)及菌落数统计(B) Fig.9 Different concentrations of rAgLEAP-2 protein inhibit the growth of E. coli (A) and CFU count (B) a:空白对照;b:100 μg/mL;c:200 μg/mL;d:300 μg/mL;e:400 μg/mL;f:500 μg/mL。下同。 a: No-treatment control; b: 100 μg/mL; c: 200 μg/mL; d: 300 μg/mL; e: 400 μg/mL; f: 500 μg/mL. The same as below. |

|
图 10 不同浓度rAgLEAP-2蛋白抑制金黄色葡萄球菌生长情况(A)及菌落数统计(B) Fig.10 Different concentrations of rAgLEAP-2 protein inhibit the growth of S. aureus (A) and CFU count (B) |

|
图 11 不同浓度rAgLEAP-2蛋白抑制链球菌生长情况(A)及菌落数统计(B) Fig.11 Different concentrations of rAgLEAP-2 protein inhibit the growth of Streptococcus (A) and CFU count (B) |
|
图 12 不同浓度rAgLEAP-2蛋白抑制鳗弧菌生长情况(A)及菌落数统计(B) Fig.12 Different concentrations of rAgLEAP-2 protein inhibit the growth of V. anguillarum (A) and CFU count (B) |
|
图 13 不同浓度rAgLEAP-2蛋白抑制希瓦氏菌生长情况(A)及菌落数统计(B) Fig.13 Different concentrations of rAgLEAP-2 protein inhibit the growth of Shewanella (A) and CFU count (B) |
本研究对AgLEAP-2进行了序列鉴定、表达模式和抗菌活性分析。AgLEAP-2基因全长622 bp,cDNA全长246 bp,编码81个氨基酸。与大多数哺乳动物和鱼类一样,由一个信号肽和一个成熟肽组成。成熟肽AgLEAP-2包含4个保守的半胱氨酸,并在2条链内形成2个二硫键。AgLEAP-2基因序列在进化中高度保守。氨基酸多重序列比对和系统发育树研究发现,AgLEAP-2与鱼类的LEAP-2C聚集为一个分支,与长江鲟和中华鲟的LEAP-2C相似性最高,表明AgLEAP-2可能属于LEAP-2C。长江鲟和中华鲟的LEAP-2B和LEAP-2C已被鉴定为其他鱼类LEAP-2B和LEAP-2C的同源物(Zhang et al, 2018)。鉴于俄罗斯鲟基因组测序研究尚未开展,从目前我们团队俄罗斯鲟性腺转录组测序和俄罗斯鲟皮肤转录组测序结果未发现AgLEAP-2的其他同源物(Chen et al, 2016; Gong et al, 2019)。
LEAP-2以不同的模式在脊椎动物的健康组织中广泛表达。研究表明,鱼类的LEAP-2在肝脏中高度表达。LEAP-2A和LEAP-2B基因在虹鳟肝脏中高度表达,而在其他组织中不表达(Zhang et al, 2004)。LEAP-2在卵形鲳鲹肝脏中的表达量最高,但肠中表达量最低(Lei et al, 2020)。LEAP-2C在健康长江鲟肝脏中高度表达(Zhang et al, 2018)。其他鱼类的LEAP-2或LEAP-2C也在肠中高度表达。例如,LEAP-2在健康大黄鱼的肝脏中高表达,而LEAP-2C在肠中高度表达(Li et al, 2014)。像大多数脊椎动物一样,AgLEAP-2在俄罗斯鲟的13种健康组织中广泛表达,在肝脏和肠道中表达水平最高。LEAP-2在不同物种中表达模式相似,说明LEAP-2在不同物种中功能可能一致,但它也具有组织和物种特异性。
感染嗜水气单胞菌后,AgLEAP-2在俄罗斯鲟的肝脏、脾脏、肠道、鳃、血液和头肾中显著上调,与其他鱼类的研究结果一致。研究表明,LEAP-2有助于抵抗病原菌的入侵。例如,经嗜水气单胞菌攻击后,长江鲟的肝脏、脾脏、血液和中肾中LEAP-2的表达量显著增加(Zhang et al, 2018)。黏膜免疫系统是鱼类整个免疫系统的重要组成部分,具有独特的结构和功能,是抵御感染的第一道防线。鳃、肠和皮肤是3个重要的粘膜免疫组织,是鱼类抵抗病原体感染的第一道屏障(Magnadottir, 2006)。例如,虹鳟感染杀鲑气单胞菌(Aeromonas salmonicida)后,LEAP-2在肠道中高度表达(Zhang et al, 2004)。草鱼感染嗜水气单胞菌后,LEAP-2在肝脏、脾脏、皮肤、肌肉和肠道中的表达水平上调(Liu et al, 2010)。鲤鱼经过腹腔注射鳗弧菌后,LEAP-2在皮肤中的表达水平显著上调(Yang et al, 2014)。本研究表明,俄罗斯鲟在感染嗜水气单胞菌72 h后,AgLEAP-2在鳃和肠中的表达水平分别为0 h的5 000倍和600倍,显著高于其他组织。综上所述,AgLEAP-2可能参与了俄罗斯鲟的粘膜免疫。
LEAP-2蛋白作为抗菌肽的一种,主要具有防止病原菌入侵和抵抗感染的作用。大多数对哺乳动物和鱼类LEAP-2的研究主要集中在抗菌和抗病毒免疫活性上。重组LEAP-2蛋白显示出针对人类、家禽和鱼类病原体的选择抗菌功能(Krause et al, 2003; Liu et al, 2010; Townes et al, 2004)。研究发现,随着草鱼LEAP-2蛋白浓度的增加,其对嗜水气单胞菌的抗菌活性更显著(Liu et al, 2010)。大菱鲆LEAP-2蛋白能有效抑制藤黄微球菌(Micrococcus luteus)、迟缓爱德华氏菌(Edwardsiella tarda)和鳗弧菌生长,且呈剂量依赖性(Zhang et al, 2014)。鲶鱼LEAP-2蛋白能有效抑制迟缓爱德华氏菌和鳗弧菌,最低抑菌浓度为6.25 mg/mL (Li et al, 2015)。卵形鲳鲹LEAP-2蛋白对无乳葡萄球菌(Streptococcus agalactiae)、枯草芽孢杆菌(Bacillus subtilis)和金黄色葡萄球菌3种革兰氏阳性菌,以及溶藻弧菌(Vibrio alginolyticus)、副溶血弧菌(Vibrio parahaemolyticus)、哈维氏弧菌(Vibrio harveyi)、美人鱼发光杆菌(Photobacterium damselae)、大肠杆菌5种革兰氏阴性菌的最低抑制浓度为312.5 μg/mL (Liu et al, 2020)。与其他鱼类LEAP-2蛋白一样,rAgLEAP-2蛋白能有效抑制革兰氏阴性菌和革兰氏阳性菌的生长,并呈剂量依赖性,从而证明AgLEAP-2蛋白具有一定的抗菌活性。
综上所述,AgLEAP-2的克隆和同源性分析证明,该基因属于LEAP-2C。AgLEAP-2在抵御细菌感染的免疫反应中发挥重要作用,rAgLEAP-2蛋白可以抑制革兰氏阳性菌和革兰氏阴性菌的生长。本研究加深了对鱼类非特异性免疫反应的认识,为深入研究俄罗斯鲟LEAP-2的抗菌机制提供了研究基础。
BAO B, PEATMAN E, XU P, et al. The catfish liver-expressed antimicrobial peptide 2 (LEAP-2) gene is expressed in a wide range of tissues and developmentally regulated. Molecular Immunology, 2006, 43(4): 367-377 DOI:10.1016/j.molimm.2005.02.014 |
BOAS L C P V, LIMA L M P D, MIGLIOLO L, et al. Linear antimicrobial peptides with activity against Herpes simplex virus 1 and Aichi virus. Biopolymers, 2017, 108(2): e22871 DOI:10.1002/bip.22871 |
CHEN Y, XIA Y, SHAO C, et al. Discovery and identification of candidate sex-related genes based on transcriptome sequencing of Russian sturgeon (Acipenser gueldenstaedtii) gonads. Physiological Genomics, 2016, 48(7): 464-476 DOI:10.1152/physiolgenomics.00113.2015 |
DI J, ZHANG S, HUANG J, et al. Isolation and identification of pathogens causing haemorrhagic septicaemia in cultured Chinese sturgeon (Acipenser sinensis). Aquaculture Research, 2018, 49: 3624-3633 DOI:10.1111/are.13830 |
FJELL C D, HIS J A, HANCOCK R E, et al. Designing antimicrobial peptides: Form follows function. Nature Reviews Drug Discovery, 2011, 11(1): 37-51 |
GE X, YANG H, BEDNAREK M A, et al. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metabolism, 2018, 27(2): 461-469 DOI:10.1016/j.cmet.2017.10.016 |
GONG Y, HU M, XU S, et al. Comparative transcriptome analysis reveals expression signatures of albino Russian sturgeon, Acipenseriformes gueldenstaedtii. Marine Genomics, 2019, 46: 1-7 DOI:10.1016/j.margen.2019.02.004 |
HONG Y, TRUONG A D, LEE J, et al. Identification of duck liver-expressed antimicrobial peptide 2 and characterization of its bactericidal activity. Asian Australasian Journal of Animal Sciences, 2019, 32(7): 1052-1061 DOI:10.5713/ajas.18.0571 |
KRAUSE A, SILLARD R, KLEEMEIER B, et al. Isolation and biochemical characterization of LEAP-2, a novel blood peptide expressed in the liver. Protein Science, 2003, 12(1): 143-152 DOI:10.1110/ps.0213603 |
LEI Y, QIU R, SHEN Y, et al. Molecular characterization and antibacterial immunity functional analysis of liver-expressed antimicrobial peptide 2 (LEAP-2) gene in golden pompano (Trachinotus ovatus). Fish and Shellfish Immunology, 2020, 106: 833-843 DOI:10.1016/j.fsi.2020.09.002 |
LI H X, LU X J, LI C H, et al. Molecular characterization and functional analysis of two distinct liver-expressed antimicrobial peptide 2 (LEAP-2) genes in large yellow croaker (Larimichthys crocea). Fish and Shellfish Immunology, 2014, 38: 330-339 DOI:10.1016/j.fsi.2014.04.004 |
LI H X, LU X J, LI C H, et al. Molecular characterization of the liver-expressed antimicrobial peptide 2 (LEAP-2) in a teleost fish, Plecoglossus altivelis: Antimicrobial activity and molecular mechanism. Molecular Immunology, 2015, 65(2): 406-415 DOI:10.1016/j.molimm.2015.02.022 |
LIANG T, JI W, ZHANG G R, et al. Molecular cloning and expression analysis of liver-expressed antimicrobial peptide 1 (LEAP-1) and LEAP-2 genes in the blunt snout bream (Megalobrama amblycephala). Fish and Shellfish Immunology, 2013, 35: 553-563 DOI:10.1016/j.fsi.2013.05.021 |
LIU F, LI J L, YUE G, et al. Molecular cloning and expression analysis of the liver-expressed antimicrobial peptide 2 (LEAP-2) gene in grass carp. Veterinary Immunology and Immunopathology, 2010, 133(2/3/4): 133-143 |
LIU B, LIU G D, GUO H Y, et al. Characterization and functional analysis of liver-expressed antimicrobial peptide-2 (LEAP-2) from golden pompano Trachinotus ovatus (Linnaeus 1758). Fish and Shellfish Immunology, 2020, 104: 419-430 DOI:10.1016/j.fsi.2020.06.029 |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods, 2001, 25(4): 402-408 DOI:10.1006/meth.2001.1262 |
LU X J, CHEN Q, YANG G J, et al. The TNFalpha converting enzyme (TACE) from ayu (Plecoglossus altivelis) exhibits TNFalpha shedding activity. Molecular Immunology, 2015, 63(2): 497-504 DOI:10.1016/j.molimm.2014.10.010 |
MAGNADOTTIR B. Innate immunity of fish (overview). Fish and Shellfish Immunology, 2006, 20(2): 137-151 DOI:10.1016/j.fsi.2004.09.006 |
MOHAMMADI M, MORADI HASAN-ABAD A, DEHGHANI P, et al. Dicentracin-like from Asian sea bass fish and moronecidine-like from Hippocampus comes: Two candidate antimicrobial peptides against Leishmanina major infection. International Journal of Peptide Research and Therapeutics, 2020, 27: 769-778 |
NGUYEN L T, HANEY E F, VOGEL H J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends in Biotechnology, 2011, 29(9): 464-472 DOI:10.1016/j.tibtech.2011.05.001 |
OMAR A M, TIMO M, MATTHIAS T, et al. Ghrelin and LEAP-2: Rivals in energy metabolism. Trends in Pharmacological Sciences, 2018, 39(8): 685-694 DOI:10.1016/j.tips.2018.06.004 |
SHAYKHIEV R, BEISSWENGER C, KANDLER K, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. American Journal of Physiology Lung Cellular and Molecular Physiology, 2005, 289(5): L842-848 DOI:10.1152/ajplung.00286.2004 |
TOWNES C L, MICHAILIDIS G, NILE C J, et al. Induction of cationic chicken liver-expressed antimicrobial peptide 2 in response to Salmonella enterica infection. Infection and Immunity, 2004, 72(12): 6987-6993 DOI:10.1128/IAI.72.12.6987-6993.2004 |
TRAVKOVA O G, MOEHWALD H, BREZESINSKI G. The interaction of antimicrobial peptides with membranes. Advances in Colloid and Interface Science, 2017, 247: 521-532 DOI:10.1016/j.cis.2017.06.001 |
VALERO Y, ARIZCUN M, CORTES J, et al. NK-lysin, dicentracin and hepcidin antimicrobial peptides in European sea bass. Ontogenetic development and modulation in juveniles by nodavirus. Developmental and Comparative Immunology, 2020, 103: 103516 |
YANG G, GUO H, LI H, et al. Molecular characterization of LEAP-2 cDNA in common carp (Cyprinus carpio L.) and the differential expression upon a Vibrio anguillarum stimulus: Indications for a significant immune role in skin. Fish and Shellfish Immunology, 2014, 37(1): 22-29 DOI:10.1016/j.fsi.2014.01.004 |
ZHANG Y A, ZOU J, CHANG C I, et al. Discovery and characterization of two types of liver-expressed antimicrobial peptide 2 (LEAP-2) genes in rainbow trout. Veterinary Immunology and Immunopathology, 2004, 101(3/4): 259-269 |
ZHANG J, YU L P, LI M F, et al. Turbot (Scophthalmus maximus) hepcidin-1 and hepcidin-2 possess antimicrobial activity and promote resistance against bacterial and viral infection. Fish and Shellfish Immunology, 2014, 38(1): 127-134 DOI:10.1016/j.fsi.2014.03.011 |
ZHANG S, XU Q, DU H, et al. Evolution, expression, and characterization of liver-expressed antimicrobial peptide genes in ancient chondrostean sturgeons. Fish and Shellfish Immunology, 2018, 79: 363-369 DOI:10.1016/j.fsi.2018.05.023 |