先天性免疫系统是鱼类抵御病原体入侵的第一道防线,由能够识别病原相关的分子模式(pathogen- associated molecular patterns, PAMPs)的模式识别受体介导(pattern recognition receptors, PPRs)(MacLeod et al, 2017)。根据PRRs结构、位置和分泌等特征,PRRs可分为膜结合受体[如Toll样受体(toll-like receptors, TLRs)、C型凝集素等]、胞内受体(如NOD样受体、RIG样受体等)及分泌型受体(如甘露糖结合凝集素、脂多糖结合蛋白) 3种类型(Lee et al, 2007)。其中,TLRs可识别多种病原体表达的脂类、碳水化合物、肽和核酸结构,是最早且研究最为广泛的受体之一(Rebl et al, 2010)。
TLR家族成员在进化过程中保守,由3个经典结构域组成:跨膜(transmembrane, TM)结构域、富含亮氨酸(leucine-rich repeat, LRR)结构域和Toll/白介素-1受体(toll/IL-1 receptor, TIR)结构域。LRR结构域参与配体识别和结合,TIR结构域与衔接蛋白[如诱导干扰素-β适配器(toll/IL-1 receptor domain-containing- adaptor inducing IFN-β, TRIF)、髓样分化因子(myeloid differentiation factor 88, MyD88)衔接样蛋白]相互作用触发信号级联反应,以诱导机体产生免疫反应(Wang et al, 2018; Shan et al, 2018)。根据Roach等(2005)的分类,TLRs被分为TLR1、TLR3、TLR4、TLR5、TLR7和TLR11六个亚家族。目前,在哺乳动物中共发现13种TLRs成员(TLR1~13),而在鱼类中已发现20多种,其中TLR5s、TLR18~20和TLR22~27是鱼类所特有的(Zhang et al, 2014)。鱼类TLRs种类增加的原因尚未可知,可能是其为适应水生养殖环境中存在的复杂的多种病原体(Liang et al, 2018)以及不同类型的TLR家族具有识别不同PAMPs的特点(Chaturvedi et al, 2009; Baoprasertkul et al, 2007)。例如,TLR2和TLR4识别革兰氏阴性菌脂多糖(lipopolysaccharide, LPS);TLR5识别细菌鞭毛蛋白(Baoprasertkul et al, 2007; Voogdt et al, 2018)。TLR13属于TLR11家族,小鼠(Mus musculus) TLR13能够识别细菌23S核糖体RNA (rRNA)中保守的核苷酸序列(5´-GGACGGAAAGACCCCGUGG-3´),并激活MyD88依赖的信号通路,诱导炎性细胞因子消除病原体(Valenzuela-Muñoz et al, 2016; Vidya et al, 2018)。鱼类TLR13的相关报道主要围绕TLR13保护机体免受细菌或病毒侵袭相关功能进行研究,例如,斜带石斑鱼(Epinephelus coioides)脾细胞(grouper spleen cells, GS)受到金黄色葡萄球菌(Staphylococcus aureus)和副溶血弧菌(Vibrio Parahemolyticus)的23S rRNA刺激后,其TLR13表达量均呈上调趋势(Liang et al, 2018; Yu X et al, 2021);小黄鱼(Larimichthys polyactis)在鳗弧菌(Vibrio anguillarum)刺激36 h后,其TLR13在脾脏的表达水平显著上调,且其白细胞受到聚肌胞苷酸[Polyinosinic: polycytidylic acid, Poly(I: C)]刺激后也表现出了表达上调(Wang et al, 2016)。
卵形鲳鲹(Trachinotus ovatus)是重要的海水经济鱼类之一,主要分布在印度洋、太平洋和大西洋等海域,因其生长速度快、肉质细嫩肥美、肌间无刺、经济效益高等优点受到大众的喜爱(李远友等, 2019)。近年来,卵形鲳鲹海水养殖规模不断扩大,在高密度养殖过程中易受到病原微生物感染[如无乳链球菌(Streptococcus agalactiae)和溶藻弧菌(Vibrio alginolyticus)],造成严重的经济损失(Cai et al, 2017; Zhou et al, 2020)。作为宿主抵御病原体入侵的第一道防线,了解鱼类先天性免疫的作用机制将有助于制定绿色高效措施,防控细菌性疾病暴发。本研究克隆了卵形鲳鲹TLR13的开放阅读框(open reading frame, ORF) (命名为ToTLR13),利用生物信息学分析其序列特征,采用实时荧光定量PCR技术(Real-time fluorescence quantitative PCR, RT-qPCR)检测其在细菌感染后免疫相关组织中的表达谱变化规律,并观察其在细胞中的亚定位。研究结果将为鱼类TLR13功能的研究奠定基础。
1 材料与方法 1.1 主要试剂RNA提取试剂(Trizol、氯仿、无水乙醇、75%乙醇和RNase-free水)、cDNA合成试剂盒和荧光定量试剂盒购自苏州近岸蛋白科技股份有限公司。无乳链球菌和溶藻弧菌菌种由本实验室保种。所用的大肠杆菌(Escherichia coli) DH5α、pMD18-T载体、DNA Marker、Premix Taq酶、Quick限制性内切酶购自TaKaRa公司。琼脂糖凝胶回收试剂盒、质粒提取试剂盒和无内毒素质粒小提中量试剂盒购自天根生化科技(北京)有限公司。LipofectamineTM 3000 Reagent试剂盒购自赛默飞世尔科技(中国)有限公司。1×PBS缓冲液(pH 7.2)、4%多聚甲醛、0.1% Triton-100、抗荧光衰减封片剂、DAPI溶液购自索莱宝科技(北京)有限公司。人非小细胞肺癌细胞(human non-small-cell lung cancer cells, A549)和pEGFP-N1绿色荧光质粒载体由本实验室保存。
1.2 实验样品制备130尾健康卵形鲳鲹购自广西钦州市三娘湾鸿钰水产科技有限公司,平均体质量为(50±5) g。实验前,将健康卵形鲳鲹于室内2 000 L水箱中暂养15 d,水温保持在(25±1) ℃,每日监测鱼体健康状况。随机取3条健康卵形鲳鲹,用MS-222麻醉后,各取8个不同组织(心脏、鳃、肾、头肾、肝、脾、脑和肌肉)装入1.5 mL无酶管中,立即投入液氮暂存后转入–80 ℃冰箱保存,用于后续基因克隆和表达分析。
将无乳链球菌和溶藻弧菌分别接种于10 mL Luria-Bertani肉汤(LB)培养基中,放置于37 ℃和28 ℃摇床200 r/min震荡培养。培养后离心收集细菌,PBS洗涤3次,并用PBS调整浓度备用。将120尾健康卵形鲳鲹分为对照组、无乳链球菌实验组和溶藻弧菌实验组,每组40尾。3组鱼分别腹腔注射100 μL的PBS、3.4×107 CFU/mL无乳链球菌和2.75×107 CFU/mL溶藻弧菌。每组分别于注射后0、6、12、24、48、72和96 h取3尾鱼,取鳃、脾、肝和肾4个组织置于1.5 mL无酶管中,立即投入液氮暂存后转入–80 ℃冰箱保存,用于后续的表达分析。
1.3 实验方法 1.3.1 ToTLR13基因克隆使用ORF finder程序(https://www.ncbi.nlm.nih.gov/orffinder)预测本实验室卵形鲳鲹脾脏转录组测序结果中TLR13基因ORF,并设计特异性引物ToTLR13-F和ToTLR13-R (表 1)扩增该基因ORF序列。按照TransZol Up说明书从鳃中提取总RNA,用1%琼脂糖凝胶和NanoDrop 2000 (Thermo)检测总RNA的质量和浓度。按照cDNA合成说明书将总RNA反转录成第一链cDNA,并储存在–80℃冰箱以备后续使用。
|
|
表 1 实验所用引物 Tab.1 Primers used in the study |
以鳃cDNA为模板,利用ToTLR13-F和ToTLR13-R引物(表 1)扩增ToTLR13基因ORF序列。扩增体系:cDNA 4 μL,引物ToTLR13-F/R各2 μL,Premix Taq酶24 μL,H2O 14 μL,反应条件:94 ℃ 4 min;35次循环(94 ℃ 30 s;56 ℃ 30 s;72 ℃ 1 min);72 ℃ 10 min;16 ℃保存。PCR产物经1%琼脂糖凝胶电泳检测后,割胶回收纯化PCR产物。将PCR产物连接至pMD18-T载体构建pMD18-ToTLR13重组质粒,并转化至E. coli DH5α。使用M13-F和M13-R引物(表 1)进行菌落PCR鉴定,将阳性菌落送至江苏东玄基因科技有限公司进行测序验证。
1.3.2 ToTLR13基因生物信息学分析根据所测得的序列用下列方法进行生物信息学分析。ExPASy Proteomics Server (http://web.expasy.org/protparam/)进行蛋白质理论等电点、分子量和亲水性等信息的推测;蛋白质的跨膜结构域预测利用TMHMM Server 2.0 (http://www.cbs.dtu.dk/services/TMHMM/);BLAST (https://blast.ncbi.nlm.nih.gov/)进行序列比对分析;使用SignalP-5.0 Server (http://www.cbs.dtu.dk/services/SignalP/)进行信号肽序列预测;运用Softberry-Psite进行氨基酸基本功能位点布预测;NCBI Conserved Domains (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)分析蛋白保守结构域;利用SWISSM-MODEL (https://swissmodel.expasy.org/)进行同源建模分析。结合Clustal X和GeneDoc软件,对氨基酸序列进行多重比对分析;利用MEGA7.0软件构建系统进化树。
1.3.3 病原菌刺激前后ToTLR13在卵形鲳鲹主要免疫相关组织中的表达模式分析按照1.3.1提取总RNA和反转录第一链cDNA的方法,提取心脏、鳃、肾、头肾、肝、脾、脑和肌肉组织的cDNA模板。利用RT-qPCR技术分析健康卵形鲳鲹和病原菌刺激后各组织ToTLR13 mRNA的表达情况,每个反应重复3次,以β-actin作为内参。引物RT-ToTLR13-F/RT-ToTLR13-R和β-actin-F/β-actin-R序列见表 1。扩增体系为10 μL:cDNA 1 μL、2×NovoStrat® SYBR qPCR SuperMix Plus 5 μL、ROX Reference Dye Ⅱ 0.2 μL、RT-ToTLR13-F/RT-ToTLR13-R或β-actin-F/β-actin-R各0.4 μL、RNase free水3 μL。反应条件:95 ℃ 30 s;(95 ℃ 5 s;56 ℃ 15 s;72 ℃ 10 s) 40个循环;95 ℃ 5 s,70 ℃ 1 min,95 ℃ 15 s。反应结束后用QuantStudioTM 6 Flex Real-Time PCR System软件进行检测,选用3个重复熔解曲线相似单峰的Ct值。
1.3.4 ToTLR13亚细胞定位将pMD18-ToTLR13和pEGFP-N1质粒分别用Hind Ⅲ和BamHⅠ限制性内切酶进行酶切纯化后,用T4连接酶将ToTLR13连接至pEGFP-N1质粒,构建重组质粒pEGFP- ToTLR13。利用无内毒素质粒提取试剂盒(天根)分别提取pEGFP-ToTLR13和pEGFP-N1质粒,使用NanoDrop 2000 (Thermo)检测浓度后,放置–20 ℃冰箱备用。
取200 μL浓度为1×106个/μL的生长状况良好的A549细胞,平铺在放置有细胞爬片的24孔细胞培养板中,5% CO2培养箱中37 ℃培养24 h,细胞铺满70%~80%时进行转染。按照LipofectamineTM 3000 Reagent试剂盒(赛默飞世尔, 中国)说明书进行转染,设置pEGFP-N1作为空载对照,pEGFP-ToTLR13作为实验组。转染24 h后,弃培养液,使用500 μL 1×PBS清洗3次后,使用4%多聚甲醛固定30 min;弃多聚甲醛后,1×PBS清洗3次,使用0.1% Triton X-100透化细胞7 min;透化后1×PBS清洗3次,0.5 μg/mL DAPI避光染色10 min。最后,用1×PBS漂洗3次后,用镊子将细胞爬片从24孔板中取出,倒扣在滴有玻片固定液的载玻片上。使用激光共聚焦显微镜(Olympus, FV10i, 日本)检测并拍照。
1.3.5 统计学分析采用SPSS软件的ANOVA和Tukey’s分析RT-qPCR结果。利用t检验对数据进行比较,差异显著性的判断标准为P < 0.05,差异极显著的判断标准为P < 0.01。
2 结果 2.1 ToTLR13基因序列分析利用PCR方法成功扩增得到ToTLR13的ORF序列(GenBank登录号:ON365560)。结果显示,ToTLR13 ORF序列为1 269 bp,编码422个氨基酸,分子量为48.09 kDa,等电点为8.13(图 1)。蛋白质结构域预测结果显示,ToTLR13含有1个TM结构域、5个LRR结构域和1个C端TIR结构域(图 1)。通过SignalP-5.0 Server预测该序列1~15为信号肽区域,信号肽剪切位点在第15~16个氨基酸之间(图 1)。运用Softberry预测得到该基因基序包括5个LRR结构域(78VDM-LDI148),4个微体C-末端定位信号序列(54SIK56、264SSR266、314SSR316和340SLR342),3个N-肉豆蔻酰化位点(29GQGIGN34、145CTLPNG150和408GGGRGG413),3个络蛋白激酶Ⅱ磷酸化位点(16ILE18、200SYEE203和213SVMD215),1个cAMP和cGMP依赖性蛋白激酶磷酸化位点(375RKKT378)。
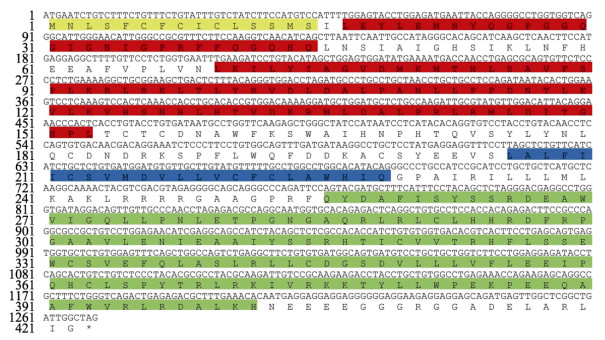
|
图 1 卵形鲳鲹ToTLR13核苷酸序列及推测的氨基酸序列 Fig.1 Nucleotide sequence and deduced amino acid sequence of ToTLR13 in T. ovatus 黄色为信号肽;红色为LRR结构域;蓝色为跨膜结构域;绿色为TIR结构域。 Signal peptide is marked in yellow; The LRR domains are marked in red; The transmembrane domain is marked with blue; The TIR domain is marked in green. |
ToTLR13蛋白半衰期为30 h,不稳定系数为42.83,将该蛋白质归类为不稳定蛋白质。经过亲水性预测得到,第405位氨基酸的指数是–2.85,为最大亲水性;而第225位氨基酸达到最大疏水性,指数为3.35,总平均亲水性系数为–0.021,归类为亲水性蛋白(图 2)。通过SWISS-MODEL和PyMOL软件对卵形鲳鲹和小鼠、大黄鱼(Larimichthys crocea) TLR13的LRR结构域和TIR结构域进行三级结构模拟和叠加。结果显示,卵形鲳鲹和大黄鱼TLR13的LRR结构域分别具有3个和2个β折叠,TIR结构域都具有5个α螺旋和4个β折叠,而小鼠TLR13的LRR结构域有3个β折叠,TIR结构域具有10个α螺旋和8个β折叠(图 3和图 4)。卵形鲳鲹与小鼠、大黄鱼TLR13的功能结构域三级结构高度重合,表明不同物种的TLR13的结构和功能具有一定的相似性。
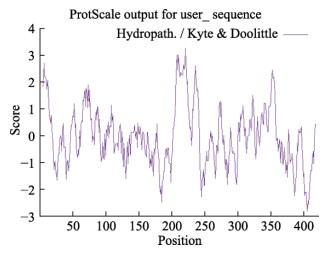
|
图 2 ToTLR13疏水性预测 Fig.2 Hydrophobicity prediction of ToTLR13 |
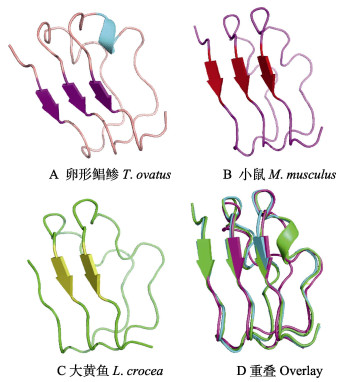
|
图 3 卵形鲳鲹、小鼠和大黄鱼TLR13的LRR结构域三级结构预测 Fig.3 Predicted 3D structure of LRR domain of TLR13 in T. ovatus, M. musculus, and L. crocea |

|
图 4 卵形鲳鲹、小鼠和大黄鱼TLR13的TIR结构域三级结构预测 Fig.4 Predicted 3D structure of TIR domain of TLR13 in T. ovatus, M. musculus, and L. crocea |
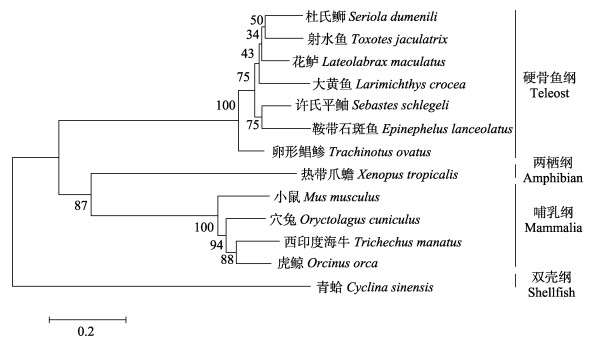
通过与NCBI数据库中的其他物种的TLR13氨基酸序列进行比对,发现ToTLR13与其他硬骨鱼的TLR13具有高度的相似性,与杜氏

|
图 5 卵形鲳鲹ToTLR13与其他物种TLR13氨基酸序列比对 Fig.5 Multiple alignments of ToTLR13 amino acid sequence of T. ovatus with other species 深蓝色标记的序列高度一致,粉色标记的序列较为相似,蓝绿色标记的序列相似度较低;红色方框为LRR结构域,蓝色方框为TM结构域,绿色方框为TIR结构域。 The same sequences are marked with dark blue, the similar sequence are marked with pink, and the sequences with relatively low similarity are marked with blue-green; the LRR domains are marked with red box, the TM domains are marked with blue box, and the TIR domains are marked with green box. |
|
图 6 N-J法构建TLR13氨基酸系统进化树
Fig.6 Phylogenetic tree of TLR13 constructed by neighbour-joining method
氨基酸序列GenBank号:杜氏 (XP_022598134.1);射水鱼(XP_040922374.1);花鲈(QDE10488.1);大黄鱼(KAE8284326.1);许氏平 (XP_022598134.1);射水鱼(XP_040922374.1);花鲈(QDE10488.1);大黄鱼(KAE8284326.1);许氏平 (QXJ40778.1);鞍带石斑鱼(XP_0033467894.1);卵形鲳鲹(ON365560);热带爪蟾(XP_002935047.2);小鼠(EDL14060.1);穴兔(XP_002720271.2);西印度海牛(XP_012411715.1);虎鲸(XP_012392423.1);青蛤(AIZ97750.1)。
Amino acid sequence GenBank number: S. dumerili (XP_022598134.1); T. jaculatrix (XP_040922374.1); Lateolabrax maculatus (QDE10488.1); L. crocea (KAE8284326.1); S. schlegelii (QXJ40778.1); E. lanceolatus (XP_0033467894.1); T. ovatus (ON365560); Xenopus tropicalis (XP_002935047.2); M. musculus (EDL14060.1); O. cuniculus (XP_002720271.2); Trichechus manatus (XP_012411715.1); Orcinus orca (XP_012392423.1); C. sinensis (AIZ97750.1). (QXJ40778.1);鞍带石斑鱼(XP_0033467894.1);卵形鲳鲹(ON365560);热带爪蟾(XP_002935047.2);小鼠(EDL14060.1);穴兔(XP_002720271.2);西印度海牛(XP_012411715.1);虎鲸(XP_012392423.1);青蛤(AIZ97750.1)。
Amino acid sequence GenBank number: S. dumerili (XP_022598134.1); T. jaculatrix (XP_040922374.1); Lateolabrax maculatus (QDE10488.1); L. crocea (KAE8284326.1); S. schlegelii (QXJ40778.1); E. lanceolatus (XP_0033467894.1); T. ovatus (ON365560); Xenopus tropicalis (XP_002935047.2); M. musculus (EDL14060.1); O. cuniculus (XP_002720271.2); Trichechus manatus (XP_012411715.1); Orcinus orca (XP_012392423.1); C. sinensis (AIZ97750.1).
|
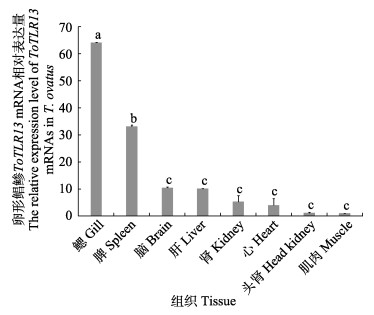
ToTLR13在健康卵形鲳鲹各组织中的表达情况见图 7。结果显示,ToTLR13在健康卵形鲳鲹的心脏、鳃、肾、头肾、肝、脾、脑和肌肉组织中均有表达,其中,在鳃组织中表达量最高,是肌肉的64.1倍(P < 0.01)。其次,在脾、脑、肝和肾中的表达量呈逐渐降低的趋势,而在心脏和头肾中的表达量相对较低,肌肉的表达水平最低。
|
图 7 卵形鲳鲹ToTLR13基因在不同组织中的表达情况 Fig.7 Expression pattern of ToTLR13 gene in different tissues of T. ovatus ToTLR13在各组织相对表达量以肌肉表达水平(设定为1)作为参照进行计算;不同字母表示差异显著(P < 0.05)。 The relative expression of ToTLR13 in each tissue was calculated based on the muscle expression level (set as 1) as a reference; Different letters on the column represent significant differences (P < 0.05). |
为检测ToTLR13是否参与机体先天性免疫防御,本研究进一步分析了无乳链球菌和溶藻弧菌刺激(0、6、12、24、48、72和96 h)后ToTLR13在免疫相关组织鳃、脾、肝和肾中的表达情况。结果显示,无乳链球菌和溶藻弧菌刺激后,ToTLR13在免疫相关组织中的表达水平均呈现显著性上调,但其在不同时间和不同组织中的表达水平差异较大。由图 8可知,在鳃中,ToTLR13表达水平在无乳链球菌刺激0~48 h时呈低水平的升高趋势,感染72 h时表达水平突然达到高峰(是攻毒前的23倍),96 h又降回到原始水平。感染溶藻弧菌时,ToTLR13表达水平则呈先上升后下降再上升的趋势,在感染12 h和96 h均表现出显著差异,且96 h达到峰值。脾ToTLR13 mRNA表达水平在2种细菌刺激后均呈现出时间依赖性上升,无乳链球菌实验组在24 h时表达水平达到峰值,而溶藻弧菌实验组在96 h时表达水平达到峰值。肝ToTLR13 mRNA表达水平在感染无乳链球菌的0~48 h时呈规律性先上升后下降趋势,在72 h时表达量达到峰值。在溶藻弧菌实验组,肝ToTLR13 mRNA表达水平则呈先下降至原始水平以下后上升的趋势,并在48 h达到峰值。在肾中,ToTLR13 mRNA表达水平在无乳链球菌感染后分别在12 h和72 h时达到峰值;而在溶藻弧菌感染后6 h时便迅速达到了峰值,随后降低至攻毒前水平。
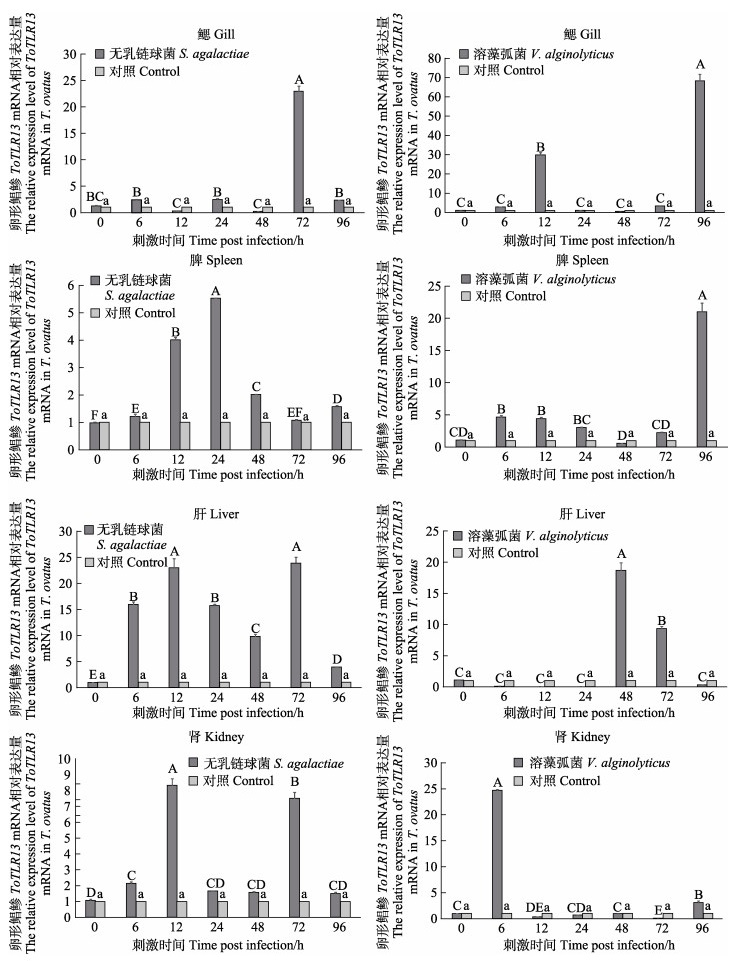
|
图 8 无乳链球菌和溶藻弧菌刺激后ToTLR13在卵形鲳鲹免疫相关组织中的表达模式 Fig.8 The relative expression of ToTLR13 mRNA in immune-related tissue of T. ovatus by S. agalactiae and V. alginolyticus 柱上不同字母(大写字母:实验组;小写字母:对照组)表示表达差异显著。 Different letters on the column (Upper-case letter: Experimental group; Lower-case letter: Control group) represent significant differences. |
为更好地理解ToTLR13在细胞中的功能,本研究通过构建pEGFP-ToTLR13重组表达荧光质粒进行其在A549细胞中的亚细胞定位研究。激光共聚焦显微镜观察发现,对照组pEGFP-N1空载质粒绿色荧光均匀地分布于整个细胞中,而实验组的绿色荧光主要分布于细胞质,说明pEGFP-ToTLR13质粒主要定位于细胞质(图 9)。
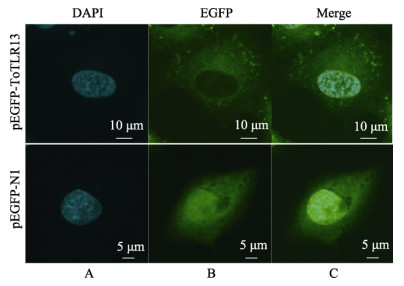
|
图 9 ToTLR13在A549细胞中的亚细胞定位 Fig.9 Subcellular localization of ToTLR13 in A549 cells A:DAPI染色,示细胞核;B:EGFP染色,示pEGFP-ToTLR13或pEGFP-N1;C:合并后的图像 A: DAPI staining, showing the nucleus; B: EGFP staining, showing pEGFP-ToTLR13 or PEGFP-N1; C: Merged images |
TLRs在宿主免疫识别各种PAMPs并触发级联免疫反应中发挥重要的作用(Qi et al, 2020a、b; Zhou et al, 2021)。本研究克隆了卵形鲳鲹ToTLR13基因的ORF序列,该序列为1 269 bp,编码422个氨基酸,具有用于配体识别的1个TM结构域、1个C端TIR结构域和5个LRR结构域。其中,TIR结构域几乎存在于所有的跨膜TLR中且序列高度保守,参与TLR的信号传递和定位(Shan et al, 2018; Dinarello, 2018)。LRR结构域参与PAMPs识别,并通过MyD88依赖性途径或非依赖性途径触发细胞内信号的激活(St Paul et al, 2011)。研究显示,哺乳动物和鱼类的LRR的内含子存在差异(Matsushima et al, 2007),并且不同物种间的TLR13的LRR结构域数量不同。例如,在哺乳动物中,小鼠和大鼠(Rattus norvegicus) TLR13有18个LRR结构域;在硬骨鱼中,斜带石斑鱼和大黄鱼TLR13有11个(Liang et al, 2018),而与其他物种TLR13相比,ToTLR13含有的LRR结构域较少,仅5个。TLRs的LRR结构域是用于识别PAMPs的结构域,不同物种间TLRs的LRR结构域差异可能在其识别不同配体方面具有重要作用(Matsushima et al, 2007)。吴羽媛等(2018)研究表明,马氏珠母贝(Pinctada martensi)和太平洋牡蛎(Crassostrea gigas)的TLR13蛋白结构高度相似。本研究通过构建和比对TLR13保守结构域的三级结构发现,硬骨鱼类的LRR结构域和TIR结构域具有高度的相似性,并与哺乳动物小鼠的空间结构重合率也较高,暗示其结构和功能在物种间具有一定的保守性。
通过同源序列比对发现,ToTLR13与其他硬骨鱼的TLR13具有较高的相似性(82.52%~84.58%),而与其他纲物种的序列相似性较低(33.30%~46.11%);构建的系统进化树发现,ToTLR13与其他硬骨鱼TLR13聚为一支,与其他物种TLR13相分离,说明ToTLR13与鱼类亲缘关系较近,与哺乳类、两栖类和贝类等亲缘关系较远。以上结果表明,ToTLR13在进化过程中具有较高保守性,可能具有与其他物种的TLR13相似的功能。
卵形鲳鲹ToTLR13 mRNA在所检测的组织中具有表达,这与其他鱼类TLR13的表达谱结果相似(Liang et al, 2018; Yu J X et al, 2021; Qi et al, 2020b; Gao et al, 2021; Tang et al, 2020)。RT-qPCR结果显示,健康卵形鲳鲹ToTLR13 mRNA在所检测的组织中均有表达,其中鳃、脾表达量较高,其次脑、肝、肾表达量中等,表达量较低的组织为心、头肾和肌肉。但是,不同鱼类TLR13在各组织中表达水平略有差异。例如,小黄鱼TLR13 mRNA在肝、头肾和脾中表达水平较高,而在脑、血液、鳃和眼睛中表达较低(Wang et al, 2016);而斜带石斑鱼TLR13 mRNA则在大脑中表达最高,其次是胸腺,在肠、头肾、鳃和肾中表达相对较低(Liang et al, 2018);另外,尼罗罗非鱼(Oreochromis niloticus)中鉴定出2种TLR13 (OnTLR13a和OnTLR13b),OnTLR13a在脾和大脑中呈高表达,而OnTLR13b主要在血液、脾和肝中表达(Gao et al, 2021)。这些研究表明,鱼类TLR13在正常组织中具有种属特异性和组织特异性,同时,当宿主鱼受到免疫刺激后,TLR13在免疫相关组织中普遍会呈现高表达的现象,暗示其可能在宿主免疫应答的过程中起到重要的作用。
TLRs大量存在于脾、肝和肾等免疫相关组织中,可在先天免疫细胞和绝大多数非造血细胞上表达,如树突状细胞、巨噬细胞、T细胞和B细胞(St Paul et al, 2011)。当受到病原微生物、病毒及寄生虫的刺激后,不同鱼类TLR13在各免疫相关组织中表达谱变化不同。如小黄鱼受到鳗弧菌和poly(I: C)刺激后,脾的TLR13 mRNA表达呈上升趋势,并且在小黄鱼白细胞受到poly(I: C)刺激后12 h时,TLR13 mRNA表达量显著增加(Wang et al, 2016)。达氏鲟(Acipenser dabryanus)头肾白细胞分别在LPS和poly(I: C)的刺激下,TLR13 mRNA表达均呈现显著上调(Tang et al, 2020),并且在斜带石斑鱼GS细胞受到病原菌23S rRNA刺激后,得到相似的结果,TLR13 mRNA的表达显著增加(Liang et al, 2018)。罗非鱼受到无乳链球菌的刺激后,OnTLR13a在肠道、鳃、脾、肾和血液中的表达有不同程度的上调,OnTLR13b在肠道、鳃、脾和肾中亦是如此(Gao et al, 2021)。褐鳟(Salmo trutta)肾脏在粘液虫感染后的4~12周内,TLR13 mRNA的表达显著上升(Sudhagar et al, 2020)。本研究发现,无乳链球菌和溶藻弧菌刺激后,ToTLR13 mRNA表达水平在鳃、脾、肝和头肾中均呈现出显著性上调,但总体上,溶藻弧菌诱导的ToTLR13 mRNA表达水平高于无乳链球菌(图 7)。值得注意的是,无乳链球菌和溶藻弧菌刺激后,ToTLR13 mRNA在鳃中呈现较高的表达上升(23和68倍),并且分别在72、12和96 h时表达量突增,该现象与小黄鱼、斜带石斑鱼和尼罗罗非鱼TLR13的结果相似(Liang et al, 2018; Wang et al, 2016; Gao et al, 2021)。由皮肤、肠道和鳃等黏膜相关组织构成的黏膜免疫系统,可作为鱼类抵御外源病原菌重要的免疫屏障(李坤明等, 2020; 胡伟等, 2022; Ángels et al, 2012; Yu J X et al, 2021)。上述研究结果提示,ToTLR13可能参与了病原菌入侵宿主时的免疫防御,但TLR13在配体识别过程中具体的免疫机制尚不清楚,需要后续开展实验进一步探究。
根据细胞定位,TLRs可分为两大类:一类位于细胞膜表面(如TLR1、TLR2、TLR4、TLR5、TLR6和TLR11),另一类定位于细胞质(内质体或溶酶体)中(如TLR3、TLR7、TLR8、TLR9和TLR10) (Qian et al, 2013)。研究证实,细胞内的TLRs可识别细菌、病毒、真菌或寄生虫等所释放的核酸(Shi et al, 2011)。Lee等(2013)发现,小鼠TLR13定位于核内体,并且能够识别保守的细菌23S rRNA。而在硬骨鱼中,斜带石斑鱼和小黄鱼的TLR13均定位于细胞质中(Liang et al, 2018; Wang et al, 2016)。硬骨鱼TLR13在内质体中的细胞定位可能表明了一种调节机制,通过内质体的微环境来调节配体的结合(Lee et al, 2013)。本研究亚细胞定位结果显示,在A549细胞的细胞质中绿色荧光信号较强,说明pEGFP-ToTLR13主要在细胞质上表达。以上结果表明,ToTLR13在进化过程中具有较高保守性,说明ToTLR13可能与其他鱼类的TLR13具有相似的生物学功能。
4 结论本研究利用PCR技术成功克隆了卵形鲳鲹ToTLR13基因的ORF序列,保守结构域分析显示其含有1个TM结构域、1个C端TIR结构域和5个LRR结构域。同源性比对、系统进化分析和三级结构比对结果表明,ToTLR13与其他鱼类TLR13同源性较高且具有较近的进化关系。RT-qPCR结果显示,ToTLR13 mRNA在卵形鲳鲹各组织中呈广谱型表达,其中鳃的表达量最高。而在病原菌刺激后,ToTLR13在一些免疫相关组织表达显著上调,表明ToTLR13基因卵形鲳鲹病原菌免疫过程中发挥了重要作用。亚细胞定位结果显示,ToTLR13定位于A549细胞的细胞质中。以上结果为进一步研究硬骨鱼TLR13的免疫调节作用提供了一定的科学参考价值。
致谢: 感谢北部湾大学海洋学院大型共享仪器支持!
ÁNGELS E M. An overview of the immunological defenses in fish skin. ISRN Immunology, 2012(2012): 853470 |
BAOPRASERTKUL P, PEATMAN E, ABERNATHY J, et al. Structural characterisation and expression analysis of toll-like receptor 2 gene from catfish. Fish and Shellfish Immunology, 2007, 22(4): 418-426 DOI:10.1016/j.fsi.2006.04.005 |
CAI X H, WANG B, PENG Y H, et al. Construction of a Streptococcus agalactiae phoB mutant and evaluation of its potential as an attenuated modified live vaccine in golden pompano, Trachinotus ovatus. Fish and Shellfish Immunology, 2017, 63: 405-416 DOI:10.1016/j.fsi.2016.11.050 |
CHATURVEDI A, PIERCE S K. How location governs toll-like receptor signaling. Traffic, 2009, 10(6): 621-628 DOI:10.1111/j.1600-0854.2009.00899.x |
DINARELLO C A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunological Reviews, 2018, 281(1): 8-27 DOI:10.1111/imr.12621 |
GAO F Y, PANG J C, WANG M, et al. Structurally diverse genes encode TLR13 in Nile tilapia: The two receptors can recognize Streptococcus 23S RNA and conduct signal transduction through MyD88. Molecular Immunology, 2021, 132: 60-78 DOI:10.1016/j.molimm.2021.01.020 |
HU W, WU H, YUAN H W, et al. Molecular cloning and expression analysis of the melatonin receptor gene in grass carp (Ctenopharyngodon idella). Progress in Fishery Sciences, 2022, 43(1): 141-152 [胡伟, 吴慧, 袁汉文, 等. 草鱼褪黑激素受体Mtnr1基因克隆及组织表达分析. 渔业科学进展, 2022, 43(1): 141-152] |
LEE B L, MOON J E, SHU J H, et al. UNC93B1 mediates differential trafficking of endosomal TLRs. elife, 2013, 2(2): e00291 |
LEE M S, KIM Y J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annual Review of Biochemistry, 2007, 76: 447-480 DOI:10.1146/annurev.biochem.76.060605.122847 |
LI K M, XU W T, FU X Q, et al. Cloning and immune response analysis of protein kinase C-alpha (PKCα) in Chinese tongue sole (Cynoglossus semilaevis). Progress in Fishery Sciences, 2020, 41(2): 69-77 [李坤明, 徐文腾, 扶晓琴, 等. 半滑舌鳎蛋白激酶C-alpha(PKCα)的基因克隆和免疫应答分析. 渔业科学进展, 2020, 41(2): 69-77] |
LI Y Y, LI M M, WANG M, et al. Research advances in nutritional requirement and feed of Trachinotus ovatus. Progress in Fishery Sciences, 2019, 40(1): 167-177 [李远友, 李孟孟, 汪萌, 等. 卵形鲳鲹营养需求与饲料研究进展. 渔业科学进展, 2019, 40(1): 167-177] |
LIANG Y S, DING X, YU X, et al. Identification and functional characterization of toll-like receptor 13 from orange-spotted grouper (Epinephelus coioides). Fish and Shellfish Immunology, 2018, 74: 309-317 DOI:10.1016/j.fsi.2017.12.054 |
MACLEOD C, BRYANT C E. Visualising pattern recognition receptor signalling. Biochemical Society Transactions, 2017, 45(5): 1077-1085 DOI:10.1042/BST20160459 |
MATSUSHIMA N, TANAKA T, ENKHBAYAR P, et al. Comparative sequence analysis of leucine-rich repeats (LRRs) within vertebrate toll-like receptors. BMC Genomics, 2007, 8: 124 DOI:10.1186/1471-2164-8-124 |
ST PAUL M, MALLICK A I, HAQ K, et al. In vivo administration of ligands for chicken toll-like receptors 4 and 21 induces the expression of immune system genes in the spleen. Veterinary Immunology and Immunopathology, 2011, 144(3/4): 228-237 |
QI Z, XU Y, WANG X, et al. Structural analysis of toll-like receptor 18 from soiny mullet (Liza haematocheila): Giving insights on the ligand binding mechanism of fish specific TLRs. Fish and Shellfish Immunology, 2020, a, 107: 490-496 |
QI Z, XU Y, WANG X, et al. TLR13, TLR22, TRAF6, and TAK1 in the soiny mullet (Liza haematocheila): Molecular characterization and expression profiling analysis. Developmental and Comparative Immunology, 2020, b, 112: 103774 |
QIAN C, CAO X. Regulation of toll-like receptor signaling pathways in innate immune responses. Annuals of the New York Academy of Sciences, 2013, 1283(1): 67-74 DOI:10.1111/j.1749-6632.2012.06786.x |
REBL A, GOLDAMMER T, SEYFERT H M. Toll-like receptor signaling in bony fish. Veterinary Immunology and Immunopathology, 2010, 134(3/4): 139-150 |
ROACH J C, GLUSMAN G, ROWEN L, et al. The evolution of vertebrate toll-like receptors. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(27): 9577-9582 DOI:10.1073/pnas.0502272102 |
SHAN S J, LIU R R, JIANG L, et al. Carp toll-like receptor 8 (Tlr8): An intracellular Tlr that recruits TIRAP as adaptor and activates AP-1 pathway in immune response. Fish and Shellfish Immunology, 2018, 82: 41-49 DOI:10.1016/j.fsi.2018.08.001 |
SHI Z C, CAI Z Y, SANCHEZ A, et al. A novel toll-like receptor that recognizes vesicular stomatitis virus. Journal of Biological Chemistry, 2011, 286(6): 4517-4524 DOI:10.1074/jbc.M110.159590 |
SUDHAGAR A, EL-MATBOULI M, KUMAR G. Identification and expression profiling of toll-like receptors of Brown trout (Salmo trutta) during proliferative kidney disease. International Journal of Molecular Sciences, 2020, 21(11): 3755 DOI:10.3390/ijms21113755 |
TANG R, WANG S S, HAN P P, et al. Toll-like receptor (TLR)2 and TLR13 from the endangered primitive-ray finned fish Dabry's sturgeon (Acipenser dabryanus) and their expression profiling upon immune stimulation. Aquaculture Reports, 2020, 16: 100247 DOI:10.1016/j.aqrep.2019.100247 |
VALENZUELA-MUÑOZ V, BOLTAÑA S, GALLARDO- ESCÁRATE C. Comparative immunity of Salmo salar and Oncorhynchus kisutch during infestation with the sea louse Caligus rogercresseyi: An enrichment transcriptome analysis. Fish and Shellfish Immunology, 2016, 59: 276-287 DOI:10.1016/j.fsi.2016.10.046 |
VIDYA M K, KUMAR V G, SEJIAN V, et al. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. International Reviews of Immunology, 2018, 37(1): 20-36 DOI:10.1080/08830185.2017.1380200 |
VOOGDT C G P, WAGENAAR J A, VAN PUTTEN J P M. Duplicated TLR5 of zebrafish functions as a heterodimeric receptor. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(14): 3221-3229 |
WANG M Q, WANG L L, JIA Z H, et al. The various components implied the diversified toll-like receptor (TLR) signaling pathway in mollusk Chlamys farreri. Fish and Shellfish Immunology, 2018, 74: 205-212 DOI:10.1016/j.fsi.2017.12.064 |
WANG Y J, BI X Y, CHU Q, et al. Discovery of toll-like receptor 13 exists in the teleost fish: Miiuy croaker (Perciformes, Sciaenidae). Developmental and Comparative Immunology, 2016, 61: 25-33 DOI:10.1016/j.dci.2016.03.005 |
WU Y Y, LIANG H Y, RUAN Y, et al. Clone and tissue expression analysis of PmTLR13 gene in pinctada fucata martensii. Genomics and Applied Biology, 2018, 37(1): 238-246 [吴羽媛, 梁海鹰, 阮尧, 等. 马氏珠母贝TLR13基因的克隆与组织表达分析. 基因组学与应用生物学, 2018, 37(1): 238-246] |
YU J X, LIU X L, YANG N, et al. Characterization of toll-like receptor 1 (TLR1) in turbot (Scophthalmus maximus L.). Fish and Shellfish Immunology, 2021, 115: 27-34 DOI:10.1016/j.fsi.2021.05.018 |
YU X, LIANG Y S, ZHOU Y, et al. 23S rRNA from Vibrio parahaemolyticus regulates the innate immune response via recognition by TLR13 in orange-spotted grouper (Epinephelus coioides). Developmental and Comparative Immunology, 2021, b, 114: 103837 |
ZHANG J, KONG X H, ZHOU C J, et al. Toll-like receptor recognition of bacteria in fish: Ligand specificity and signal pathways. Fish and Shellfish Immunology, 2014, 41(2): 380-388 DOI:10.1016/j.fsi.2014.09.022 |
ZHOU S, TU X, PANG H, et al. A T3SS regulator mutant of Vibrio alginolyticus affects antibiotic susceptibilities and provides significant protection to Danio rerio as a live attenuated vaccine. Frontiers in Cellular and Infection Microbiology, 2020, 10: 183 DOI:10.3389/fcimb.2020.00183 |
ZHOU Y C, CHEN X J, CAO Z J, et al. R848 is involved in the antibacterial immune response of golden pompano (Trachinotus ovatus) through TLR7/8-MyD88-NF-κb- signaling pathway. Frontiers in Immunology, 2021, 11: 617522 |



