2. 崂山实验室海洋渔业科学与食物产出过程功能研究室 山东 青岛 266237
2. Laboratory for Marine Fisheries Science and Food Production Processes, Laoshan Laboratory, Qingdao 266237, China
白介素-1 (IL-1)家族是调节免疫系统炎症反应的重要细胞因子,在哺乳动物中,该家族包含IL-1α、IL-1β、IL-18、IL-1Ra、IL-36Ra、IL-38和IL-17等11个成员(Wang et al, 2013),按功能主要分为三类,分别为具有促炎活性的细胞因子、天然受体拮抗剂和抗炎细胞因子(Dinarello, 2018)。IL-1β作为一个典型的促炎细胞因子,广泛存在于后生动物中,在病原感染引起的免疫防御中发挥重要作用。哺乳动物成熟的IL-1β蛋白由IL前体和IL1两个结构域构成,12个β折叠片折叠形成三叶草状结构,其C-末端含有IL-1家族的特征基序(Dinarello, 2013)。正常情况下,IL-1β以无活性的前体形式存在于细胞基质中,当接收到外界刺激信号后,IL-1β前体经半胱氨酸蛋白酶-1 (caspase-1)特异性切割,形成具有生物学活性的成熟IL-1β,通过非经典分泌途径从细胞中释放出来(Wewers et al, 1997)。IL-1β与受体结合后,通过衔接蛋白髓样分化因子(MyD88)激活下游的白介素1受体相关激酶(IRAKs)和肿瘤坏死因子受体相关因子(TRAF6),调控核因子κB (NF-κB)、c-Jun氨基末端激酶(JNK)等转录因子的激活,进而在机体免疫应答、炎症响应、代谢调节、肿瘤转移等一系列生理过程中发挥作用(Dinarello, 2018)。
IL-1β的结构和功能在进化中较为保守。目前,已在多种鱼类中鉴定出IL-1β基因,且发现在新真骨鱼亚群、原棘鳍总目等鱼类中存在1个以上的IL-1β亚型(Engelsma et al, 2003; Zou et al, 1999)。溶藻弧菌(Vibrio alginolyticus)、耶尔森氏菌(Yersinia ruckeri)、神经坏死病病毒(VNNV)和寄生性血内鞭毛虫(Trypanoplasma borreli)感染能够引起鱼类IL-1β表达响应(Wu et al, 2015; Pleguezuelos et al, 2000; Wu et al, 2019)。个体注射重组IL-1β蛋白能够增强鱼类单核/巨噬细胞迁移和吞噬活性,抑制细菌增殖(Gao et al, 2019; Hong et al, 2003)。在鲤鱼(Cyprinus carpio)、牙鲆(Paralichthys olivaceus)中使用重组IL-1β作为佐剂可增强疫苗注射后的免疫应答,提高抗体效价(Guo et al, 2018; Taechavasonyoo et al, 2013)。
黄条鰤(Seriola aureovittata)肉质鲜嫩,具有极大的消费市场潜力(Wang et al, 2021)。目前,黄条鰤的人工繁育和养殖技术已日渐成熟,但其疾病防控技术尚待完善(Purcell et al, 2018)。本研究通过RACE克隆获得黄条鰤 SaIL-1β1和SaIL-1β2的cDNA全长,并基于序列特征进行了生物信息学分析,进而通过荧光定量PCR (qRT-PCR)技术对SaIL-1β1和SaIL-1β2的组织分布和免疫应答模式进行了探究,以期为黄条鰤疾病综合防控技术提供基础信息。
1 材料与方法 1.1 实验材料实验用黄条鰤(平均体重约430 g)于实验1周前购自辽宁省大连富谷水产有限公司,置于18~25 ℃、盐度20~23、pH 8.0~8.3的无菌海水中暂养,期间持续充氧,并投喂商品化基础饲料以维持良好状态。
1.2 实验方法 1.2.1 动物刺激实验、样品收集将40尾黄条鰤随机等分为2组,MS222麻醉后称重,实验组按照1 μg/g体重注射脂多糖(LPS),对照组注射磷酸盐缓冲液(PBS),分别于6、12、24、48、72 h后每组随机取4尾鱼,解剖取头肾和脾脏组织,保存于液氮中,用于总RNA提取。此外,取对照组黄条鰤的脑、肝脏、肌肉、垂体、胃、心脏、脾脏、头肾和鳃组织保存于液氮中,用于基因克隆和组织表达分析。
1.2.2 总RNA提取和cDNA合成采用Trizol法提取上述黄条鰤各组织样品总RNA。通过琼脂糖凝胶电泳检测RNA质量,使用NanoDrop2000定量并调整至相同浓度备用。以黄条鰤 9个组织混合RNA为模板,使用SMARTer RACE 5′/3′试剂盒(TaKaRa),按照说明书操作步骤分别合成5′RACE及3′RACE cDNA,用于基因片段克隆。使用Primer ScriptTM RT Master Mix试剂盒(TaKaRa)将黄条鰤 LPS刺激实验的组织RNA分别反转录为cDNA,用于不同组织及LPS不同时期基因表达情况的检测。
1.2.3 SaIL-1β1和SaIL-1β2基因的克隆与测序根据NCBI基因组数据库中注释的基因序列信息,使用Primer Premier 5.0软件设计SaIL-1β1和SaIL-1β2的特异引物(表 1),并利用ExTaq高保真酶进行扩增。扩增体系(25 μL):ExTaq酶0.15 μL,cDNA模板1 μL,正反向引物各1 μL,10×Buffer 2.5 μL,dNTP 2.0 μL、MgCl2 1.5 μL,ddH2O 15.85 μL。反应程序:94 ℃预变性5 min;94 ℃变性30 s,55 ℃退火30 s,72 ℃延伸3 min 40 s,35个循环。扩增产物经1.5%琼脂糖凝胶电泳检测后,切胶回收目的条带连接T载体并转化至克隆菌pMD19-T,挑取阳性单克隆菌株,送生工生物(上海)股份有限公司进行测序。
|
|
表 1 本研究用到的引物序列 Tab.1 Nucleotide sequence of primers used in this study |
利用DNAMAN对SaIL-1β1和SaIL-1β2的氨基酸序列进行序列比对;利用SMART软件预测SaIL-1β1和SaIL-1β2的蛋白结构域;利用在线蛋白分析软件(https://www.expasy.org)预测蛋白分子量和等电点;通过SWISS-MODEL (https://swissmodel.expasy.org/)预测SaIL-1β1和SaIL-1β2蛋白的三级结构;通过NCBI查找相关脊椎动物IL-1氨基酸序列(表 2),利用SeaView4软件(Gouy et al, 2010)采用最大似然法构建进化树。
|
|
表 2 序列比对及进化树构建所用IL-1β蛋白序列 Tab.2 The amino acid sequences of IL-1β used in multi-sequence alignment and phylogenetic analyses |
根据SaIL-1β1和SaIL-1β2序列设计qRT-PCR引物,选用18S rRNA作为内参基因进行qRT-PCR扩增。反应条件:95 ℃ 2 min;95 ℃ 15 s,60 ℃ 45 s,40个循环。每个待测样品均设置3次重复,保证准确性。实验得到的数据采用2−ΔΔCt法处理数据,经计算得到SaIL-1β1和SaIL-1β的相对表达量(Schmittgen et al, 2008)。
1.2.6 数据分析本实验所涉及数据的差异性分析均利用SPSS软件,使用单因素方差分析(one-way ANOVA)进行比较。所有数据以平均值±标准差(Mean±SD)(n=3)表示,P < 0.05为差异显著。
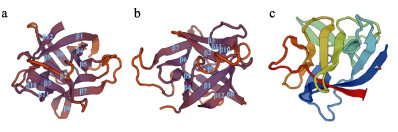
2 结果 2.1 SaIL-1β1和SaIL-1β2的编码序列分析以黄条鰤脑、肝脏和肌肉等混合组织为模板,扩增得到候选白介素基因序列,分别命名为SaIL-1β1和SaIL-1β2,SaIL-1β1基因全长cDNA为1 292 bp,其中,5′UTR为25 bp,3′UTR为439 bp,编码区长度为828 bp,编码275个氨基酸,预测蛋白质分子量为30.75 kDa,理论等电点为6.14 (图 1)。SMART保守结构域分析显示,SaIL-1β1第121~268个氨基酸区域构成IL-1结构域(图 3a)。SaIL-1β2基因全长cDNA序列为1 337 bp,其中,5′UTR为122 bp,3′UTR为255 bp,编码区长度为960 bp,编码319个氨基酸,预测蛋白质分子量为36.66 kDa,理论等电点为6.40 (图 2)。SMART保守结构域分析显示,SaIL-1β1第91~245个氨基酸区域构成IL-1结构域(图 3b)。通过SWISS-MODEL网站预测获得,SaIL-1β1和SaIL-1β2蛋白均为由12个β折叠构成的三叶草结构(图 4)。

|
图 1 黄条鰤 SaIL-1β1基因核苷酸序列及推测的氨基酸序列 Fig.1 Nucleotide and deduced amino acid sequences of SaIL-1β1 in S. aureovittata 小写字母为核苷酸序列,大写字母为推导的氨基酸序列,阴影表示起始密码子和终止密码子;下划线表示保守结构域;双下划线表示polyA信号。 Lowercases indicate the nucleotides, and uppercases indicate the amino acids. Shadow indicates the start and stop codons. Underline indicates the conserved domain. Double underline indicates the polyA tail sequence. |
|
图 3 SaIL-1β1和SaIL-1β2蛋白结构域预测 Fig.3 The predicted domain of SaIL-1β1 and SaIL-1β2 a:SaIL-1β1结构域;b:SaIL-1β2结构域a: Domain of SaIL-1β1; b: Domain of SaIL-1β2 |

|
图 2 黄条鰤 SaIL-1β2基因核苷酸序列及推测的氨基酸序列 Fig.2 Nucleotide and deduced amino acid sequences of SaIL-1β2 in S. aureovittata 小写字母为核苷酸序列,大写字母为推导的氨基酸序列,阴影表示起始密码子和终止密码子;下划线表示保守结构域;双下划线表示polyA信号。 Lowercases indicate the nucleotides, and uppercases indicate the amino acids. Shadow indicates the start and stop codons. Underline indicates the conserved domain. Double underline indicates the polyA tail sequence. |
|
图 4 SaIL-1β1和SaIL-1β2蛋白三级结构预测 Fig.4 The tertiary structure of SaIL-1β1 and SaIL-1β2 a:SaIL-1β1蛋白;b:SaIL-1β2蛋白;c:人类IL-1β蛋白 a: SaIL-1β1 protein; b: SaIL-1β2 protein; c: Human IL-1β protein |
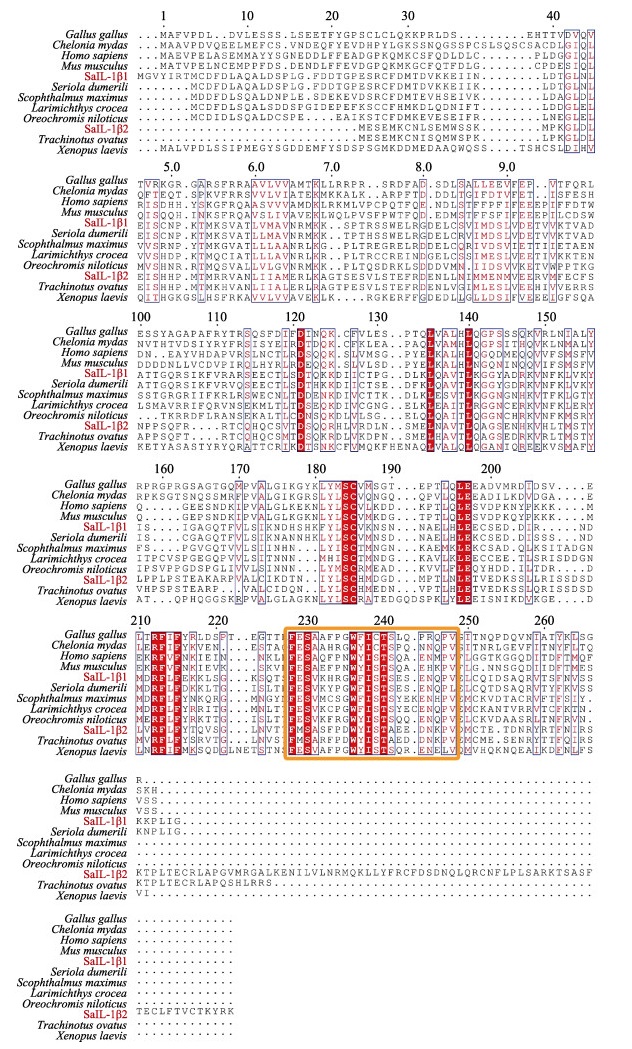
SaIL-1β1和SaIL-1β2蛋白序列与其他物种的同源性分析显示,SaIL-1β1与高体鰤(Seriola dumerili, XP_022625284.1)、大菱鲆(Scophthalmus maximus, XP_035481228.1)、大黄鱼(Larimichthys crocea, KAE8285371.1)和罗非鱼(Oreochromis niloticus, XP_005457944.1)蛋白的相似性分别为87.04%、53.01%、50.55%和47.58%。SaIL-1β2与高体鰤 (BAT57261.1)、卵形鲳鲹(Trachinotus ovatus, AZL87291.1)、大菱鲆(XP_035496710.1)和大黄鱼(KAE8291795.1)蛋白的相似性分别是97.25%、77.43%、65.18%和60.16%。氨基酸多序列比对揭示,SaIL-1β1和SaIL-1β2的C端均含有IL-1超家族典型的[FC]-x-S-[ASLV]-x(2)-P-x(2)-[FYLIV]-[LI]-[SCA]-T-x(7)-[LIVM]基序(图 5)。进化树分析结果表明,脊椎动物IL-1β蛋白分为两支,哺乳动物、爬行动物和鸟类聚为一支,鱼类聚为一支,SaIL-1β1与高体鰤 IL-1β亲缘关系较近,SaIL-1β2与卵形鲳鲹IL-1β亲缘关系较近(图 6)。
|
图 5 黄条鰤 SaIL-1β1和SaIL-1β2与其他物种IL-1β氨基酸序列比对 Fig.5 Multi-sequence alignment of SaIL-1β1 and SaIL-1β2 with IL-1β in other species 黄框为IL-1超家族C端保守基序。 Yellow box indicates the conserved motifs of IL-1 superfamily. |

|
图 6 黄条鰤 SaIL-1β1和SaIL-1β2与其他脊椎动物IL-1β2的系统进化树 Fig.6 Phylogenetic relationship of SaIL-1β1 and SaIL-1β2 with other IL-1βs in vertebrates |
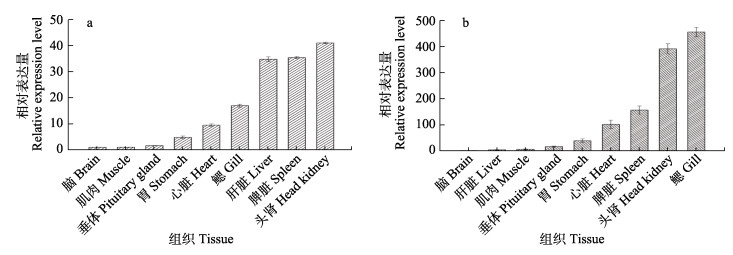
通过qRT-PCR检测SaIL-1β1和SaIL-1β2在黄条鰤脑、肝脏、肌肉、垂体、胃、心脏、脾脏、头肾和鳃中的表达水平。结果显示,9种组织中均检测到SaIL-1β1和SaIL-1β2转录水平表达。其中,SaIL-1β1在头肾中表达量最高,脾脏和肝脏次之,随后依次是鳃、心脏、胃、垂体、肌肉和脑(图 7a)。SaIL-1β2在鳃中表达量最高,头肾和脾脏次之,随后依次是心脏、胃、垂体、肌肉、肝脏和脑(图 7b)。
|
图 7 黄条鰤主要组织中SaIL-1β1(a)和SaIL-1β2(b)转录水平表达 Fig.7 The expression of SaIL-1β1 (a) and SaIL-1β2 (b) transcripts in different tissues of S. aureovittata |
在LPS刺激后的黄条鰤头肾中,SaIL-1β1各时间点转录水平表达均显著高于对照组,在LPS刺激后6 h,SaIL-1β1急剧上升至对照组的10.03倍(P < 0.01),随后逐渐回落,在12、24、48、72 h分别为对照组的7.15、4.09、2.71、3.03倍(P < 0.05)(图 8a)。SaIL-1β2转录水平呈现出先上升后下降的趋势,在LPS刺激后6 h,急剧上升至对照组的11.49倍(P < 0.05),最后逐渐回落,48 h恢复至正常水平,72 h下降至对照组的0.29倍(P < 0.05)(图 8b)。

|
图 8 LPS刺激后SaIL-1β1和SaIL-1β2的转录水平变化 Fig.8 The expression of SaIL-1β1 and SaIL-1β2 transcripts after LPS stimulation |
在LPS刺激后的黄条鰤脾脏中,SaIL-1β1转录水平显著升高,在LPS刺激后6 h,SaIL-1β1急剧上升至对照组的6.59倍(P < 0.05),随后逐渐回落,在12、24、48 h分别为对照组的3.85、4.09、2.17、2.65倍(P < 0.05),72 h恢复正常水平(图 8c)。SaIL-1β2转录水平表达模式与SaIL-1β1相似,在6、12、24、48 h分别为对照组的7.25、3.20、2.02、1.59倍,72 h恢复至正常水平(图 8d)。
3 讨论本研究在黄条鰤中克隆获得了SaIL-1β1和SaIL-1β2两个基因,分别编码275和319个氨基酸。经预测,2个蛋白均不含信号肽,具有白介素-1结构域并含有12个β折叠片。与其他脊椎动物相同,鱼类IL-1β蛋白在前体阶段被半胱天冬-1酶分解后分泌至细胞外,因此,分解过程无信号肽参与(Wewers et al, 1997)。SaIL-1β1和SaIL-1β2两个蛋白含有的12个β折叠是成熟IL-1β的典型特征,可能形成对受体结合至关重要的β三叶草结构。高等脊椎动物IL-1β由IL1前体结构域和IL1结构域组成(Dinarello, 2013)。已有研究提出,部分鱼类物种中缺少的IL1前体结构域功能性较弱,且其保守性远低于IL1结构域(Hailey et al, 2009)。哺乳动物IL-1超家族的C端经典基序为[FC]- x-S-[ASLV]-x(2)-P-x(2)-[FYLIV]-[LI]-[SCA]-T-x(7)- [LIVM] (Bird et al, 2002),多序列比对结果显示,IL-1β的C端典型基序在SaIL-1β1和SaIL-1β蛋白中均存在,但在黄条鰤中存在细微变异,为[FC]-x-S- [ASLV]-x(2)-[PRS]-x(2)-[FYLIV]-[LI]-[SCA]-T-x(7)- [LIVM]形式。相较于哺乳动物IL-1β,鱼类的IL-1β存在基因多态性(Eggestøl et al, 2020; Engelsma et al, 2003),本研究在黄条鰤中发现了SaIL-1β1和SaIL-1β2两种亚型,可能与鱼类基因组复制有关。进一步分析脊椎动物IL-1β分子的进化关系,结果表明,鱼类的IL-1β分子发生了独立复制,单独聚为一支,而在鱼类中SaIL-1β1和SaIL-1β2分属于2个分支,其中,SaIL-1β1与高体鰤 IL-1β进化关系最近,SaIL-1β2与卵形鲳鲹进化关系最近。以上结果表明,SaIL-1β1和SaIL-1β2基本具有脊椎动物IL-1β的结构特征,可能属于白介素-1超家族成员。
IL-1β是一种重要的促炎症细胞因子,在免疫响应中起着重要的调节作用(Dinarello, 2018)。本研究显示SaIL-1β1和SaIL-1β2 mRNA在脑、肝脏、肌肉、垂体、胃、心脏、脾脏、头肾和鳃组织广泛表达,表明SaIL-1β1和SaIL-1β2可能广泛参与机体各种生理活动,其中SaIL-1β1在头肾、脾脏、肝脏和鳃中表达较高,而SaIL-1β2主要在鳃、头肾、脾脏和心脏中表达较高,在肝脏中较少。头肾和脾脏是鱼类主要的免疫器官,鳃是鱼类接触水环境微生物的主要器官,其中的黏膜中含有大量的T淋巴细胞抵御病原感染(Klosterhoff et al, 2015)。以上结果暗示了SaIL-1β1和SaIL-1β2在黄条鰤免疫响应中具有重要作用。
头肾和脾脏是硬骨鱼类免疫细胞产生和分化的重要器官(Kumar et al, 2016)。LPS是革兰氏阴性菌细胞壁外壁的主要成分,能够模拟细菌感染、激活机体炎症信号通路、诱导炎症因子的释放(Lu et al, 2008)。为探究SaIL-1β1和SaIL-1β2是否参与黄条鰤免疫应答,本研究检测了LPS刺激后黄条鰤头肾和脾脏中SaIL-1β1和SaIL-1β2转录水平时序变化。qRT-PCR结果显示,SaIL-1β1和SaIL-1β2均在LPS刺激后大量表达,且在刺激6 h时表达量最高,达到对照组的10倍以上。斑马鱼和大西洋鲑(Salmo salar)等硬骨鱼类
在LPS刺激后,激活Toll通路上调il-1β表达,进而诱导其他炎症细胞因子、趋化因子的合成,激活淋巴细胞和巨噬细胞等,介导机体促炎反应(Pleguezuelos et al, 2000; Vojtech et al, 2012)。因此,可以推测,在LPS刺激后,SaIL-1β1和SaIL-1β2在黄条鰤头肾和脾脏中表达量上调以参与免疫反应,且在免疫防御早期的促炎反应中发挥重要作用。
本研究首次在黄条鰤中克隆得到SaIL-1β1和SaIL-1β2,通过序列结构、组织表达分析等手段鉴定了其作为IL-1β分子的特征。同时,探明了SaIL-1β1和SaIL-1β2参与黄条鰤免疫响应的表达规律,为今后深入研究细胞因子白介素在黄条鰤免疫防御中的作用提供了基础信息。
BIRD S, ZOU J, WANG T, et al. Evolution of interleukin-1β. Cytokine and Growth Factor Reviews, 2002, 13(6): 483-502 DOI:10.1016/S1359-6101(02)00028-X |
DINARELLO C A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunological Reviews, 2018, 281(1): 8-27 DOI:10.1111/imr.12621 |
DINARELLO C A. Overview of the interleukin-1 family of ligands and receptors. In "Seminars in immunology", 2013, 25(1): 389-393 |
EGGESTØL H Ø, LUNDE H S, KNUTSEN T M, et al. Interleukin-1 ligands and receptors in lumpfish (Cyclopterus lumpus L.): Molecular characterization, phylogeny, gene expression, and transcriptome analyses. Frontiers in Immunology, 2020, 11: 502 DOI:10.3389/fimmu.2020.00502 |
ENGELSMA M Y, STET R J, SAEIJ J P, et al. Differential expression and haplotypic variation of two interleukin-1β genes in the common carp (Cyprinus carpio L.). Cytokine, 2003, 22(1): 21-32 |
GAO J, JIANG X, WANG J, et al. Phylogeny and expression modulation of interleukin 1 receptors in grass carp (Ctenopharyngodon idella). Developmental and Comparative Immunology, 2019, 99: 103401 DOI:10.1016/j.dci.2019.103401 |
GOUY M, GUINDON S, GASCUEL O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular and Biological Evolution, 2010, 27: 221-224 DOI:10.1093/molbev/msp259 |
GUO M, TANG X, SHENG X, et al. The effects of IL-1β, IL-8, G-CSF and TNF-α as molecular adjuvant on the immune response to an E. tarda subunit vaccine in flounder (Paralichthys olivaceus). Fish and Shellfish Immunology, 2018, 77: 374-384 DOI:10.1016/j.fsi.2018.04.009 |
HAILEY K L, LI S, ANDERSEN M D, et al. Pro-interleukin (IL)-1β shares a core region of stability as compared with mature IL-1β while maintaining a distinctly different configurational landscape: A comparative hydrogen/deuterium exchange mass spectrometry study. Journal of Biological Chemistry, 2009, 284(38): 26137-26148 DOI:10.1074/jbc.M109.027375 |
HONG S, PEDDIE S, CAMPOS-PÉREZ J J, et al. The effect of intraperitoneally administered recombinant IL-1β on immune parameters and resistance to Aeromonas salmonicida in the rainbow trout (Oncorhynchus mykiss). Developmental and Comparative Immunology, 2003, 27(9): 801-812 DOI:10.1016/S0145-305X(03)00056-9 |
KLOSTERHOFF M C, PEREIRA Jr J, RODRIGUES R V, et al. Ontogenic development of kidney, thymus and spleen and phenotypic expression of CD3 and CD4 receptors on the lymphocytes of cobia (Rachycentron canadum). Anais da Academia Brasileira de Ciências, 2015, 87: 2111-2121 DOI:10.1590/0001-3765201520140623 |
KUMAR R, JOY K, SINGH S. Morpho-histology of head kidney of female catfish Heteropneustes fossilis: Seasonal variations in melano-macrophage centers, melanin contents and effects of lipopolysaccharide and dexamethasone on melanins. Fish Physiology and Biochemistry, 2016, 42(5): 1287-1306 DOI:10.1007/s10695-016-0218-2 |
LU Y C, YEH W C, OHASHI P S. LPS/TLR4 signal transduction pathway. Cytokine, 2008, 42(2): 145-151 DOI:10.1016/j.cyto.2008.01.006 |
PLEGUEZUELOS O, ZOU J, SECOMBES C J. Cloning, sequencing, and analysis of expression of a second IL-1 β gene in rainbow trout (Oncorhynchus mykiss). Immunogenetics, 2000, 51(12): 1002-1011 DOI:10.1007/s002510000240 |
PURCELL C M, SEETHARAM A S, SNODGRASS O, et al. Insights into teleost sex determination from the Seriola dorsalis genome assembly. BMC Genomics, 2018, 19(1): 1-11 DOI:10.1186/s12864-017-4368-0 |
SCHMITTGEN T D, LIVAK K J. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols, 2008, 3: 1101-1108 DOI:10.1038/nprot.2008.73 |
TAECHAVASONYOO A, KONDO H, NOZAKI R, et al. Identification of novel interleukin 1 beta family genes in Japanese flounder Paralichthys olivaceus. Fish and Shellfish Immunology, 2013, 34(1): 393-396 DOI:10.1016/j.fsi.2012.10.001 |
VOJTECH L N, SCHARPING N, WOODSON J C. Roles of inflammatory caspases during processing of zebrafish interleukin-1 beta during Francisella noatunensis infection. Infection and Immunity, 2012, 80(8): 2878-2885 DOI:10.1128/IAI.00543-12 |
WANG B, WANG K, CUI A, et al. LPXRFa down-regulates brain reproductive genes in yellowtail kingfish (Seriola lalandi). Aquaculture, 2021, 545: 737251 DOI:10.1016/j.aquaculture.2021.737251 |
WANG P, LU Y Q, WEN Y, et al. IL-16 induces intestinal inflammation via PepT1 upregulation in a pufferfish model: New insights into the molecular mechanism of inflammatory bowel disease. Journal of Immunology, 2013, 191(3): 1413-1427 DOI:10.4049/jimmunol.1202598 |
WEWERS M D, DARE H A, WINNARD A V, et al. IL-1 beta-converting enzyme (ICE) is present and functional in human alveolar macrophages: Macrophage IL-1 beta release limitation is ICE independent. Journal of Immunology, 1997, 159: 5964-5972 DOI:10.4049/jimmunol.159.12.5964 |
WU J, SHI Y H, ZHANG X H, et al. Molecular characterization of an IL-1β gene from the large yellow croaker (Larimichthys crocea) and its effect on fish defense against Vibrio alginolyticus infection. Zoological Research, 2015, 36(3): 133-141 |
WU Y, ZHOU Y, CAO Z, et al. Comparative analysis of the expression patterns of IL-1β, IL-11, and IL-34 in golden pompano (Trachinotus ovatus) following different pathogens challenge. Fish and Shellfish Immunology, 2019, 93: 863-870 DOI:10.1016/j.fsi.2019.08.018 |
ZOU J, CUNNINGHAM C, SECOMBES C J. The rainbow trout Oncorhynchus mykiss interleukin-1β gene has a different organization to mammals and undergoes incomplete splicing. European Journal of Biochemistry, 1999, 259: 901-908 |



