2. 上海海洋大学食品学院 上海 201306
2. College of Food Science and Technology, Shanghai Ocean University, Shanghai 201306, China
呋喃西林是人工合成的广谱抗菌药物,能破坏细菌的糖代谢和氧化酶系统,早期在水产和畜牧养殖中用来治疗畜禽疾病和防治病虫害。呋喃西林进入动物体内后迅速代谢,其代谢物氨基脲(semicarbazide, SEM)与蛋白质结合后在动物体内长期稳定存在(范清涛等, 2020)。Vass等(2008a、b)研究发现,动物源性食品中残留的SEM可通过食物链传递给人类,长期摄入对人体有致癌、致畸等副作用。对SD大鼠(Rattus norvegicus)的研究表明,SEM造成大鼠多个组织器官发生病变,包括子宫、胰脏和甲状腺等,同时,表现出致变性和弱遗传毒性效应(Maranghi et al, 2009; 朱乐玫等, 2012)。对妊娠期大鼠腹腔注射40 mg/kg SEM,21 d后大鼠腹中胎儿组织和骨骼发生畸变,同时多个脏器出现核酸水平显著降低的现象(李嘉, 2008)。SEM在组织形态学上导致多种组织器官形态改变,也对神经系统、内分泌系统的功能产生影响。美国、澳大利亚、加拿大、日本、新加坡、欧盟等已禁止在食品工业中使用该类药物,并严格执行对水产品中呋喃西林的残留监测(龚珞军等, 2019)。呋喃西林也已被我国列入首批《兽药地方标准废止目录》中,在《关于开展2016年国家产地水产品质量安全监督抽查工作的通知》中,将SEM的残留限量值定为1.0 µg/kg。
近年来,我国对甲壳类水产品药物残留进行检测发现,SEM的检出超标率高达50% (于慧娟等, 2012)。2002—2003年期间,欧盟食品和饲料快速预警系统(Rapid Alert System for Food and Feed, RASFF)发布了300余份来自泰国、文莱、巴西等国家的虾类等SEM残留事件的通报,数百吨水产品因此被销毁。SEM在不同甲壳类水生动物中的本底含量差异较大,几乎在所有甲壳中均不同程度的检出SEM,包括日本沼虾(Macrobrachium nipponense)、罗氏沼虾(Macrobrachium rosenbergii)、凡纳滨对虾(Litopenaeus vannamei)、克氏原螯虾(Procambarus clarkii)、中华绒螯蟹(Eriocheir sinensis)等,其壳中SEM检出率为100%,且含量远高于1.0 µg/kg。在甲壳类肌肉可食组织中,SEM含量差异较大,如凡纳滨对虾、中华绒螯蟹等肌肉中未检出SEM,而日本沼虾和罗氏沼虾肌肉中SEM检出率极高(于慧娟等, 2012; 张睿等, 2012; 李东利等, 2015; 王鼎南等, 2016; 彭婕等, 2019; 曹爱玲等, 2020; 范清涛等, 2020) (表 1)。SEM严重超标的现状引发了人们对甲壳类水产品安全性的思考。本文针对甲壳类水产品中SEM的不同来源进行总结,概述有关SEM来源的研究现状及生成机理,以期为甲壳类水产品中SEM的检测提供理论根据。
|
|
表 1 不同品种甲壳类水产品不同检测部位SEM含量(μg/kg) Tab.1 SEM content in different test sites of different crustacean aquatic products (μg/kg) |
赵芸等(2019)研究发现,许多不曾接触呋喃西林药物的养殖甲壳类水生动物体内(以虾蟹为代表)含有SEM。此外,研究人员多次从生活在天然水域的虾蟹中检测出SEM,推测SEM具有内源性。Saari等(2004)在未食用呋喃西林的克氏原螯虾中检测到SEM最高含量为12 µg/kg,首次提出了甲壳动物自身可以产生SEM的观点。McCracken等(2013)通过对孟加拉国野生淡水明虾(Macrobrachium agwi)研究发现,虾肉和虾壳均有SEM检出,相较于虾肉,虾壳中SEM含量更高,这与van Poucke等(2011)对罗氏沼虾、王鼎南等(2016)对日本沼虾、倪永付等(2012)对微山湖小青虾SEM检测结果一致。这些结果证明了甲壳类水产品中内源性SEM的存在。彭婕等(2019)在中华绒螯蟹脱壳后新长出的软壳中检测到了SEM,而蟹肉中未检出SEM,推测水生动物甲壳可能是其内源性SEM的主要来源。
1.2 甲壳类水产品中外源性SEM的来源 1.2.1 生长环境或食物摄入SEM自然界中,甲壳类动物的生存环境会因人类经济活动的影响而受到SEM污染。于召强等(2013)研究发现,SEM作为一种新的水体污染物在水体和沉积物中长时间存在并不断富集,最终进入生物体内。徐英江等(2010)在潮河河口水和河流沉积物中检测到SEM大量存在;田秀慧(2018)分别在山东省金城湾、四十里湾和莱州湾西部3个水域的水体、沉积物中检测到SEM存在,且所监测水域中的贝类、虾蟹等SEM均有不同程度的检出,表明水体环境中的SEM对水生动物的富集污染。其次,甲壳类水生动物可通过摄食自然界中的藻类或喂食饲料引入SEM,田秀慧(2018)通过对月菱形藻(Nitzschia closterium)、扁藻(Platymonas)和叉鞭金藻(Dicrateria sp.)中的SEM进行监测,发现SEM在藻类中有很强的富集能力,富集系数为145.3~200.0。同样,Hoenicke等(2004)在红藻(Rhodophyta)、褐藻(Phaeophyta)中检测到了SEM。水生动物食用这些天然食物后,体内会产生SEM残留。
1.2.2 养殖过程中非法使用呋喃西林引入SEM呋喃西林作为广谱抗菌药物,具有良好的抗菌效果和价格低廉等优点。某些养殖户为了降低水产品疾病发生率(比较典型的疾病:赤皮、烂鳃和肠炎)、获得更大的经济利益,违反呋喃西林使用规定。呋喃西林进入动物体内后,可以在弱酸性条件下迅速分解成SEM (Leitner et al, 2001),从而与蛋白形成难以代谢的结合体。Kwon(2017)研究表明,在混合养殖的过程中,一些养殖户会用从出生开始就被投喂了呋喃西林等抗生素的家禽垫料对池塘施肥,从而对养殖水体造成SEM污染进而进入养殖动物体内。索纹纹等(2013)研究发现,通过对养殖斑点叉尾
次氯酸盐因具有强氧化性广泛用于水产品的卫生消毒(李会生等, 2001)。袁涛等(2011)研究发现,次氯酸盐浸泡的水产品中SEM含量显著高于清水浸泡的水产品。杨曦等(2011)、王建(2015)发现水产品中SEM含量与次氯酸盐的质量浓度、作用持续时间和食品接触物表面积成正比。Zhang等(2016)采用次氯酸盐处理凡纳滨对虾、三疣梭子蟹(Portunus trituberculatus)和哈氏仿对虾(Parapenaeopsis hardwickii)后发现,SEM含量与次氯酸盐浓度存在剂量依赖性且次氯酸盐对不同水产品的SEM含量具有差异。
1.2.4 偶氮二甲酰胺加工产生SEM偶氮二甲酰胺(azodicarbonamide, ADC)在人类生产生活中有2种用途:一是用作食品添加剂,增强面粉团的柔韧性;二是作为食品级玻璃罐密封垫圈的原材料(黄晓姗等, 2018)。ADC在高温高压条件下,受热快速分解产生SEM,对与其接触的食品产生污染。
某些生产厂家为迎合消费者口感需求会对水产品裹粉以促进销售,如面包虾等,使用含ADC的面粉作为甲壳类水产品的裹粉原料是导致甲壳类水产品SEM超标的原因之一。Pereira等(2004)在含有ADC添加剂的面粉中检出SEM,含量约为2~5 µg/kg。胡菏等(2018)、阮莎莎等(2019)等对市售的面粉及面制品中的ADC和SEM的含量进行监测发现,面粉及面制品中均有ADC检出,面制品中SEM的含量高于面粉,且面包中SEM的含量高达139~1 288 µg/kg,说明ADC经高温反应生成了SEM。另外,SEM可通过包装材料对与之接触的食品造成污染。Stadler等(2004)从水产品罐头、蜂蜜、调味品和婴儿食品罐头中均检测到了SEM,最高含量可达25 µg/kg;陈志锋等(2009)研究发现,食品接触垫圈经过加热可分解产生SEM;赵天祎等(2019)在蜂蜜中检测出SEM,通过模拟性实验推断是蜂蜜罐垫圈的内溶物外溶所致。Hoenicke等(2004)将玻璃罐垫圈进行加热并检测SEM含量,发现垫圈中的ADC在高温条件下降解成SEM,且SEM可从垫圈迁移到食品中。因此,甲壳类水产品在加工运输过程中应当尽量避免与ADC接触,以防受到外源性SEM的污染。
2 甲壳类水产品中SEM的生成机理 2.1 内源性SEM生成机理甲壳类水产品中壳的SEM含量普遍大于肉,而壳的主要成分是甲壳素(几丁聚糖)。因此,相关研究对内源性SEM是否来源于甲壳中的甲壳素进行了论证。
周萍等(2008)研究发现,未使用任何药物的蜂蛹在生长后期体内的SEM含量随着甲壳素含量的增加而逐渐增加,推测内源性SEM的形成或与内源甲壳素有关。McCracken等(2013)研究发现,在虾壳和虾肉之间存在着一层分泌甲壳素的单细胞表皮层,靠近该表皮层虾肉的SEM含量是内层虾肉检出量的3倍以上,由此推断虾肉中的SEM主要来自于产生甲壳素的细胞表皮层。然而,彭婕等(2015)通过对中华绒螯蟹蟹壳中的甲壳素研究发现,内源性SEM的产生与甲壳素无关,可能是蟹壳中的结合蛋白水解后,产生了在一定条件下能转化为SEM的氨基酸。对于以上实验结果,彭婕等(2019)进一步研究了不同蟹壳中的主要成分与SEM残留的相关性,发现不同部位蟹壳中甲壳素含量与SEM残留水平呈明显的正相关性,蛋白质含量及氨基酸组成与SEM残留水平呈负相关性,这可能是甲壳素和氨基酸在之前处理过程中产生了SEM,是导致中华绒螯蟹中内源性SEM残留的原因。
目前,关于甲壳类水产品内源性SEM的形成机理已有一定进展。一种被认可的说法是内源性SEM形成可能与甲壳中的结合蛋白及氨基酸有关。曹爱玲等(2019、2020)研究发现,中华鳖壳中SEM含量随着烘干温度的上升而上升,但其蛋白含量随烘干温度的上升而下降,对其丰度差异蛋白质进行筛选,发现球蛋白、角蛋白等13种蛋白与SEM含量的变化显著相关,此类蛋白涉及细胞碳代谢、焦点黏附等生物过程,由此猜测这些蛋白随热能的增加转化成了SEM。Samsonova等(2008)对动物体内含有SEM残基的蛋白进行鉴定,发现白蛋白中含有高浓度SEM残基,并且在以谷胱甘肽S-转移酶为主要成分的蛋白质混合物中也发现含有高浓度的SEM残基,表明SEM的形成可能与SEM结合的谷胱甘肽有关联。谢冬冬等(2014)研究表明,甲壳类水产品中内源SEM不仅与样品中的蛋白质有关,而且与样品中的氨基酸组成有关联。Noonan等(2008)证实甲壳类水产品中含量最丰富的氨基酸是精氨酸。精氨酸酶可将精氨酸水解为鸟氨酸和尿素进入尿素循环,尿素和SEM在结构上有一定的相似性。Hoenicke等(2004)认为SEM是由含氮物质(如精氨酸、组氨酸、瓜氨酸和肌酐)与酰胺或尿素反应形成。Abernethy等(2015)研究表明,肼作为参与尿素循环的重要中间物质,可与酸根离子反应生成SEM,因此,某些高氨氮(NH4+-N)含量的食品可在酸性条件下形成SEM。由此推测,精氨酸可能是动物内源性SEM的前体。Yu等(2019)在凡纳滨对虾生长期间对参与尿素循环的相关物质含量进行检测后认为,虾体内的精氨酸等物质通过尿素循环最终形成SEM。
精氨酸是内源性SEM形成的重要潜在前体。精氨酸等参与甲壳类水生动物的尿素循环,通过恶嗪啶中间体产生了SEM,结合尿素循环中主要物质的含量变化分析,内源性SEM的形成与精氨酸的胍基和酰胺基、瓜氨酸和尿素的酰胺结构密切相关,内源性SEM可能的生成机理如图 1所示。

|
图 1 内源性SEM在甲壳类水产品中可能的生成机理 Fig.1 Possible formation mechanism of endogenous SEM in crustacean aquatic products A: 虾肉;B: 虾表皮层;C: 虾壳。 A: Shrimp meat; B: Shrimp epidermal layer; C: Shrimp shell. |
Hoenicke等(2004)研究不同浓度的次氯酸盐对北海虾、鱼制品、蛋白粉、红藻、鸡肉和蜂蜜的SEM检出的影响发现,随着有效氯含量不断增加,6种样品的SEM含量均有不同程度的增加,其中,以蛋白粉最为显著,检出量为20 μg/kg。考虑到水产品中富含的精氨酸、组氨酸、瓜氨酸、肌酸酐等多种具有脒基或脲基结构的含氮化学物质结构与SEM化学结构相近,Hoenickek等(2004)认为这些含氮化学物质经过次氯酸盐溶液的处理后,发生降解反应最终形成了SEM。Abernethy等(2015)推测次氯酸盐溶液中的氨基甲酸根离子可能与水产品中的氨或酸性酰胺反应生成肼,肼通过尿素循环与尿素及其他化合物反应生成SEM。Bendall (2009)认为SEM是次氯酸盐和尿素在一定条件下发生霍夫曼反应产生的。由此推测,水产品中的SEM可能来自体内以及次氯酸盐中的氨,次氯酸盐引入SEM可能生成机理见图 2。
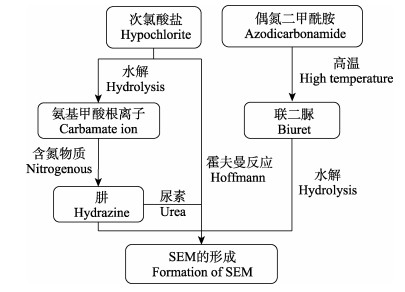
|
图 2 外源性SEM在甲壳类水产品中可能的生成机理 Fig.2 Possible formation mechanism of exogenous SEM in crustacean aquatic products |
温度可能对SEM的生成有影响。Pereira等(2004)研究发现,在面粉中添加10 µg/kg的ADC,所检样品的SEM含量为12 µg/kg,转化率约为0.1%。Ye等(2011)研究发现,经高温烘焙后,面粉中添加的ADC会分解成SEM,且面制品外部的SEM含量要高于内部,说明温度对SEM的生成有积极影响。姚敬等(2016)、蒋志红等(2014)通过实验证明了ADC在湿热条件下可生成SEM。Noonan等(2008)研究ADC面粉及面制品中的SEM生成情况时发现,生面团不含SEM,而面制品中有SEM检出,进一步研究发现SEM含量与温度成正比。
ADC在高温条件下先降解为联二脲,联二脲再经过水解反应转化为SEM (李金强等, 2009)。考虑到呋喃西林与联二脲分子结构的差异性,可排除呋喃西林代谢产生联二脲的推测。从现有的研究看,ADC是联二脲的唯一生物来源,因此,可将联二脲作为ADC相应的目标检出物,ADC引入SEM生成机理见图 2。
3 结论与展望SEM在自然界中的来源广泛,甲壳类水产品中检出的SEM非呋喃西林唯一代谢产生,水体环境及加工过程的污染等均可导致SEM超标。对于SEM的生成机理,目前研究相对较少,当前对于内源性SEM的生成机理有2种推测:一是富含蛋白质的甲壳类水产品中的含氮物质如精氨酸等参与尿素循环等过程形成SEM;二是来源于单细胞表皮层的甲壳素转化。对于外源性SEM的生成机理也有2种推断:一是甲壳类水产品经次氯酸盐的处理生成的肼进入尿素循环,增加了SEM生成量;二是加工过程中外加的ADC经高温降解为SEM,导致SEM过量残留。深入分析研究甲壳类水产品中SEM的主要来源及形成作用机理对于保障我国水产养殖业健康发展具有重大意义。对甲壳类水产品内源性SEM的形成机理进行研究,可为我国制定甲壳类水产品中SEM最大残留限量提供科学数据。
ABERNETHY G A. Generation of semicarbazide from natural azine development in foods, followed by reaction with urea compounds. Food Additives and Contaminants, Part A. Chemistry Analysis Control Exposure and Risk Assessment, 2015, 32(9): 1416-1430 |
BENDALL J. Semicarbazide is non-specific as a marker metabolite to reveal nitrofurazone abuse as it can form under Hofmann conditions. Food Additives and Contaminants, Part A. Chemistry Analysis Control Exposure and Risk Assessment, 2009, 26(1): 47-56 |
CAO A L, CHEN Y L, CAI L Y, et al. The effects of different drying temperatures on the detectable amount of semicarbazide in crustacean aquatic products. Chinese Journal of Animal Quarantine, 2020, 37(1): 94-99 [曹爱玲, 陈怡琳, 蔡路昀, 等. 不同烘干温度对甲壳类水产品中氨基脲检出量的影响. 中国动物检疫, 2020, 37(1): 94-99 DOI:10.3969/j.issn.1005-944X.2020.01.020] |
CAO A L, YU Z F, CHEN Y L, et al. Research progress on nitrofurazone and its metabolite of semicarbazide in animal-derived food. Chinese Journal of Animal Quarantine, 2019, 36(6): 62-67 [曹爱玲, 余招锋, 陈怡琳, 等. 动物源食品中呋喃西林及其代谢物氨基脲研究进展. 中国动物检疫, 2019, 36(6): 62-67 DOI:10.3969/j.issn.1005-944X.2019.06.015] |
CHEN Z F, LI C, SUN L, et al. SEM problems in food contact materials. Food and Machinery, 2009, 25(2): 5-7 [陈志锋, 李成, 孙利, 等. 食品接触材料中的氨基脲问题. 食品与机械, 2009, 25(2): 5-7] |
DING C Y. Study on the detection of nitrofuran metabolites residues in shrimps. Master′s Thesis of Zhejiang University of Technology, 2019, 48-51 [丁春燕. 青虾中硝基呋喃代谢物残留的检测研究. 浙江工业大学硕士研究生学位论文, 2019, 48-51] |
DING J W, DENG J C, YANG X Q, et al. Accumulation and elimination of four nitrofuran metabolites in muscle of grouper (Epinephelus awoara). South China Fisheries Science, 2018, 14(1): 60-67 [丁军伟, 邓建朝, 杨贤庆, 等. 4种硝基呋喃类代谢物在青石斑鱼肌肉中的富集与消除规律. 南方水产科学, 2018, 14(1): 60-67] |
FAN Q T, CHEN S J, DENG J C, et al. Research progress on the production and control of semicarbazide in crustacean aquatic products. Journal of Food Safety and Quality, 2020, 11(5): 1439-1445 [范清涛, 陈胜军, 邓建朝, 等. 甲壳类水产品中氨基脲产生及控制方法研究进展. 食品安全质量检测学报, 2020, 11(5): 1439-1445] |
FAN X H, ZHEN H, QIAN W, et al. Study on SEM attenuation of nitrofurazone metabolites in Eriocheir sinensis. Jiangsu Agricultural Science, 2010(6): 368-370 [樊新华, 郑浩, 钱伟, 等. 呋喃西林代谢物氨基脲在中华绒螯蟹体内的衰减研究. 江苏农业科学, 2010(6): 368-370 DOI:10.3969/j.issn.1002-1302.2010.06.146] |
GONG L J, YANG L S, WANG J L, et al. Lecture on quality and safety of aquatic products: Lecture Ⅱ Nitrofurans and quality and safety of aquatic products (1). Guide to Enrichment in Fisheries, 2019(10): 58-60 [龚珞军, 杨兰松, 王将来, 等. 《水产品质量安全》讲座第二讲: 硝基呋喃类药物与水产品质量安全(1). 渔业致富指南, 2019(10): 58-60] |
HOENICKE K, GATERMANN R, HARTIG L, et al. Formation of semicarbazide (SEM) in food by hypochlorite treatment: Is SEM a specific marker for nitrofurazone abuse?. Food Additives and Contaminants, 2004, 21(6): 526-537 DOI:10.1080/02652030410001712484 |
HU H, LIU X L, YU H, et al. Monitoring and analysis of semicarbazide contamination in flour products in Jiangxi. Chinese Journal of Health Inspection, 2018, 28(3): 340-342 [胡菏, 刘小玲, 于晖, 等. 江西省面制品中氨基脲污染监测分析. 中国卫生检验杂志, 2018, 28(3): 340-342] |
HUANG X S, GUAN M X, HANG Y P, et al. Study on degradation of flour additive azodicarbazide to semicarbazide under heat treatment. Journal of Instrumental Analysis, 2018, 37(8): 977-980 [黄晓姗, 关铭鑫, 杭义萍. 面制品添加剂偶氮甲酰胺热处理下转化为氨基脲的研究. 分析测试学报, 2018, 37(8): 977-980 DOI:10.3969/j.issn.1004-4957.2018.08.019] |
HUANG X Y, SHEN X S, HUANG D M, et al. Elimination rules of nitrofurazone metabolite residue in Eriocheir sinensis cultured in outdoor ponds. Marine Fisheries, 2017, 39(6): 674-681 [黄宣运, 沈晓盛, 黄冬梅, 等. 室外池塘自然养殖条件下呋喃西林代谢物在中华绒螯蟹体内残留和消除规律. 海洋渔业, 2017, 39(6): 674-681 DOI:10.3969/j.issn.1004-2490.2017.06.009] |
JIANG Y, DING T, XU J Z, et al. Dynamical changes of Nitrfuran metabolites in crawfish. Animal Husbandry and Veterinarian, 2008(2): 34-37 [蒋原, 丁涛, 徐锦忠, 等. 硝基呋喃类药物在克氏螯虾组织中消除规律的研究. 畜牧与兽医, 2008(2): 34-37] |
JIANG Z H, WU X P, WANG M X, et al. relationship of added azodicarbonamide with the formation of semicarbazide in heated flour and deep-fried breaded shrimp. Food Science, 2014, 35(19): 91-95 [蒋志红, 吴晓萍, 王明兴, 等. 偶氮甲酰胺与氨基脲在面粉及面包虾中的形成关系. 食品科学, 2014, 35(19): 91-95] |
KWON J W. Semicarbazide: Natural occurrence and uncertain evidence of its formation from food processing. Food Control, 2017(72): 268-275 |
LEITNER A, ZOLLNER P, LINDNER W. Determination of the metabolites of nitrofuran antibiotics in animal tissue by high-performance liquid chromatography tandem mass spectrometry. Journal of Chromatography A, 2001, 939(1/2): 49-58 |
LI D L, LI J, CHANG Z Q, et al. The elimination rules of nitrofurazone metabolites and its effects on activities of metabolic enzymes in Fenneropenaeus chinensis. Progress in Fishery Sciences, 2015, 36(5): 87-94 [李东利, 李健, 常志强, 等. 呋喃西林代谢产物在中国对虾(Fennero- penaeus chinensis)体内的消除规律及其对代谢酶活性的影响. 渔业科学进展, 2015, 36(5): 87-94] |
LI J Q, GUO H X, CAO P, et al. Study of the correlation about azodicarbonamide decomposing to semicarbazide. Chemical Analysis and Meterage, 2009, 18(6): 34-36 [李金强, 郭海霞, 曹鹏, 等. 偶氮甲酰胺分解产生呋喃西林代谢物的相关性研究. 化学分析计量, 2009, 18(6): 34-36] |
LI H S, WANG L. Application of chlorine dioxide in aquaculture industry. Inland Fisheries, 2001(1): 39-39 [李会生, 王玲. 二氧化氯在水产养殖业中的应用. 内陆水产, 2001(1): 39-39] |
LI J. Toxicology study on semicarbazide of food additive by-production. Master′s Thesis of Jilin Agricultural University, 2008 [李嘉. 食品添加剂副产物氨基脲的毒理学研究. 吉林农业大学硕士研究生学位论文, 2008]
|
MARANGHI F, TASSINA R, LAG A V, et al. Effects of the food contaminant semicarbazide following oral administration in juvenile Sprague-Dawley rats. Food and Chemical Toxicology, 2009, 47(2): 472-479 |
MCCRACKEN R, HANNA B, ENNIS D, et al. The occurrence of semicarbazide in the meat and shell of Bangladeshi fresh-water shrimp. Food Chemistry, 2013, 136(3/4): 825-832 |
NI Y F, ZHU L P, WANG Y, et al. Determination of nitrofurazone metabolite in every part of Weishan Lake shrimp. Food and Fermentation Science and Technology, 2012, 48(1): 86-88 [倪永付, 朱莉萍, 王勇, 等. 微山湖小青虾各部分呋喃西林代谢物含量测定. 食品与发酵科技, 2012, 48(1): 86-88] |
NOONAN G O, BEGLEY T H, DIACHENKO G W. Semicarbazide formation in flour and bread. Journal of Agricultural and Food Chemistry, 2008, 56(6): 2064-2067 |
PAK-SIN C, MAYDA I L, ANN A, et al. Residue depletion of nitrofuran drugs and their tissue-bound metabolites in channel catfish (Ictalurus punctatus) after oral dosing. Journal of Agricultural and Food Chemistry, 2008, 56(17): 8030-8034 |
PENG J, GAN J H, CHEN J W, et al. Distribution and formation mechanism of semicarbazide in Ericocheir sinensis. Freshwater Fisheries, 2015, 45(4): 108-112 [彭婕, 甘金华, 陈建武, 等. 中华绒螯蟹中氨基脲的分布及产生机理分析. 淡水渔业, 2015, 45(4): 108-112] |
PENG J, LÜ L, YU Y L, et al. Study on the formation approach of endogenous semicarbazide in Eriocheir sinensis. Freshwater Fisheries, 2019, 49(3): 108-112 [彭婕, 吕磊, 喻亚丽, 等. 中华绒螯蟹中内源性氨基脲的产生途径研究. 淡水渔业, 2019, 49(3): 108-112] |
PEREIRA A S, DONATO J L, DE N G. Implications of the use of semicarbazide as a metabolic target of nitrofurazone contamination in coated products. Food Additives and Contaminants, 2004, 21(1): 63-69 |
RUAN S S, LIU G H, ZHU Z, et al. Dietary exposure assessment of azocarbamide and its transformation products in flour and flour products in Shenzhen. Journal of Food Safety Quality, 2019, 10(12): 3857-3862 [阮莎莎, 刘桂华, 朱舟, 等. 深圳市面粉与面制品中偶氮甲酰胺及其转化产物膳食暴露评估. 食品安全质量检测学报, 2019, 10(12): 3857-3862] |
SAARI L, PELTONEN K. Novel source of semicarbazide: Levels of semicarbazide in cooked crayfish samples determined by LC/MS/MS. Food Additives and Contaminants, 2004, 21(9): 825-832 |
SAMSONOVA J V, DOUGLAS A J, COPER K M, et al. The identification of potential alternative biomarkers of nitrofurazone abuse in animal derived food products. Food and Chemical Toxicology, 2008, 46(5): 1548-1554 |
STADLER R H, MOTTIER P, GUY P, et al. Semicarbazide is a minor thermal decomposition product of azodicarbonamide sed in the gaskets of certain food jars. Analyst, 2004, 29(3): 276-281 |
SUO W W, LIU Y T, AI X H, et al. Elimination rules of the semicarbazide in environment and assessment of semiearbazide in the channel catfish (Ietalurus netaus) tissue. Journal of Agricultural Environmental Science, 2013, 32(4): 681-688 [索纹纹, 刘永涛, 艾晓辉, 等. 环境中氨基脲消解规律及对斑点叉尾  残留评估. 农业环境科学学报, 2013, 32(4): 681-688] 残留评估. 农业环境科学学报, 2013, 32(4): 681-688] |
TAN Z J, ZHAI Y X, LENG K L, et al. The depuration rules of the metabolites of furazolidone and nitrofurazone in turbot Scophthalmus maximus. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2008, 47(S1): 63-69 [谭志军, 翟毓秀, 冷凯良, 等. 呋喃西林和呋喃唑酮代谢物在大菱鲆组织中的消除规律. 中山大学学报(自然科学版), 2008, 47(S1): 63-69] |
TIAN X H. Temporal and spatial distribution in three typical mariculture bays of northern Shandong Province and biological toxicity in Apostichopus japonicus semicarbazide. Doctoral Dissertation of University of Chinese Academy Sciences (Yantai Coastal Institute, Chinese Academy of Sciences), 2018, 73-89 [田秀慧. 山东北部三个典型养殖海湾氨基脲的时空分布及对刺参的生物毒性效应研究. 中国科学院大学(中国科学院烟台海岸带研究所)博士研究生学位论文, 2018, 73-89] |
VAN POUCKE C, DATEVERNIER C, WILLE M, et al. Investgation into the possible natural occurance of semicarbazide icarbazide in prawn Macrobrachium rosenbergii. Journal of Agricultural and Food Chemistry, 2011, 59(5): 2107-2112 |
VASS M I, DIBLIKOVA I, CERNOCH M, et al. ELISA for semicarbazide and its application for screening in food contamination. Analytica Chimica Acta, 2008a, 608(1): 86-94 |
VASS M, HRUSKA K, FRANEK M. Nitrofuran antjbiotjcs: A review on the application prohibition and residual analysis. Journal of Veterinary Medical, 2008b, 53(9): 469-500 |
WANG D N, ZHOU F, LI S Y, et al. Background value survey and source analysis of semicarbazide in shellfish. China Fisheries Quality and Standards, 2016, 6(6): 6-11 [王鼎南, 周凡, 李诗言, 等. 甲壳类水产品中呋喃西林代谢物氨基脲的本底调查及来源分析. 中国渔业质量与标准, 2016, 6(6): 6-11] |
WANG J. Researches of nitrofurazone and semicarbazide in marine crustaceans. Master′s Thesis of Zhejiang Gongshang University, 2015, 53-59 [王建. 甲壳类水产中呋喃西林及氨基脲的研究. 浙江工商大学硕士研究生学位论文, 2015, 53-59] |
XIE D D, WAN Z G, SHEN J C, et al. Study on semicarbazide content difference from different organizations chicken generated by sodium hypochlorite. Journal of Food Safety and Quality, 2014, 5(11): 3394-3399 [谢冬冬, 万志刚, 沈金灿, 等. 不同鸡组织经次氯酸钠处理后氨基脲生成量差异研究. 食品安全质量检测学报, 2014, 5(11): 3394-3399] |
XU Y J, SUN Y Z, SONG X K, et al. Survey of semicarbazide contamination in coastal waters adjacent to the Chaohe River estuary. Oceanologia et Limnologia Sinica, 2010, 41(4): 538-542 [徐英江, 孙玉增, 宋秀凯, 等. 潮河口邻近海域氨基脲污染现状调查研究. 海洋与湖沼, 2010, 41(4): 538-542] |
YANG X, LI H G. Study on formation of semicarbazide (SEM) in aquatic products by hypochlorite treatment. Science and Technology for Food Industry, 2011, 32(4): 158-159 [杨曦, 李红光. 水产品在消毒水作用下产生呋喃西林代谢物的研究. 食品工业科技, 2011, 32(4): 158-159] |
YAO J, HUANG W X, LI S X, et al. Detection of SEM in wheat flour products and study on the conversion rule with azocarbamide. Chinese Journal of Health Inspection, 2016, 26(18): 2600-2602 [姚敬, 黄伟雄, 李少霞, 等. 小麦粉制品中氨基脲的检测及与偶氮甲酰胺的转化规律研究. 中国卫生检验杂志, 2016, 26(18): 2600-2602] |
YE J, WANG X H, SANG Y X, et al. Study on the determination of semicarbazide in flour products and the transformation law between azodicarbazide and semicarbazide. Journal of Agricultural and Food Chemistry, 2011, 59(17): 9313-9318 |
YU H J, LI B, CAI Y Q, et al. Determination of semicarbazide content in crustaceans by liquid chromatography tandem mass spectrometry. Chinese Journal of Analytical Chemistry, 2012, 40(10): 1530-1535 [于慧娟, 李冰, 蔡友琼, 等. 液相色谱–串联质谱法测定甲壳类水产品中氨基脲的含量. 分析化学, 2012, 40(10): 1530-1535] |
YU W L, LIU W H, SANG Y X, et al. Analysis of endogenous semicarbazide during the whole growth cycle of Litopenaeus vannamei and its possible biosynthetic pathway. Journal of Agricultural and Food Chemistry, 2019, 67(29): 8235-8242 |
YU Z Q, XU Y J, TIAN X H, et al. Semicarbazide bioaccumulation in seashells of Sishili Bay. Marine Environmental Science, 2013, 32(1): 39-42 [于召强, 徐英江, 田秀慧, 等. 四十里湾海洋贝类对氨基脲的生物富集特性. 海洋环境科学, 2013, 32(1): 39-42] |
YUAN T, TIAN G H, WU W, et al. Effect of sodium hypochlorite, alcohol and bamboo swab on nitrofurazone metabolite in chicken products. Journal of Anhui Agricultural Sciences, 2011, 39(30): 18733-18735 [袁涛, 田国华, 吴伟, 等. 次氯酸钠、酒精及穿串用竹签对鸡肉产品中呋喃西林代谢物检测结果的影响. 安徽农业科学, 2011, 39(30): 18733-18735] |
ZHANG R, ZHANG X Y, WU B, et al. Determination and source analysis of semicarbazide in crustacean aquatic products. Environmental Chemistry, 2012, 31(6): 915-916 [张睿, 张晓燕, 吴斌, 等. 甲壳类水产品中氨基脲的测定和来源分析. 环境化学, 2012, 31(6): 915-916] |
ZHANG X J, CHEN X, XU H, et al. Identification and occurrence of endogenous semicarbazide in prawns and rabs from Zhejiang Province, China. Food Additives and Contaminants, PartA: Chemistry Analysis Control Exposure and Risk Assessment, 2016, 33(2): 252-258 |
ZHAO T Y, MA Z F, ZHANG Y. Study on the source of semicarbazide in honey. Journal of Food Safety Quality Testing, 2019, 10(11): 3401-3404 [赵天祎, 马占峰, 张莹. 蜂蜜中氨基脲本底来源研究. 食品安全质量检测学报, 2019, 10(11): 3401-3404] |
ZHAO Y, WENG L P, ZHANG L. Preliminary study on the source of SEM in river shrimp. Hangzhou Agriculture and Science and Technology, 2019(4): 43-44 [赵芸, 翁丽萍, 张乐. 河虾中氨基脲的来源初探. 杭州农业与科技, 2019(4): 43-44] |
ZHOU P, HU F L, ZHANG Z T, et al. Determination of nitrofuran metabolites in drone pupae and reasons for their exceeding the standards. Apiculture of China, 2008, 59(7): 5-9 [周萍, 胡福良, 章征天, 等. 雄蜂蛹中硝基呋喃类代谢物含量的测定及超标原因分析. 中国蜂业, 2008, 59(7): 5-9] |
ZHU L M, YUAN P, ZHANG B B, et al. Antagonistic of procyanidins on semicarbazide-induced reproductive toxicity in male mice. Practical Preventive Medicine, 2012, 19(2): 165-168 [朱乐玫, 袁萍, 张贝贝, 等. 原花青素对氨基脲致雄性小鼠生殖毒性的拮抗作用. 实用预防医学, 2012, 19(2): 165-168] |



