2. 南方海洋科学与工程广东省实验室(广州) 广东 广州 511458
2. Guangdong Provincial Laboratory of Southern Marine Science and Engineering (Guangzhou), Guangzhou 511458, China
刺参(Apostichopus japonicus)具有较高的经济价值,是我国北方重要的海水养殖品种(Roggatz et al, 2018)。随着刺参养殖产业的不断发展,封闭或半封闭的池塘养殖已成为刺参增养殖的主要模式(Yuan et al, 2007)。
冻融期是我国北方地区因初春气温回升导致池塘表面冰层融化的一段特殊时期。冻融期冰层的融化使养殖池塘水体环境由封闭状态逐渐转变为对外交换状态,由此引起养殖池塘水体出现温跃层、盐跃层和溶解氧分层等现象(Giliehinsky et al, 2002)。调查显示,冰层融化导致的池塘跃层会引起池塘底部水质恶化及病原菌暴发,进而危害养殖生物的健康(黄华伟等, 2011)。
沉积物菌群在养殖池塘生态系统中占据着重要地位,在生态系统平衡、水质调控、养殖生物疾病防控等方面均发挥着重要作用(Deng et al, 2009; Michaud et al, 2009; Blancheton et al, 2013; 范立民, 2015; 姜燕等, 2022)。李步先(2015)研究表明,从虾蟹养殖池溏沉积物中分离得到的盐单胞菌属(Halomonas)能改善养殖环境、有效降解养殖环境中的氨态氮、防止因氮污染滋生病原菌引起养殖生物患病,且具有修复环境污染的功能。从刺参养殖池塘沉积物中分离得到的益生菌菌株YQ-2和DY-6能有效抑制刺参病原菌——灿烂弧菌(Vibrio splendidus)和假交替单胞菌(Psedoalteromonas nigrifaciens)的生长繁殖(杜佗等, 2017; 王金燕等, 2018)。然而,养殖池塘生态系统极易受到外部理化因子的影响,季节变动、营养物质输入等变化均可造成池塘养殖环境菌群种类和丰度的改变(Bentzon-Tilia et al, 2016; 谭八梅等, 2021)。例如,高温条件能提高微生物体内的酶活性、提高微生物代谢水平、加速有机物分解,进而促进养殖环境物质循环(李彬等, 2010)。水体透明度直接决定光照射入池塘水体的深度,控制了水中微生物的光合作用强度,进而改变养殖环境物质循环状态(龚骏等, 2013)。pH是生物地球转换的重要调控因子,能够通过调节菌群对水中离子和微量金属的利用率,从而改变代谢效率(Lindstöm et al, 2012)。外部理化环境因子变化剧烈的冻融期是刺参健康养殖的关键时期,但关于养殖池塘冻融期菌群结构方面的研究还鲜有报道。
因此,本研究以中国北方典型岸基半开放刺参养殖池塘为对象,利用高通量测序技术解析冻融期刺参养殖池塘沉积物菌群结构特征及其影响因素,旨在为刺参养殖池塘精细化管理提供理论依据。
1 材料与方法 1.1 采集样品本研究选取中国北方典型岸基半开放刺参养殖池塘(40°37′46″N;122°8′59″E)为对象,利用采泥器分别采集封冰期、融冰期和化冰期同一刺参养殖池塘表层3~5 cm沉积物样品,采样信息见表 1。样品装入无菌离心管中,–80 ℃冰箱冷冻保存,用于细菌总DNA的提取。所有样品采自同一池塘并设置3个平行,池塘面积约为0.07 km2,水深约为4 m,刺参密度约为5头/m2,全年未使用微生物制剂。
|
|
表 1 样品信息 Tab.1 Sampling information |
采用HACH (HQd)水质分析仪器(HACH公司, 美国),现场测定养殖池水的温度(T)、盐度(S)和酸碱度(pH),其他理化参数测定均依据中华人民共和国《海洋沉积物质量标准(GB18668-2002)》进行。沉积物总有机碳(TOC)的测定采用重铬酸钾氧化–还原容量法,总氮(TN)采用过硫酸钾氧化法,总磷(TP)采用钼锑抗分光光度法,氨氮(NH4+-N)采用次溴酸盐氧化法,硝酸盐(NO3–-N)采用镉柱还原法,亚硝酸盐(NO2–-N)采用萘乙二胺分光光度法,磷酸盐(PO3–-P)采用磷钼蓝分光光度法,硫化物采用亚甲基蓝分光光度法。
1.3 DNA提取、扩增和测序选用OMEGA Soil DNA试剂盒(D5625)提取沉积物总DNA。提取的DNA用NanoDrop2000分光光度计检测浓度和纯度,为确保DNA无降解无污染,DNA浓度要求≥50 ng/μL,纯度OD260 nm/OD280 nm=1.8~2.0。利用1%琼脂糖凝胶电泳检测DNA质量,DNA条带清晰且带型完整无拖尾现象,表明所提DNA无蛋白质污染、无降解发生。
以细菌16S rRNA基因V3~V4片段341F (5´-CCT ACGGGNGGCWGCAG-3´)和805R(5´-GACTACHVG GGTATCTAATCC-3´)作为扩增引物进行PCR扩增,使用带barcode的特异引物,TaKaRa公司的Tks Gflex DNA聚合酶进行PCR,确保扩增效率和准确性。
将上述PCR扩增后获得的细菌16S rRNA基因产物送生工生物工程(上海)股份有限公司,采用Illumina Miseq 2×300 bp测序平台进行高通量测序。
1.4 数据处理与分析用FASTP (版本0.18.0) (Guo , 2017)对Illumina平台的原始数据进行过滤,标准如下:去除含有未知核苷酸(N)≥10%的reads;去除phred质量评分≤20的碱基≥50%的reads;删除含接头的reads。对低质量tag过滤得到clean tag (Bokulich et al, 2013)。参照QIIME (Caporaso et al, 2010)的tags质量控制流程。使用UCHIME算法(Edgar et al, 2011)进行tag的嵌合体检查。过滤嵌合体后得到的effective tag进行OTU丰度统计和其他后续分析。选取丰度最高的tag序列作为每个操作分类单元(operational taxonomic units, OTUs)的代表序列。采用Usearch (8.1.1831)软件,按照序列相似性为97%的阈值进行OTUs聚类。采用Mothur (1.30.1)软件对样品序列进行α多样性分析。采用RDP Classifier (2.12)软件对样品合格序列进行物种分类操作,阈值设置为0.8,低于该阈值的分类结果被划归为unclassified一类。使用R语言(版本2.5.3)进行环境因子相关性分析,以明确环境因素对群落组成的影响,获得不同水平各rank的丰度值。环境因素与物种之间的Pearson相关系数使用R语言psych包(版本1.8.4)计算(Revelle et al, 2010)。使用SPSS19.0软件对样本进行单因素方差分析(one-way ANOVA)。
2 结果 2.1 环境因子监测本研究对中国北方典型岸基半开放刺参养殖池塘封冰期、融冰期和化冰期沉积物环境理化因子进行监测(表 2)。结果显示,融冰期温度和盐度呈现显著性升高(P < 0.05),TOC在融冰期第1天达到最高,TOC在第3天开始显著下降,至融冰期达到最低点(P < 0.05),随后保持稳定。TP、NO3-N和PO43-P含量在冻融期间呈逐渐下降的趋势。化冰后期环境因子波动较小,温度和盐度基本保持稳定。
|
|
表 2 冻融期刺参养殖池塘沉积物环境理化因子 Tab.2 Environment factors of the sediment of ponds for culturing sea cucumber during ice-melting period |
冻融期不同阶段共18个样品所得原始测序序列为45 106~79 040条,原始序列经质量控制、嵌合体和非靶区域序列去除后,所得有效序列为42 993~ 76 635条(表 3)。
|
|
表 3 刺参养殖池塘沉积物菌群高通量测序结果 Tab.3 High throughput sequencing results of bacteria in the sediment of ponds for culturing sea cucumber |
通过比较不同时期样本α多样性指数得出(图 1),刺参养殖池塘冻融期间沉积物菌群丰度和多样性呈总体下降的趋势,融冰前期则呈显著性波动(P < 0.05),融冰后期菌群呈回升趋势,在冰层完全消失的化冰期菌群丰度和多样性再次下调。
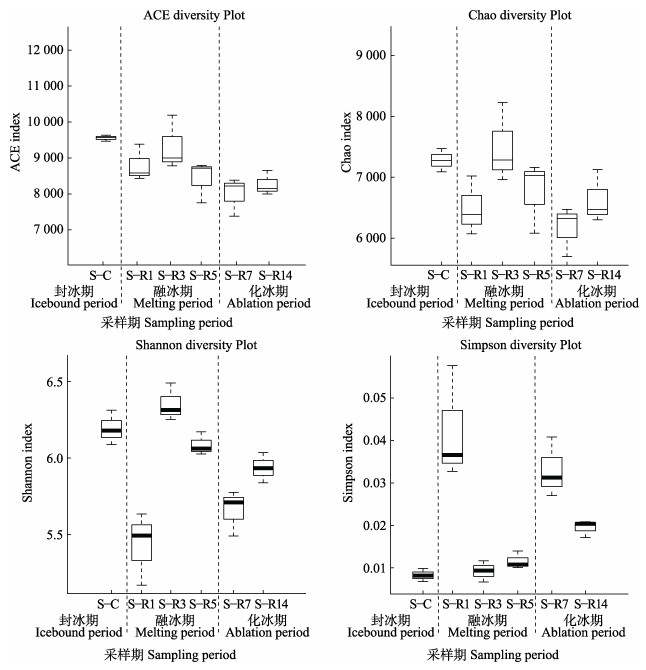
|
图 1 刺参养殖池塘沉积物菌群α多样性指数 Fig.1 α Diversity index in sea cucumber culture pond during ice-melting period S-R1、S-R3、S-R5分别为融冰期的第1、3、5天;S-R7、S-R14分别为化冰期的第7、14天。 S-R1, S-R3, and S-R5 indicate the first, third and fifth day of the melting period, respectively; S-R7 and S-R14 indicate the 7th and 14th day of the ablation period. |
在OTU水平上,根据加权Unifrac距离不同对封冰期、融冰期和化冰期样本进行主成分分析(图 2)。其中,PCA1 (principal component analysis)、PCA2和PCA3的贡献率分别为94%、2%和1%,总贡献率为97%。各组分样品聚集在PCA1,显示出较好的生物学重复性。不同时期样品分布分散,说明组间群落结构存在差异。

|
图 2 刺参养殖池塘沉积物菌群PCA分析图 Fig.2 PCA analysis diagram in sea cucumber culture pond during ice-melting period |
门水平上(图 3a),相对丰度排名前5的菌门分别为变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)、放线菌门(Actinobacteria)、浮霉菌门(Planctomycetes)、酸杆菌门(Acidobacteria)。冻融期刺参养殖池塘沉积物第一优势菌门均为变形菌门(相对丰度 > 49.04%)。次优势菌门存在显著性差异,其中,封冰期次优势菌门为拟杆菌门(相对丰度 > 6.80%),融冰期次优势菌门为绿弯菌门(Chloroflexi)和放线菌门;化冰期次优势菌门则演替为浮霉菌门。
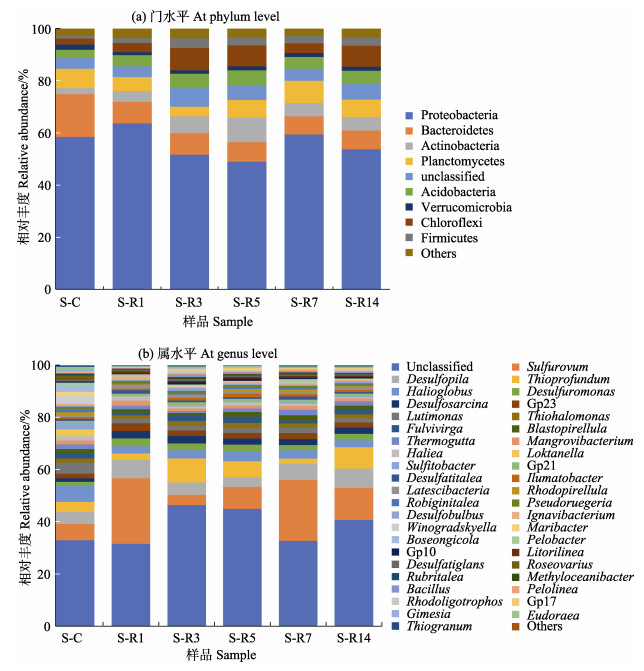
|
图 3 沉积物菌群结构相对丰度图 Fig.3 Relative abundance of sediment bacterial community |
属水平上(图 3b),封冰期优势菌属主要隶属于海泥大海球菌(Halioglobus)、硫卵菌属(Sulfurovum)、Desulfopila、Lutimonas、硫深海菌属(Thioprofundum);融冰期优势菌属主要隶属于Sulfurovum、Thioprofundum、Desulfopila;化冰期优势菌属主要隶属于Sulfurovum、Desulfopila、Thioprofundum。值得注意的是,在冻融期间检测出刺参条件致病菌假交替单胞杆菌(Pseudoalteromonas) (相对丰度为0.9%)。
线性判别法LEfSe分析显示(图 4),不同分类水平下共有244个细菌类群在冻融期存在差异。封冰期刺参养殖池塘沉积物差异菌门主要隶属于厚壁菌门,其代表菌属为盐厌氧杆菌(Halanaerobium)和嗜盐菌属(Halocella)。融冰期沉积物差异菌门隶属于酸杆菌门,其代表性差异菌属分别为GP7、GP17和GP26。化冰期沉积物差异菌门隶属于软壁菌门(Tenericutes),其代表性差异菌属为Haloplasma。
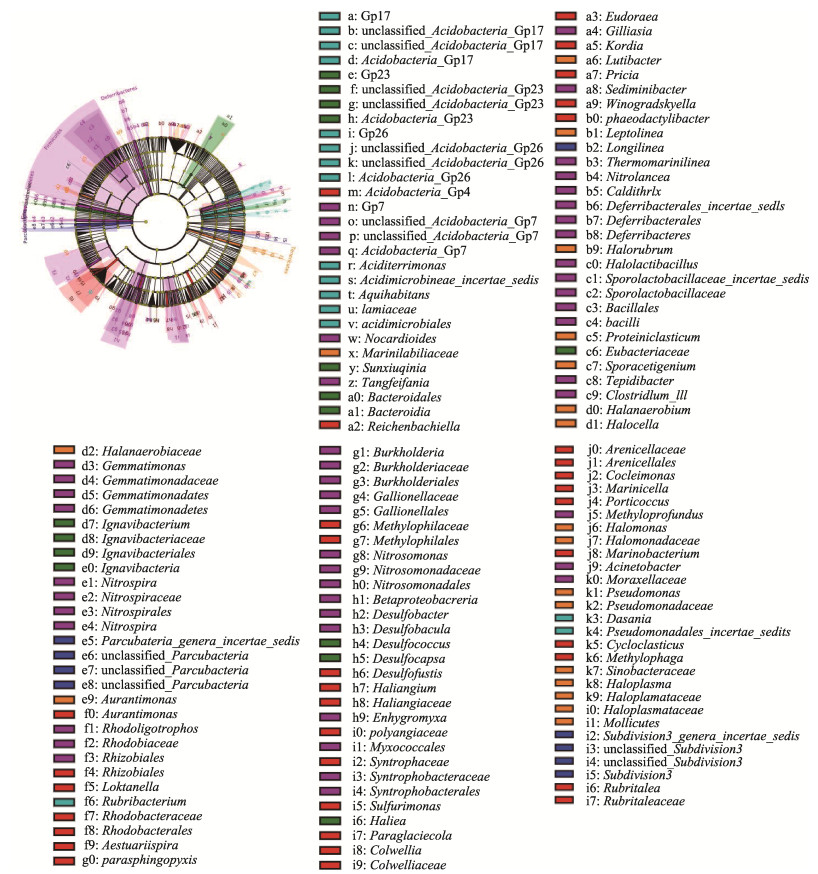
|
图 4 冻融期刺参养殖池塘沉积物菌群LEfSe分析 Fig.4 LEfSe analysis of the sediment bacterial community in sea cucumber culture pond during ice-melting period |
基于COG数据库(图 5),对比25组功能蛋白,刺参养殖池塘沉积物菌群在冻融期共有18组功能蛋白存在显著性差异(P < 0.05)。与封冰期相比,融冰期沉积物菌群在一般功能预测(general function prediction only) [R],次生代谢物生物合成、运输和分解代谢(secondary metabolites biosynthesis, transport, and catabolism) [Q],辅酶的运输和代谢(coenzyme transport and metabolism) [H],碳水化合物的运输和代谢(carbohydrate transport and metabolism) [G],核苷酸的运输和代谢(nucleotide transport and metabolism) [F],能量产生与转化(energy production and conversion) [C],复制、重组和修复(replication, recombination and repair) [L],转录(transcription) [K],染色质结构及作用方式(chromatin structure and dynamics) [B],RNA加工与修饰(RNA processing and modification) [A],细胞外结构功能(extracellular structures) [W],细胞骨架(cytoskeleton) [Z],防御机制(defense mechanisms) [V],信息转导机制(signal transduction mechanisms) [T],细胞膜、细胞壁、包膜生物发生(cell wall/membrane/ envelope biogenesis) [M],细胞周期控制、细胞分裂、染色体分区(cell cycle control, cell division, chromosome partitioning) [D] 16个方面呈现出显著性上调(P < 0.05)。化冰期,沉积物菌群在染色质结构及作用方式(chromatin structure and dynamics) [B],RNA加工与修饰(RNA processing and modification) [A],碳水化合物的运输和代谢(carbohydrate transport and metabolism) [G],细胞周期控制、细胞分裂、染色体分区(cell cycle control, cell division, chromosome partitioning) [D],细胞外结构功能(extracellular structures) [W],信息转导机制(signal transduction mechanisms) [T],一般功能预测(general function prediction only) [R],转录(transcription) [K],次生代谢物生物合成、运输和分解代谢(secondary metabolites biosynthesis, transport, and catabolism) [Q] 9组功能蛋白中表现为显著性下调(P < 0.05)。

|
图 5 基于COG数据库注释沉积物菌群功能 Fig.5 Function abundances of sediment bacterial based on COG database 同一组中上标不同字母表示平均值间差异显著(P < 0.05),各组上标相同字母表示组间差异不显著(P > 0.05)。 In the same group, there is a significant difference between the mean values indicated by different superscripts (P < 0.05), while there is no significant difference between groups indicated by the same superscripts (P > 0.05). |
冻融期刺参养殖池塘沉积物菌群与环境因子CCA分析结果见图 6。如图 6所示,封冰期刺参养殖池塘沉积物菌群单独聚为一类,融冰期和化冰期沉积物菌群则聚为另一类。根据环境因子箭头与原点连线的长度可知,刺参养殖池塘沉积物菌群排名前4的主导环境因子分别为温度、盐度、TN和TOC。其中,温度和盐度与封冰期沉积物菌群呈负相关,与融冰期沉积物菌群呈正相关。TOC和TN与封冰期沉积物菌群相对丰度呈正相关。
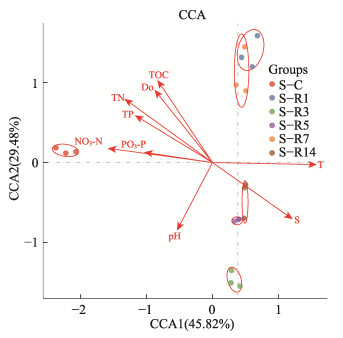
|
图 6 沉积物菌群与环境因子CCA分析图 Fig.6 CCA analysis of sediment bacterial community and environmental factors |
冻融期刺参养殖池塘沉积物优势菌门与主导环境因子间相关性分析结果见表 4。其中,拟杆菌门与盐度呈极显著负相关(P < 0.001),表明拟杆菌门受沉积物盐度影响较大。绿弯菌门与温度和盐度呈极显著正相关(P < 0.001),与TOC含量呈极显著负相关(P < 0.001)。浮霉菌门与温度和盐度等环境因子均未表现出显著相关性(P > 0.05)。放线菌门与盐度呈极显著正相关(P < 0.01),与TOC含量呈极显著负相关(P < 0.01)。
|
|
表 4 沉积物优势菌群与主导环境因子相关性分析 Tab.4 Correlation analysis between dominant bacterial community and environment factors in sediment |
微生物作为养殖池塘生态系统的生产者和分解者,在生态系统物质循环和能量流动过程中发挥着重要作用(Langenheder et al, 2006; Adams et al, 2010; 金笑等, 2017)。菌群生长繁殖所需的营养物质主要依靠环境中的无机盐和有机物,其群落组成对环境因子的变化有较强的敏感性(王轶南等, 2010; Bell et al, 2013; 李建光等, 2014; Yang et al, 2015; Amabebe et al, 2020)。本研究表明,冻融期刺参养殖池塘沉积物菌群丰度和多样性总体呈下调趋势,但融冰初期则出现显著波动。相较于封冰期,温跃层及盐跃层的出现引起底部水层环境因子发生剧烈变化(姜森颢等, 2015)。温度的升高为更多微生物提供了适宜的生境,能加速酶促反应,进而促进微生物代谢(Tang et al, 2014; Cardona et al, 2016),造成融冰初期菌群丰度及多样性上调。同时,盐度作为海水养殖中的重要环境因素,能干扰水体微生物新陈代谢,影响沉积物中微生物的多样性(Jackson et al, 2009)。倪蒙等(2019)对不同盐度罗氏沼虾(Macrobrachium rosenbergii)养殖池塘调查显示,高盐度组菌群丰度与多样性显著高于低盐度组。环境因子的大幅改变,破坏了封冰期沉积物原有的菌群结构,引起冻融期微生物丰度和多样性出现波动,随着气温回升,养殖环境温度和盐度变化趋势减小,养殖池塘环境因子和菌群结构也趋于稳定。
相比于融冰期,化冰期菌群丰度及多样性呈下调趋势。微生物能通过碳循环获取能量,而有机碳作为环境中碳源的指标之一,能在一定程度上反映环境微生物丰度。李谷等(2013)对养殖池塘群落的碳源代谢特性和功能多样性进行调查,发现养殖池塘水体微生物群落对不同类型的碳源有不同程度的利用。高浓度有机碳更适合微生物大量繁殖栖息(Ram et al, 1982)。本研究中,化冰期沉积物TOC含量显著下降(仅为0.014 mg/kg)。TOC含量过低抑制了微生物的生长繁殖,进而限制了微生物的丰度及其多样性,可能是引起化冰期微生物丰度及多样性出现下调的原因。
3.2 冻融期刺参养殖池塘沉积物菌群结构特征及其影响因素本研究中,冻融期刺参养殖池塘沉积物菌群结构呈现显著的演替特征,但变形菌门始终为冻融期沉积物第一优势菌门。变形菌门作为刺参养殖池塘丰度最高的菌群(丁斯予等, 2019; 任利华等, 2015; 窦妍等, 2016),主要是由其在原核生物分子生物学分类中的绝对优势所决定的(Gupta, 2000)。Auguet等(2009)研究表明,α-变形菌纲通过光合作用储存能量,用于生长代谢、CO2和N的固定。厌氧条件下,一些γ-变形菌可以和厌氧环境中的动物产生共生关系,对环境中的C、N和S循环起着重要作用(Olav et al, 1989; Bakunina et al, 2000; Manzoni et al, 2008)。
此外,不同时期菌群存在显著性差异,且各时期差异菌群与其生境环境因子之间显著相关。闫法军等(2014)研究表明,微生物作为生境的反映,与环境理化性质密切相关且相互限制影响。其中,封冰期刺参养殖池塘沉积物差异菌以厚壁菌门为代表。厚壁菌门可以形成抗逆性芽孢,具有极强的环境适应性,温度越低,厚壁菌门的相对丰度越大(宋兆齐等, 2015,与封冰期的低温条件相符。酸杆菌门作为融冰期特异性菌群的代表,与pH值密切相关。Jones等(2009)对北美和南美的共87个土壤样品大尺度分析发现,酸杆菌门中的GP7和GP17相对丰度与pH呈显著正相关关系。结合环境因子数据,与融冰期沉积物较高pH条件相符。化冰期刺参养殖池塘沉积物差异菌以软壁菌门为代表,而目前有关于软壁菌门的研究还鲜有报道。值得注意的是,冻融期间刺参养殖池塘沉积物中发现假交替单胞杆菌,相对丰度为0.9%,该菌是刺参腐皮综合征的主要致病菌(李强等, 2013),发病时的死亡率可达90%。因此,冻融期养殖环境管理十分重要,建议定期向水中投放水质改良剂,防止水质恶化。同时加入免疫增强剂促进诱发宿主防御反应,增强刺参抗病能力。
3.3 冻融期刺参养殖池塘沉积物菌群COG功能分析微生物参与多种物质循环,与次生代谢物生物合成、运输和分解代谢、碳水化合物的运输和代谢、能量产生与转化、转录、RNA加工与修饰等功能息息相关(Rungrassamee et al, 2016; Xiong et al, 2016)。放线菌门与绿弯菌门作为融冰期的两大优势菌门与该时期COG功能蛋白的上调具有一定相关性。放线菌门具有降解淀粉和蛋白质等大分子的功能,在氮磷循环、有机物降解及矿化过程中也具有重要作用,其发酵产物能抗菌抗病毒,能够抑制部分革兰氏阳性菌发挥功能(李文均等, 2002; 凌春耀等, 2020; Bull et al, 2005)。绿弯菌门营养方式和代谢途径十分丰富,是C、N、S等元素的生物地球化学循环的重要参与者(鲜文东等, 2020),如CO2固定、CO、CH4、NO2–氧化及纤维素等大分子的降解,这些功能与碳水化合物的运输和代谢、能量产生与转化等功能息息相关。其部分次级代谢产物具有高效的细胞毒性和抗菌作用,具有成为益生菌的潜力(Nett et al, 2006)。这些优势菌群功能与COG蛋白中次生代谢物合成、运输和分解代谢、碳水化合物的运输代谢、能量产生与转化等功能相吻合,说明冻融期独特的优势菌门可能是其功能蛋白显著上调的原因。开展冻融期沉积物菌群COG功能分析,能够反映出菌群整体代谢水平及其生理偏向。
4 结论本研究利用高通量测序技术首次探究冻融期刺参养殖池塘沉积物菌群结构和功能的演替特征,并查明影响沉积物菌群结构的主导环境因子。研究表明,环境因子改变不利于养殖池塘沉积物菌群结构的稳定,易导致潜在致病菌增殖,加大养殖生物患病风险。因此,在养殖过程中应注重融冰期养殖池塘环境的管理,避免水质恶化与病害暴发。
ADAMS H E, CRUMP B C, KLING G W. Temperature controls on aquatic bacterial production and community dynamics in arctic lakes and streams. Environmental Microbiology, 2010, 12(5): 1319-1333 DOI:10.1111/j.1462-2920.2010.02176.x |
AMABEBE E, ROBERT F O, AGBALALAH T. et al. Microbial dysbiosis-induced obesity role of gut microbiota in homeostasis of energy metabolism. British Journal of Nutrition, 2020, 123(10): 1127-1137 |
AUGUET J C, BARBERAN A, CASAMAYOR E O. Global ecological patterns in uncultured archaea. International Society for Microbial Ecology Journal, 2009, 4(2): 182-190 |
BAKUNINA I, SHEVCHENKO L S, NEDASHKOVSKAIA O I, et al. Screening of marine bacteria for fucoidan hydrolases. Mikrobiologiia, 2000, 69(3): 370-376 |
BELL T H, YERGEAU E, MAYNARD C, et al. Predictable bacterial composition and hydrocarbon degradation in Arctic soils following diesel and nutrient disturbance. International Society for Microbial Ecology Journal, 2013, 7(6): 1200-1210 |
BENTZON-TILIA M, SONNENSCHEIN E C, GRAM L. Monitoring and managing microbes in aquaculture-towards a sustainable industry. Microbial Biotechnology, 2016, 9(5): 576-584 DOI:10.1111/1751-7915.12392 |
BLANCHETON J P, ATTRAMADAL K J K, MICHAUD L, et al. Insight into bacterial population in aquaculture systems and its implication. Aquacultural Engineering, 2013, 53: 30-39 DOI:10.1016/j.aquaeng.2012.11.009 |
BOKULICH N A, SUBRAMANIAN S, FAITH J J, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nature Methods, 2013, 10(1): 57-59 DOI:10.1038/nmeth.2276 |
BULL A T, STACH J E M, WARD A C, et al. Marine actinobacteria: Perspectives challenges future directions. Antonie van Leeuwenhoek, 2005, 87(1): 65-79 DOI:10.1007/s10482-004-6562-8 |
CAPORASO J G, KUCZYNSKI J, STOMBAUGH J, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 2010, 7(5): 335-336 DOI:10.1038/nmeth.f.303 |
CARDONA E, GURGUEN Y, MAGRÉ K, et al. Bacterial community characterization of water and intestine of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbiology, 2016, 16: 157 DOI:10.1186/s12866-016-0770-z |
DENG H, HE C B, ZHOU Z C, et al. Isolation and pathogenicity of pathogens from skin ulceration disease and viscera ejection syndrome of the sea cucumber Apostichopus japonicus. Aquaculture, 2009, 287(1/2): 18-27 |
DING S Y, WANG L, XU H C, et al. Bacterial community structure and function in the intestinal tracts and culture environment of sea cucumber (Apostichopus japonicus). Chinese Journal of Ecology, 2019, 38(1): 210-220 [丁斯予, 王荦, 徐翰晨, 等. 刺参肠道及养殖环境菌群结构与功能. 生态学杂志, 2019, 38(1): 210-220] |
DOU Y, ZHAO X W, DING J, et al. Application of high-throughput sequencing for analyzing bacterial communities in earthen ponds of sea cucumber aquaculture in northern China. Oceanologia et Limnologia Sinica, 2016, 47(1): 122-129 [窦妍, 赵晓伟, 丁君, 等. 应用高通量测序技术分析北方刺参养殖池塘环境菌群结构. 海洋与湖沼, 2016, 47(1): 122-129] |
DU T, LI B, WANG Y G, et al. Screening and characteristic analysis of potential probiotics from large water ponds used for sea cucumber (Apostichopus japonicus) farming. Progress in Fishery Sciences, 2017, 38(3): 180-187 [杜佗, 李彬, 王印庚, 等. 刺参(Apostichopus japonicus)大水面养殖池塘环境中优势益生菌筛选及其特性分析. 渔业科学进展, 2017, 38(3): 180-187] |
EDGAR R C, HASS B J, CLEMENTE J C, et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 2011, 27(16): 2194-2200 DOI:10.1093/bioinformatics/btr381 |
FAN L M. Microbial community in tilapia (Oreochromis niloticus) cultural ponds. Doctoral Dissertation of Nanjing Agricultural University, 2015 [范立民. 吉富罗非鱼养殖池塘微生物群落研究. 南京农业大学博士研究生学位论文, 2015]
|
GILIEHINSKY D A. Permafrost model of extraterrestrial habitat. Astrobiology, 2002, 125-142 |
GONG J, ZHANG X L. Contribution and mechanism of microbe-driving nitrogen cycling processes in coastal ecosystems. Microbiology China, 2013, 40(1): 44-58 [龚骏, 张晓黎. 微生物在近海氮循环过程的贡献与驱动机制. 微生物学通报, 2013, 40(1): 44-58] |
GUPTA R S. The phylogeny of proteobacteria: Relationships to other eubacterial phyla and eukaryotes. FEMS Microbiology Reviews, 2000, 24(4): 367-402 DOI:10.1111/j.1574-6976.2000.tb00547.x |
HUANG H W, WANG Y G, CHEN X, et al. Relationship between the occurrence of rotten skin syndrome and environmental factors in cultured Apostichopus japonicus at low temperature. Fisheries Science and Technology Information, 2011, 38(6): 292-297 [黄华伟, 王印庚, 陈霞, 等. 低温期养殖刺参腐皮综合征的发生与环境因子间的关系. 水产科技情报, 2011, 38(6): 292-297] |
JACKSON C, VALLAIRE S. Effects of salinity and nutrients on microbial assemblages in Louisiana wetland sediments. Wetlands, 2009, 29(1): 277-287 DOI:10.1672/08-86.1 |
JIANG S H, LIU S F, CAI X, et al. Annual variation characteristics of main ecological factors in the culture ponds of Apostichopus japonicus Selenka. Fishery Modernization, 2015, 42(5): 1-7 [姜森颢, 刘双凤, 蔡勋. 刺参养殖池塘主要生态因子周年变化特征. 渔业现代化, 2015, 42(5): 1-7 DOI:10.3969/j.issn.1007-9580.2015.05.001] |
JIANG Y, LI C Y, XU Y J, et al. Responses of microbiota structure in the intestinal tract and pond culture environment of Japanese flounder (Paralichthys olivaceus) to probiotics. Progress in Fishery Sciences, 2022, 43(2): 137-146 [姜燕, 李存玉, 徐永江, 等. 池塘养殖牙鲆肠道和环境菌群结构对益生菌制剂的响应. 渔业科学进展, 2022, 43(2): 137-146] |
JIN X, KOU W B, YU H T, et al. Environmental factors influencing the spatial distribution of sediment bacterial community structure and function in Poyang Lake. Research of Environmental Sciences, 2017, 30(4): 529-536 [金笑, 寇文伯, 于昊天, 等. 鄱阳湖不同区域沉积物细菌群落结构、功能变化及其与环境因子的关系. 环境科学研究, 2017, 30(4): 529-536] |
JONES R T, ROBESON M S, LAUBER C L, et al. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME Journal, 2009, 3: 442-453 DOI:10.1038/ismej.2008.127 |
LANGENHEDER S, LINDSTROM E S, TRANVIK L J. Structure and function of bacterial communities emerging from different sources under identical conditions. Applied and Environmental Microbiology, 2006, 72(1): 212-220 DOI:10.1128/AEM.72.1.212-220.2006 |
LI B X. A primary study on isolation, screening and removal characteristics of probiotics in ponds of shrimp and crab. Master´s Thesis of Ocean University of China, 2015 [李步先. 虾蟹养殖池塘益生菌的分离筛选及降解特性初步分析研究. 中国海洋大学硕士研究生学位论文, 2015]
|
LI B, RONG X J, LIAO M J, et al. Bacteria community in the intestine and culture environment of Apostichopus japonicus in winter. Marine Sciences, 2010, 34(4): 64-69 [李彬, 荣小军, 廖梅杰, 等. 冬季刺参养殖环境与肠道内细菌菌群的研究. 海洋科学, 2010, 34(4): 64-69] |
LI G, SONG J H, LI X L, et al. Metabolic characteristics and functional diversity of carbon source in microflora of ponds with recirculating aquaculture system. Agricultural Science and Technology, 2014, 15(2): 278–282, 299 [李谷, 宋景华, 李晓莉, 等. 循环水养殖池塘微生物群落的碳源代谢特性和功能多样性研究. 农业科学与技术(英文版), 2014, 15(2): 278–282, 299] |
LI J G, XU Y P, LI X Y, et al. Characterization of bacterial communities associated with the intestinal tracts and culture environment of sea cucumber (Apostichopus japonicus) in different culture seasons. Fisheries Science, 2014, 33(9): 562-568 [李建光, 徐永平, 李晓宇, 等. 不同养殖季节仿刺参肠道与养殖环境中菌群结构的特点. 水产科学, 2014, 33(9): 562-568] |
LI Q, SUN K T, ZHANG X Y, et al. Research progress on "skin ulceration syndrome" of Aposichopus japonica. Journal of Agricultural Science and Technology, 2013, 15(6): 40-45 [李强, 孙康泰, 张显昱. 刺参"腐皮综合征"研究进展. 中国农业科技导报, 2013, 15(6): 40-45] |
LI W J, ZHANG Z Z, JIANG C L. The advance on taxonomic of genus Thermoactinomyces. Acta Microbiologica Sinica, 2002, 42(6): 759-763 [李文均, 张忠泽, 姜成林. 高温放线菌属分类研究进展. 微生物学报, 2002, 42(6): 759-763] |
LIN C Y, HUANG H B, GAO C H, et al. Antibacterial anthracimycins from Streptomyces pratensis SCSIO LCY05 isolated from Ascidian. Chinese Journal of Marine Drugs, 2020, 39(3): 7-14 [凌春耀, 黄洪波, 高程海, 等. 海鞘来源放线菌Streptomyces pratensis SCSIO LCY05中anthracimycin类化合物及其抗菌活性研究. 中国海洋药物, 2020, 39(3): 7-14] |
LINDSTÖM E S, LANGENHEDER S. Local and regional factors influencing bacterial community assembly. Environmental Microbiology Reports, 2012, 4(1): 1-9 |
MANZONI S, JACKSON R B, TROFYMOW J A, et al. The global stoichiometry of litter nitrogen mineralization. Science, 2008, 321(5889): 684-686 |
MICHAUD L, LO GIUDICE A, TROUSSELLIER M, et al. Phylogenetic characterization of the heterotrophic bacterial communities inhabiting a marine recirculating aquaculture system. Journal of Applied Microbiology, 2009, 107(6): 1935-1946 |
NETT M, EROL Ö, KEHRAUS S, et al. Siphonazole, an unusual metabolite from Herpetosiphon sp. Angewandte Chemie-International Edition, 2006, 45(23): 3863-3867 |
NI M, GAO Q, YUAN J L, et al. Effect of salintity on the water quality and mircrobial community structure of the water for young Macrobrachium rosenbergii rearing. Acta Agriculturae Universitatis Jiangxiensis, 2019, 41(5): 976-985 [倪蒙, 高强, 原居林, 等. 不同盐度罗氏沼虾育苗水体水质及微生物群落结构研究. 江西农业大学学报, 2019, 41(5): 976-985] |
OLAV V, OLSEN Y. Chemical composition and phosphate uptake kinetics of limnetic bacterial communities cultured in chemostats under phosphorus limitation. Limnology and Oceanography, 1989, 34(5): 939-946 |
RAM N M, ZUR O, AVNIMELECH Y. Microbial changes occurring at the sediment-water interface in an intensively stocked and fed fish pond. Aquaculture, 1982, 27(1): 63-72 |
REN L H, LI B, SUN G H, et al. Structure of bacterial community in biofloc from Apostichopus japonicus breeding ponds revealed by 16S rRNA clone library. Oceanologia et Limnologia Sinica, 2015, 46(1): 197-205 [任利华, 李斌, 孙国华, 等. 16S rDNA克隆文库解析仿刺参(Apostichopus japonicus)苗种培育池中生物絮团的细菌群落结构. 海洋与湖沼, 2015, 46(1): 197-205] |
REVELLE W, REYELLE M W. Package 'psych'. The Comprehensive R Archive Network, 2010
|
ROGGATZ C C, GONZÁLEZ-WANGÜEMERT M, PEREIRA H, et al. A first glance into the nutritional properties of the sea cucumber Parastichopus regalis from the Mediterranean Sea (SE Spain). Natural Product Research, 2018, 32(1): 116-120 |
RUNGRASSAMEE W, KLANCHUI A, MAIBUNKAEW S, et al. Bacterial dynamics in intestines of black tiger shrimp and the Pacific white shrimp during Vibrio harveyi exposure. Journal of Invertebrate Pathology, 2016, 133: 12-19 |
SONG Z Q, WANG L, LIU X H, et al. Diversities of Firmicutes in four hot springs in Yunnan and Tibet. Biotechnology, 2015, 25(5): 481–486, 436 [宋兆齐, 王莉, 刘秀花, 等. 云南和西藏四处热泉中的厚壁菌门多样性. 生物技术, 2015, 25(5): 481–487, 436] |
TAN B M, WANG L, PEI H L, et al. The seasonal structural and functional characteristics of bacterial community in Apostichopus japonicus culture pond water. Progress in Fishery Sciences, 2021, 42(3): 77-88 [谭八梅, 王荦, 裴泓霖, 等. 不同季节刺参养殖池塘水体菌群结构与功能特征研究. 渔业科学进展, 2021, 42(3): 77-88] |
TANG Y Y, TAO P Y, TIAN J G, et al. Identification of bacterial community composition in freshwater aquaculture system farming of Litopenaeus vannamei reveals distinct temperature-driven patterns. International Journal of Molecular Sciences, 2014, 15(8): 13663-13680 |
WANG J Y, LI B, WANG Y G, et al. Screening and characteristic analysis of Bacillus velezensis from sea cucumber (Apostichopus japonicus) ponds. Journal of Fishery Sciences of China, 2018, 25(3): 567-575 [王金燕, 李彬, 王印庚, 等. 刺参养殖池塘一株贝莱斯芽孢杆菌的分离及其生理特性. 中国水产科学, 2018, 25(3): 567-575] |
WANG Y N, ZHU S W, CHANG Y Q, et al. PCR-DGGE analysis of bacterial community composition in the intestine and aquaculture pond of Apostichopus japonicus. Progress in Fishery Sciences, 2010, 31(3): 119-122 [王轶南, 朱世伟, 常亚青, 等. 刺参肠道及养殖池塘菌群组成的PCR- DGGE指纹图谱分析. 渔业科学进展, 2010, 31(3): 119-122] |
XIAN W D, ZHANG X T, LI W J, et al. Research status and prospect on bacterial phylum Chloroflexi. Acta Microbiologica Sinica, 2020, 60(9): 1801-1820 [鲜文东, 张潇橦, 李文均. 绿弯菌的研究现状及展望. 微生物技术与生态理论, 2020, 60(9): 1801-1820] |
XIONG J B, DAI W F, LI C H. Advances, challenges, and directions in shrimp disease control: The guidelines from an ecological perspective. Applied Microbiology and Biotechnology, 2016, 100(16): 6947-6954 |
YAN F J, TIAN X L, DONG S L, et al. Seasonal variation of functional diversity of aquatic microbial community in Apostichopus japonicus cultural pond. Chinese Journal of Applied Ecology, 2014, 25(5): 1499-1505 [闫法军, 田相利, 董双林, 等. 刺参养殖池塘水体微生物群落功能多样性的季节变化. 应用生态学报, 2014, 25(5): 1499-1505] |
YANG Y Y, WANG Z, HE T, et al. Sediment bacterial communities associated with anaerobic biodegradation of bisphenol A. Microbial Ecology, 2015, 70(1): 97-104 |
YUAN X T, YANG H S, WANG L L, et al. Effects of aestivation on the energy budget of sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Acta Ecologica Sinica, 2007, 27(8): 3155-3161 |



