2. 中国海洋大学海洋生命学院 海洋生物遗传学与育种教育部重点实验室 山东 青岛 266003;
3. 烟台丰泓海洋苗业有限公司 山东 烟台 265617
2. Key Laboratory of Marine Genetics and Breeding, Ministry of Education, College of Marine Life Science, Ocean University of China, Qingdao 266003, China;
3. Yantai Fenghong Marine Seeds Co., LTD, Yantai 265617, China
海带(Saccharina japonica)的生活史属于异型世代交替类型,包含大型的孢子体世代和微观的配子体世代。成熟期的海带孢子体在表面形成具有生殖能力的孢子囊结构,孢子囊作为海带孢子体重要的生殖器官,对于完成海带生活史、保证群体延续等具有重要作用。海带杂交新品种具有优良的经济性状,在养殖生产中的应用潜力巨大,但一些杂交新品种尤其是远缘杂交品种形成孢子囊的特点十分接近其野生亲本,而不同于常规养殖品种的亲本,即相较于常规的海带品种晚熟,其形成孢子囊的时间大大延迟,错过了海带育苗生产中的种海带采孢子时间(苏丽等, 2017),限制了具有优良生产性状的海带杂交品种的应用与推广。因此,了解海带孢子囊发育过程中的外观形态、组织结构和生理生化变化过程,对研究海带杂交品种晚熟的调控机制具有重要指导意义。
苏丽等(2018)研究表明,提前采苗时间不能促进海带杂交品种提前产生孢子,但晚熟海带的孢子囊可通过改变孢子体的养殖条件获得。关于环境影响海带孢子囊发育的研究有较多报道,其中,温度(Su et al, 2020)、营养盐浓度(Kumura et al, 2010; Mizuta et al, 1998)等多种环境因素均影响海带孢子囊的形成。除培养条件的影响因素外,海带孢子体组织部位(Lüning et al, 1988; Shao et al, 2004)、脱落酸含量(Nimura et al, 2002; Schaffelke et al, 1995)和孢子体的碳氮比值(C: N)(Harries et al, 1932)也是孢子囊发育的内在影响因素。另外,形成孢子囊的孢子体长时间在培养温度为23 ℃时受到胁迫,影响游孢子的释放和发育(李霞等, 2016)。在大型褐藻孢子囊发育过程中,伴随着光合作用减弱、呼吸作用增强(Aruga et al, 1990; Matsuyama et al, 1983; Nimura et al, 2001; Sakanishi et al, 1991)、褐藻多酚积累(Alstyne et al, 1999)以及RNA/DNA比率增加(Nimura et al, 2001)等生物学过程。Mizuta等(2010)研究表明,活性氧(ROS)清除机制和碘外排的抗氧化机制在海带孢子囊发育过程中也发挥着重要作用。在以往对海带孢子囊的研究中,多以野生海带为研究对象,对人工培育的海带杂交品种孢子囊形成的研究较少。Su等(2020)研究了光周期和温度等对海带杂交品种孢子囊的诱导作用,尚未有关于人工培育的海带杂交品种孢子囊形成过程与生理生化特征的研究。
本研究通过室内培养海带孢子体的组织块样品,系统观察海带孢子囊形成与发育过程中的外观形态和组织结构变化,探明海带孢子囊发育过程中生化组分的变化过程与特征,相关结果有利于加深对海带杂交品种孢子囊形成的过程及生理生化特征的理解,可为进一步解决海带杂交品种孢子囊晚熟问题、探索孢子囊形成诱导新方法提供理论依据和数据支持。
1 材料与方法 1.1 实验材料本研究的海带是以中国养殖海带品种海带“黄官1号”和日本野生海带为父母本的杂交后代,具有孢子囊晚熟的典型特征。2021年6月在海带养殖筏架上选取3株长势相近、长度为3.0~3.5 m的厚成期海带孢子体,于保温箱中12 h内带回实验室,使用灭菌海水清洗干净后,在孢子体叶片距离基部40~60 cm处的中带部靠近边缘部分,使用打孔器获得直径为2 cm的海带孢子体圆片状的组织块。圆片状的海带组织块于冷光源低温光照培养箱(DGXM-508B)中使用2 L充气烧瓶(自制)作为培养容器进行培养。充气装置为V-30型超静强力气泵。
1.2 实验设计 1.2.1 培养条件设置海带孢子体形成孢子囊的环境条件:白光,光周期8 (L)∶16 (D),光照强度为0~100 μmol photons/(m2·s),温度为17 ℃,煮沸冷却的自然海水中添加外源N浓度为5.0 mg/L,外源P浓度0.5 mg/L作为培养液。每天观察海带孢子体变化。
1.2.2 生化指标测定样品的取样基于前期研究对孢子囊发育过程的观察,在1.2.1条件下分别选取营养生长期(SA)样品:培养2 d,孢子体未形成孢子囊;孢子囊发育初期(SB)样品:培养约10 d,显微镜下观察发现,海带表面形成轻度磨砂状突起(肉眼观察情况下不明显);孢子囊发育中期(SC)样品: 培养约19 d,海带表面形成明显突起,且在水中观察到由褐色向黄色转变;孢子囊成熟期(SD)样品:培养约24 d,海带孢子囊表皮形成泡状胶质突起并逐渐脱落;游动孢子释放期(SE):培养约30 d,阴干后孢子囊释放游动孢子。每个阶段的样品均设置3个生物学重复,取样的时间为涵盖海带孢子囊发育前、中、后的整个过程。
1.3 组织结构观测与生化指标测定对SA、SB、SC、SD和SE样品制作组织切片,使用苏木精-伊红(HE染色)对组织染色,使用OLYMPUS SZ61解剖镜(日本)、Nikon ECLIPSE E800显微镜(日本)对染色组织切片观察,并通过NIS- EIements D 3.1软件拍照记录,使用Image-Pro Plus 5.1软件对孢子体最外层细胞(后期发育为隔丝细胞)和孢子母细胞(发育形成孢子囊)长度进行测量。
将无孢子囊结构的孢子体(SA)和表面带有孢子囊的孢子体样品(SB~SE)使用液氮冷冻15~20 min,随后–80 ℃保存。总氮(TN)使用凯氏定氮法测定,可溶性蛋白(BCA法)、叶绿素、1, 5-二磷酸核酮糖(RUBP)羧化酶(RUBP carboxylase, RubisCO)、超氧阴离子(superoxide anion)使用比色法测定,相关试剂盒均购于苏州科铭生物技术有限公司。超氧化物歧化酶(SOD) (WST-1法)、过氧化氢酶(CAT)(钼酸铵法)、丙二醛(MDA)(硫代巴比妥酸法,TBA法),抗坏血酸过氧化物酶(APX)、过氧化物酶(POD)、过氧化氢(H2O2)、植物苹果酸脱氢酶(MDH)等使用比色法,各指标测定使用的试剂盒购于南京建成生物公司。每个测量实验均设置3个平行,测定TN含量时,每个平行称取0.3 g样品,其余指标称取0.1 g样品,并按照组织质量(g)∶提取液体积(mL)为1∶10的比例获取提取液,测定叶绿素时,组织质量(g)∶提取液体积(mL)为1∶100,严格按照对应试剂盒操作步骤进行各个指标的测定。
1.4 数据处理使用IBM SPSS Statistics 19.0软件对孢子囊发育过程中细胞长度变化和各项生理生化指标进行单因素方差分析(onw-way ANOVA)和Duncan多重比较,P < 0.05表示差异显著,并使用GraphPad Prism 8.3.0软件作图。
2 结果 2.1 孢子囊不同发育阶段的外观形态和组织结构变化在孢子囊发育过程中,不同阶段的表观形态发生了明显变化(图 1)。海带孢子体在形成孢子囊前表面光滑(SA);随着孢子囊逐渐形成,表皮细胞开始向外突出,海带表面开始形成磨砂状凸起(SB),随后表皮细胞继续延伸,在表面形成增厚突起,海带表面在水体中观察由褐色变黄色(SC);随着培养时间的延长,胶质组成的保护膜形成凸起并开始脱落,此时,阴干后不释放游动孢子(SD);随着保护层的脱落,海带孢子囊表面变得光滑,阴干处理后大量释放游动孢子(SE)。

|
图 1 海带孢子囊发育过程中的外观形态特征变化 Fig.1 Changes in morphological characteristics during the sorus formation process of S. japonica SA:营养生长期;SB:孢子囊发育初期;SC: 孢子囊发育中期;SD:孢子囊成熟期;SE:游动孢子释放期;下同。 SA: Vegetative stage; SB: Early development stage of sorus; SC: Middle development of sorus; SD: Sorus maturity stage; SE: Release stage of zoospores. The same as below. |
海带孢子囊的组织结构变化见图 2。从图 2可以看出,由表皮细胞分化形成的上层细胞延伸形成隔丝细胞(p),组织结构切片显示,隔丝细胞在孢子囊发育整个时期不断伸长,其顶端形成透明胶质薄膜(d);隔丝之间形成隔丝腔(pc),在隔丝腔中,基部细部分化形成孢子母细胞,随后孢子母细胞经历减数和有丝分裂,孢子母细胞不断伸长;在孢子母细胞发育过程中发现,SD阶段形成孢子囊帽状结构(cl),此时,未形成游动孢子;当在单个孢子母细胞形成32个细胞核时,每个细胞核与一个色素体及其周边的原生质结合形成一个游动孢子,孢子囊帽状结构(cl)结构不显著,隔丝腔空间被孢子母细胞填充而减少。此时,能释放游动孢子,但基细胞仍在分化形成孢子母细胞,说明孢子母细胞发育的不同步,这可能是孢子囊能连续和多次释放游动孢子的原因。
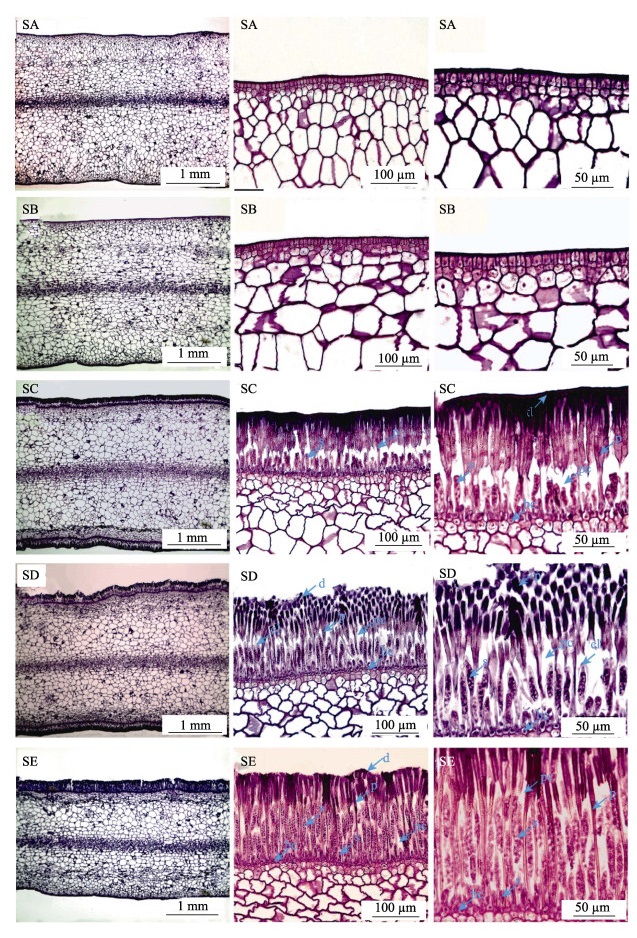
|
图 2 海带孢子囊发育过程中的组织结构变化 Fig.2 Changes of tissue structure during the sorus formation of S. japonica bc:基细胞;cl:孢子囊帽状结构;d:隔丝细胞顶端的胶质层;p:隔丝;s:孢子母细胞或孢子囊。 bc: Basal cell; cl: Cap-like structures of sours; d: Cuticle at the apex of the paraphysis cells; p: Paraphyses; s: Sporoblast or sporangium. |
对孢子囊发育不同阶段的最外层细胞(后期分化为隔丝细胞)和孢子母细胞(后期发育成孢子囊细胞)长度变化统计结果见图 3。从图 3可以看出,在孢子囊发育整个时期,分化成隔丝的细胞长度在各阶段均显著增加(P < 0.05),在SC阶段分化形成的孢子母细胞不断延伸,在各阶段显著增加(P < 0.05),尤其在游动孢子形成阶段,孢子囊细胞长度约为SD阶段的2倍。

|
图 3 海带孢子囊发育阶段细胞长度的变化 Fig.3 Changes of cell length during sorus development of S. japonica 柱状图上的字母下标为相同数字的作为同一组进行多重比较,同一组的不同字母表示在P < 0.05水平具有显著性差异。 The same subscript number at the letters represent the same group. The measured value in the same group are compared. The different letters in the same group represent significant differences (P < 0.05). |
从图 4a可以看出,在孢子囊发育过程中,可溶性蛋白含量呈先升高后降低的变化趋势。其中,从SA~SC阶段蛋白含量逐渐增加,SB>SA,但相比差异不显著(P < 0.05),SC阶段蛋白含量显著高于其他阶段(P < 0.05)。SC~SE阶段蛋白含量逐渐降低,且SE阶段蛋白含量最低,与SC阶段相比差异显著(P < 0.05)。从图 4b可以看出,在孢子囊发育过程中,氮含量总体呈上升趋势,SA与SB阶段、SB与SC阶段海带组织氮含量相比无显著差异(P > 0.05),SC阶段相对于SA阶段氮含量显著升高(P < 0.05),随着孢子囊的持续形成,SD和SE阶段氮含量显著高于SC阶段(P < 0.05),SD发育至SE阶段过程中的氮含量相比无显著变化(P > 0.05)。

|
图 4 海带孢子囊发育不同阶段蛋白和氮含量变化 Fig.4 Changes of souluble protein and nitrogen content during different development stages of S. japonica sorus 不同字母表示差异显著(P < 0.05),下同。 Different letters indicate significant difference (P < 0.05), the same as below. |
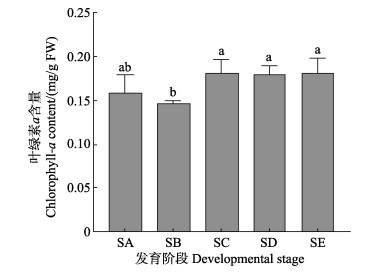
从图 5可以看出,在孢子囊发育过程中,SA与SB阶段的叶绿素a含量无显著变化(P > 0.05),发育到SC阶段时,叶绿素a含量相比于SB阶段显著增加(P < 0.05)。同时,叶绿素a含量在孢子囊发育的SC~SE阶段相比无显著变化(P > 0.05)。
|
图 5 海带孢子囊发育阶段叶绿素a含量变化 Fig.5 Changes of Chlorophyll-a content during different development stages of S. japonica sorus |
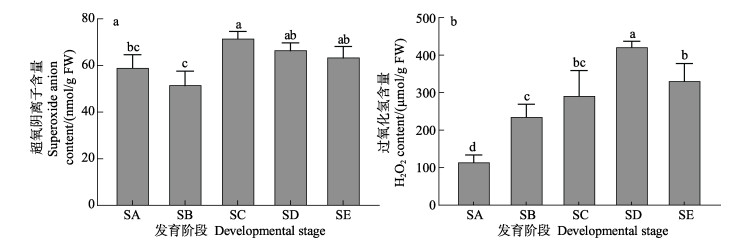
从图 6a可以看出,海带孢子囊发育前期的SA阶段到形成初期的SB阶段,超氧阴离子含量有所下降(P > 0.05),SB阶段发育至SC阶段超氧阴离子含量显著升高(P < 0.05),并在SD和SE阶段保持较高水平,且显著高于SB阶段(P < 0.05)。从图 6b可以看出,H2O2含量变化呈明显的先升高后下降趋势。孢子囊未形成前的SA阶段H2O2含量最低,随着孢子囊的发育,SB阶段H2O2含量显著升高(P < 0.05),在发育到SD阶段时达到峰值,随后在SE阶段显著降低(P < 0.05),但仍显著高于SA阶段(P < 0.05)。
|
图 6 海带孢子囊发育阶段超氧阴离子和H2O2含量变化 Fig.6 Changes of superoxide anion and H2O2 content during different development stages of S. japonica sorus |
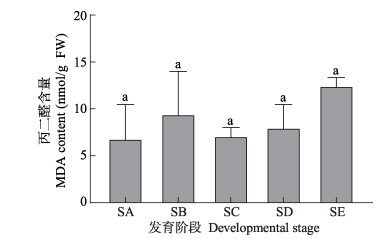
从图 7可以看出,MDA含量在海带表皮形成磨砂状突起阶段(SB)相对于SA阶段有所增加,在SB到SC阶段MDA含量下降,从SC~SE阶段MDA含量持续增加,但整个过程相比无显著变化(P > 0.05)。鉴于海带孢子囊发育过程中活性氧水平升高,但未出现膜脂氧化而导致MDA含量升高,这说明活性氧受到抗氧化系统的合理调控。
|
图 7 海带孢子囊发育阶段MDA含量变化 Fig.7 Changes of malondialdehyde (MDA) content during different development stages of S. japonica sorus |
孢子囊发育不同阶段抗氧化酶类变化见图 8。从图 8a可以看出,SOD活力随着孢子囊的发育而降低,在SA和SB阶段SOD活力下降,但相比无显著差异(P > 0.05),当发育到SC阶段时,SOD活力与SA阶段相比显著降低(P < 0.05),并始终低于未形成孢子囊时的酶活力。CAT与POD活力在孢子囊发育前期有所增加,在形成隔丝细胞与孢子母细胞阶段(SC)明显降低,透明胶状保护膜脱落和孢子母细胞发育阶段(SD)升高,在释放游动孢子阶段(SE)时降低(图 8b和图 8c)。APX活力与POD活力变化趋势相似(图 8d),但在孢子囊发育的SD和SE阶段的酶活性显著下降(P < 0.05)。
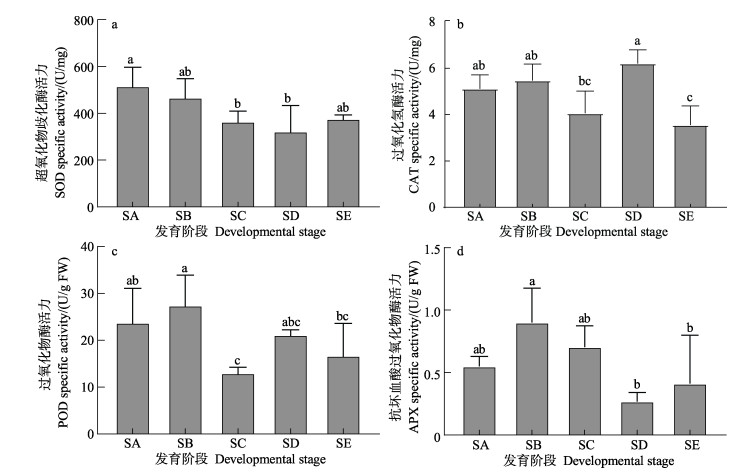
|
图 8 海带孢子囊发育阶段抗氧化酶类的酶活力 Fig.8 Enzyme activity of antioxidant enzymes during different stages of S. japonica sorus development |
从图 9可以看出,RubisCO活力在孢子囊发育过程中表现为先降低后升高的趋势。海带表面由光滑(SA)到表面形成磨砂状突起(SB)阶段显著降低(P < 0.05),SC阶段相比于SB阶段有所降低,但相比无显著差异(P > 0.05)。随着孢子囊发育到SD阶段,RubisCO酶活力显著增加(P < 0.05)且达到整个发育过程的最大值。MDH活力在孢子囊发育整个过程中变化不显著(P > 0.05)。
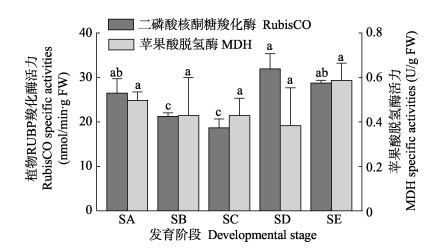
|
图 9 海带孢子囊发育阶段RubisCO和MDH活性 Fig.9 The activities variation of RubisCO and MDH during different stages of S.a japonica sorus development |
海带孢子囊的发育伴随着隔丝细胞与孢子母细胞的形成(Abe et al, 1939)。本研究发现,孢子囊开始发育时,隔丝细胞开始伸长,此时,表观结构发生变化,表现为孢子体表面变成磨砂状表面,直到能够释放游动孢子,隔丝细胞长度持续增加。当基细胞形成孢子母细胞时,孢子母细胞在隔丝腔中不断延伸,且在不同发育阶段表现出显著变化,尤其在游动孢子形成及释放阶段,孢子母细胞长度比未释放游动孢子(表层保护膜凸起并脱落阶段)时延长近1倍。
可溶性蛋白主要是植物体中生理代谢相关酶类等物质,可溶性蛋白含量的多少可以反映植物体内代谢水平的高低。蛋白含量在孢子囊发育过程中,SA~SC阶段逐渐升高,SC~SE阶段逐步下降。孢子囊发育过程中的表皮形成突起、细胞伸长形成单细胞隔丝及孢子母细胞形成等生命活动都需要较高的代谢水平来支撑,而在孢子囊发育过程后期,主要包括游孢子的形成和释放等生命过程,可溶性蛋白含量降低。在高等植物中,Kong等(2016)研究指出,营养期积累的氮会在繁殖和衰老期通过高分子物质降解,如蛋白含量降低,从而实现物质转移。本研究发现,在释放游孢子阶段,蛋白含量降低到未形成孢子囊(SA)阶段时的含量以下,这可能与孢子囊发育过程中对氮的需求较大,从而出现对氮的再分配,使蛋白含量有所降低有关。
海带中叶绿素含量的高低反映出光合作用的强弱,并影响植物体内能量和物质的转化(黄健等, 2002; 文滨滨等, 2019),同时,影响着光合作用的捕获、传递和转化(胡单等, 2011; 杨昕宇等, 2019; 周竹青等, 2001)。Aruga等(1990)研究表明,孢子囊部分和非孢子囊部分叶绿素a含量相比无明显差异。本研究发现,叶绿素a含量在孢子囊发育前期有所降低,但在发育后期显著升高。叶绿素a在隔丝细胞形成后均显著高于未形成孢子囊时的初始值(P < 0.05),说明孢子囊在发育过程中,尤其在隔丝细胞、孢子母细胞、游孢子的形成和释放阶段伴随着叶绿素的大量积累。但也有研究认为,在孢子囊发育过程中,光合作用降低,呼吸作用增强(Aruga et al, 1990; Matsuyama et al, 1983; Nimura et al, 2001; Sakanishi et al, 1991),孢子囊部分的光补偿点升高。活化的RubisCO在一定程度上能表征光合作用的强弱。本研究发现,RubisCO活性在孢子囊发育前期降低,但发育到SD和SE阶段时,酶活性升高。由于净光合速率的强弱不仅与叶绿素含量有关,对于光需求量较少的阴生植物来说,光照强度较高时,光合作用的强弱主要受到光合电子传递关键组分和碳同化关键酶等限制因素的影响(许大全等, 2002)。因此,在孢子囊发育过程中,叶绿素含量虽然显著升高,但可能由其他限速条件引起光合作用在孢子囊发育前期(SB和SC阶段)下降。MDH参与线粒体能量代谢(汪新颖等, 2009),本研究发现,MDH活力在孢子囊发育过程中变化不大,仅在SE阶段活性有所升高,可能与释放游孢子等生命活动需要能量支撑有关。
孢子囊发育受到氮和磷含量的影响,较低的氮含量能延迟孢子囊的形成并减少孢子囊的面积,孢子囊发育时间、位置与孢子体的生长程度和氮含量有关(Mizuta et al, 1998),孢子体中足够的营养物质积累能促进孢子囊发育(Kumura et al, 2010)。本研究发现,在孢子囊发育过程中,氮积累持续增加,尤其在SC与SA阶段氮含量显著增多,SD和SE阶段持续保持较高水平。研究显示,长海带(L. longissimi)孢子囊中的蛋白质、脂质和糖类含量高于其他部分,当叶片组织中的C/N值较低时,会形成孢子囊(Harries et al, 1932; Hosoda et al, 1970)。另外,Sasaki等(1969)研究发现,当单位面积鲜重比超过58 mg/cm2时,L. angustata var. longissima开始形成孢子囊,说明孢子囊的形成需要营养物质积累为前提。
ROS包括单线态氧(1O2)、超氧阴离子(O2–)、H2O2和羟基自由基(HO•),对植物细胞既有毒害作用,也在植物生长、防御、激素信号传导方面发挥着重要作用(Mittler et al, 2004)。Mizuta等(2010)研究发现,相比于未形成孢子囊的部分,孢子囊部分细胞内ROS产量升高并伴随着ROS的释放,抗氧化物和ROS清除酶活性显著升高。另外,ROS能促进细胞壁松动诱导细胞生长(Fry et al, 1998; Liszkay et al, 2004; Potikha et al, 1999),并在孢子囊发育时促进细胞的伸长。本研究发现,在整个发育过程中,SOD活性逐渐降低,而H2O2含量持续增加,这表明海带孢子囊发育可能需要H2O2的参与。在高温胁迫下,海带孢子体中MDA含量显著增加(凌晶宇等, 2016),但在温度为17 ℃培养孢子囊的过程中,MDA含量在孢子囊发育过程虽然呈动态变化,与未形成孢子囊时期相比无显著差异,说明在此过程中H2O2的变化并未对海带孢子体生长发育形成胁迫。另外,ROS不仅与胁迫耐受性有关(Choo et al, 2004; Collén et al, 2010a、b、c),ROS作为生物和非生物胁迫的信号物质发挥作用(Breusegem et al, 2006)。因此,在孢子囊发育过程中,各种抗氧化酶类的活性在孢子囊发育不同阶段的动态变化,精准调控ROS保持在合理的水平,既不会对细胞造成伤害,又能保障ROS作为信号分子参与孢子囊发育的分子调控过程。
4 结论海带孢子囊形成时伴随着孢子体表皮细胞的分化,隔丝细胞和孢子母细胞的变化引起海带孢子体表面特征的变化。在孢子囊发育过程中,伴随着N、蛋白含量、叶绿素含量的增加,在抗氧化酶类的动态调节下H2O2含量持续增加,说明在孢子囊发育过程中,较高含量的ROS可能在孢子囊发育过程中发挥重要作用。
ABE K. Mitosen im sporangium von Laminaria japonica Aresch. Science Reports of the Tohoku Imperial University (Biology), 1939, 8(14): 327-329 |
ALSTYNE K L V, MCCARTHY J J, HUSTEAD C L, et al. Phlorotannin allocation among tissues of northeastern Pacific kelps and rockweeds. Journal of Phycology, 1999, 35(3): 483-492 DOI:10.1046/j.1529-8817.1999.3530483.x |
ARUGA Y, TOYOSHIMA M, YOKOHAMA Y. Comparative photosynthetic studies of Ecklonia cava (Laminariales, Phaeophyta) bladelets with and without zoosporangial sori. Hydrobiologia, 1990, 204/205(1): 473-477 DOI:10.1007/BF00040273 |
BREUSEGEM F V. Reactive oxygen species in plant cell death. Plant Physiology, 2006, 141(2): 384-390 DOI:10.1104/pp.106.078295 |
CHOO K S, SNOEIJS P, PEDERSEN M. Oxidative stress tolerance in the filamentous green algae Cladophora glomerata and Enteromorpha ahlneriana. Journal of Experimental Marine Biology and Ecology, 2004, 298(1): 111-123 DOI:10.1016/j.jembe.2003.08.007 |
COLLÉN J, DAVISON I R. Reactive oxygen metabolism in intertidal Fucus spp. (Phaeophyceae). Journal of Phycology, 2010b, 35(1): 62-69 |
COLLÉN J, DAVISON I R. Reactive oxygen production and damage in intertidal Fucus spp. (Phaeophyceae). Journal of Phycology, 2010a, 35(1): 54-61 |
COLLÉN J, DAVISON I R. Stress tolerance and reactive oxygen metabolism in the intertidal red seaweeds Mastocarpus stellatus and Chondrus crispus. Plant, Cell and Environment, 2010c, 22(9): 1143-1151 |
FRY S C. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochemical Journal, 1998, 332: 507-515 DOI:10.1042/bj3320507 |
HARRIES, R. An investigation bu cultural methods of some of the factors influencing the development of the gametophytes and the early stages of the sporophytes of Laminaria digitata, L. saccharina, and L. clonstoni. Annals of Botany, 1932, 46(4): 893-928 |
HOSODA K. Studies on the components of Naga-kombu, Laminaria longissima-I general components. Nippon Suisan Gakkaishi, 1970, 36(7): 698-701 DOI:10.2331/suisan.36.698 |
HU D, YANG Y H. Effects of different application rates of nitrogen on photosynthetic pigment, biomass and yield of winter highland barley seedlings. Journal of Anhui Agricultural Sciences, 2011, 39(24): 14561–14563, 14585 [胡单, 杨永红. 不同施氮对冬青稞幼苗光合色素·生物量及产量的影响. 安徽农业科学, 2011, 39(24): 14561–14563, 14585 DOI:10.3969/j.issn.0517-6611.2011.24.017] |
HUANG J, TANG X X, DUAN D L, et al. Physiological effect of light on kelp. Marine Sciences, 2002, 26(4): 55-58 [黄健, 唐学玺, 段德麟, 等. 不同光照条件下海带体内各种化合物的含量及光合作用和呼吸作用的变化. 海洋科学, 2002, 26(4): 55-58 DOI:10.3969/j.issn.1000-3096.2002.04.015] |
KONG L A, XIE Y, HU L, et al. Remobilization of vegetative nitrogen to developing grain in wheat (Triticum aestivum L. ). Field Crops Research, 2016, 196: 134-144 DOI:10.1016/j.fcr.2016.06.015 |
KUMURA T, YASUI H, MIZUTA H. Nutrient requirement for zoospore formation in two alariaceous plants Undaria pinnatifida (Harvey) Suringar and Alaria crassifolia Kjellman (Phaeophyceae: Laminariales). Fisheries Science, 2010, 72(4): 860-869 |
LI X, PANG S J, SU L. Performance of spore release, gametogenesis and growth of sporophytes resulted from sorus cultured under different temperature and culture periods. Progress in Fishery Sciences, 2016, 37(1): 111-119 [李霞, 逄少军, 苏丽. 温度和时间对海带(Saccharina japonica)孢子囊孢子放散及后期生长发育的影响. 渔业科学进展, 2016, 37(1): 111-119] |
LING J Y, LIANG Z R, WANG F J, et al. Effects of high temperature on the antioxidant enzymes and chlorophyll fluorescence parameters of Saccharina japonica. Progress in Fishery Sciences, 2016, 37(3): 120-125 [凌晶宇, 梁洲瑞, 王飞久, 等. 高温对海带(Saccharina japonica)抗氧化酶及叶绿素荧光参数的影响. 渔业科学进展, 2016, 37(3): 120-125] |
LISZKAY A, ZALM E, SCHOPFER P. Production of reactive oxygen intermediates (O2•2, H2O2, and •OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiology, 2004, 136(2): 3114-3123 DOI:10.1104/pp.104.044784 |
LÜNING K. Photoperiodic control of sorus formation in the brown alga Laminaria saccharina. Marine Ecology Progress Series, 1988, 45(1/2): 137-144 |
MATSUYAMA K. Photosynthesis of Undaria pinnatifida Suringar f. Distans Miyabe et Okamura (Phaeophyceae) from Oshoro Bay. Ⅱ. Photosynthetic in several portions of the thallus. Hokkaido-ritsu Suisan Shikenjo hokoku=Scientific reports of Hokkaido Fisheries Experimental Station, 1983, 25: 195–200
|
MITTLER R, VANDERAUWERA S, GOLLERY M, et al. Reactive oxygen gene network of plants. Trends in Plant Science, 2004, 9(10): 490-498 DOI:10.1016/j.tplants.2004.08.009 |
MIZUTA H, HAYASAKI J, YAMAMOTO H. Relationship between Nitrogen content and sorus formation in the brown alga Laminaria japonica cultivated in Southern Hokkaido. Japanese Fisheries Science, 1998, 64(6): 909-911 DOI:10.2331/fishsci.64.909 |
MIZUTA H, YASUI H. Significance of radical oxygen production in sorus development and zoospore germination in Saccharina japonica (Phaeophyceae). Botanica Marina, 2010, 53(5): 409-416 |
NIMURA K, MIZUTA H. Differences in photosynthesis and nucleic acid content between sterile and fertile parts of the sporophyte of Laminaria japonica (Phaeophyceae). Algae, 2001, 16: 151-155 |
NIMURA K, MIZUTA H. Inducible effects of abscisic acid on sporophyte discs from Laminaria japonica Areschoug (Laminariales, Phaeophyceae). Journal of Applied Phycology, 2002, 14(3): 159-163 DOI:10.1023/A:1019929712633 |
POTIKHA T S, COLLINS C C, JOHNSON D I, et al. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiology, 1999, 119(3): 849-858 DOI:10.1104/pp.119.3.849 |
SAKANISHI Y, YOKOHAMA Y, ARUGA Y. Photosynthetic capacity of various parts of the blade of Laminaria longissima Miyabe (Phaeophyceae). Japanese Journal of Phycology, 1991, 39: 239-243 |
SASAKI, S. An ecological study of Laminaria angustata var. Longissima (M.) MIY ABE on the coast of Kushiro Prov, Hokkaido. 1. On the life of winter population. Scientific Reports, 1969, 10: 1-42 |
SCHAFFELKE B. Abscisic acid in sporophytes of three laminaria species (Phaeophyta). Journal of Plant Physiology, 1995, 146(4): 453-458 DOI:10.1016/S0176-1617(11)82008-0 |
SHAO J P, LÜNING K. Breaking seasonal limitation: Year- round sporogenesis in the brown alga Laminaria saccharina by blocking the transport of putative sporulation inhibitors. Aquaculture, 2004, 240(1/2/3/4): 531-541 |
SU L, PANG S J, GAO S Q. Investigation of sorus formation in the new Saccharina japonica hybrid cultivar b013. Journal of Agricultural Science and Technology, 2017, 19(2): 119-123 [苏丽, 逄少军, 高素芹. 杂交海带新品系b013孢子囊形成规律初探. 中国农业科技导报, 2017, 19(2): 119-123] |
SU L, SHAN T F, LI J, et al. Aquaculture of the hybrid cultivars of Saccharina japonica: Removing the obstacle of sori production by photoperiodic control. Aquaculture, 2020, 519: 734917 DOI:10.1016/j.aquaculture.2019.734917 |
SU L. The study on reproduction and cultivation problems releated to variety breeding of the economic seaweed Saccharina japonica. Doctoral Dissertation of the University of Chinese Academy of Sciences, 2018 [苏丽. 与海带品种培育相关的繁殖与栽培问题的研究. 中国科学院大学(中国科学院海洋研究所)博士研究生学位论文, 2018]
|
WANG X Y, WANG B, HOU S T. Structure and function of malate dehydrogenases. Journal of Biology, 2009, 26(4): 69-72 [汪新颖, 王波, 侯松涛, 等. 苹果酸脱氢酶的结构及功能. 生物学杂志, 2009, 26(4): 69-72] |
WEN B B, ZHANG X H, SHEN H Y, et al. Effects of nitrate nitrogen on chlorophyll synthesis and related genes expression of 'Gala 3' in tissue culture. Journal of Shandong Agricultural University (Natural Science), 2019, 50(2): 179-185 [文滨滨, 张新昊, 沈红艳, 等. 硝态氮对组培'嘎拉3'叶绿素合成及相关基因表达的影响. 山东农业大学学报(自然科学版), 2019, 50(2): 179-185] |
XU D Q. Photosynthetic efficiency. 4th edition. Shanghai: Shanghai Scientific and Technical Publishers, 2002, 47 [许大全. 光合作用效率. 第4版. 上海: 上海科学技术出版社, 2002, 47] |
YANG X Y, KANG Y C, CAO L. Nitrogen application and slow-release urea are beneficial to the improvement of yield and quality of pepper. Molecular Plant Breeding, 2019, 17(22): 7507-7512 [杨昕宇, 康益晨, 曹莉, 等. 施氮与缓释尿素有利于辣椒产量及品质的提升. 分子植物育种, 2019, 17(22): 7507-7512] |
ZHOU Z Q, ZHANG Q L. Study on the change of chlorophyll contents and its relationships with photosynthetic leaf area among the different types of wheat cultivars (Lines). Journal of Hubei Engineering University, 2001(6): 5-8 [周竹青, 张清良. 小麦品种(系)叶绿素含量变化及其与光合叶面积关系研究. 孝感学院学报, 2001(6): 5-8] |



