2. 中国水产科学研究院黄海水产研究所青岛海洋科学与技术试点国家实验室深蓝渔业工程联合实验室 山东 青岛 266071
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences; Joint Laboratory for Deep Blue Fishery Engineering of Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266071, China
鱼类的摄食行为不仅与外界因素有关,还受到多种内源性摄食因子的调控(Volkoff et al, 2020)。下丘脑神经内分泌因子,如神经肽Y (neuropeptide Y, NPY)、黑皮质素(melanocortin, MCR)及食欲肽(orexin)等在鱼类摄食中发挥重要的调控作用(Bertucci et al, 2019; Soengas et al, 2018)。其中,NPY在鱼类中是一种强有力的促食欲因子(Bertucci et al, 2019)。
1982年,NPY首次从猪脑中提取出来,因其富含酪氨酸而被称为神经肽酪氨酸或神经肽Y,具有显著促进摄食的功能(Tatemoto et al, 1982)。在硬骨鱼类中,npy于1989年首次在金鱼(Carassius curatus)中克隆获得(Kah et al, 1989)。目前,已在大西洋鲑(Salmo salar) (Murashita et al, 2009)、重口裂腹鱼(Schizothorax davidi) (Deng et al, 2019)、银鲫(Carassius auratus gibelio) (Lei et al, 2019)等硬骨鱼中相继克隆获得了npy基因。已有研究表明,鱼类npy mRNA主要在脑中表达(Lei et al, 2019; Deng et al, 2019),参与摄食调控、糖脂代谢等多种生理功能(Horio et al, 2021)。
黄条鰤(Seriola aureovittata)是一种大型长距离洄游的掠食性鱼类(Booth et al, 2022; 柳学周等, 2017)。本团队于2017年突破了黄条鰤苗种繁育技术,并开展了其生长和生殖功能基因的相关研究(Wang et al, 2021; 徐永江等, 2019)。黄条鰤食量较大且摄食凶猛,在养殖过程中,养殖者需了解黄条鰤的摄食特性及其内在调控机制,以便对投喂策略做出调整,在节约养殖成本的同时,促进养殖鱼类的生长,从而获得更大的经济效益。NPY作为一种强有力的促食欲因子(Bertucci et al, 2019),在硬骨鱼中被证明有促摄食的作用(Lei et al, 2019; Tolås et al, 2021),但尚未在黄条鰤中鉴定出该基因。本研究拟探究npy基因在黄条鰤摄食与饥饿补偿机制中的规律,以期为进一步研究黄条鰤的摄食和能量代谢提供基础。
1 材料与方法 1.1 实验设计与样品采集所有动物均经中国水产科学研究院黄海水产研究所实验动物福利伦理和动物实验安全委员会批准。本研究所用黄条鰤购自大连富谷食品有限公司,克隆及组织表达所用的黄条鰤为3龄鱼,取样3尾雌鱼[体重为(5.58±0.21) kg,全长为(85.67±2.18 cm)]和3尾雄鱼[体重为(4.28±0.04) kg,全长为(80.33±1.36) cm]。用MS-222 (120 mg/L)将黄条鰤麻醉,分别取脑、垂体、鳃、心、肝、脾、肾、胃、肠、肌肉和性腺等组织样品,置于液氮中保存备用。
饥饿再投喂实验所用的黄条鰤为1龄鱼,使用180尾实验鱼[体重为(616.25±24.23) g,全长为(41.5± 0.65 cm)],设置对照组(持续投喂)和实验组(饥饿再投喂),每组设置3个平行,每个平行30尾鱼,实验正式开始前驯化7 d。实验鱼被饲养在容积为3 m3玻璃钢水槽中,充气流水养殖(盐度为27~30,pH值为7.8~8.2,溶氧 > 6 mg/L,水温为24~26 ℃),日换水率为300%。饵料为冰鲜玉筋鱼(Ammodytes personatus),每天投喂2次(07:00和18:00)至饱食,1 h后清理底部残饵。饥饿再投喂实验组先进行21 d饥饿处理,随后7 d与对照组同步投喂。在实验开始后的第7、14、21和28天进行取样,每次每组取样6尾。取样前12 h统一停止投喂,采用MS-222麻醉实验鱼(身体失去平衡,腹部向上,眼睑有反应),解剖后取出脑、垂体和胃,置于液氮中保存备用。
1.2 总RNA提取与cDNA反转录使用RNAiso Plus Total RNA提取试剂盒(TaKaRa, 日本)提取黄条鰤的组织总RNA,通过NanoDrop 2000C分光光度计(Thermo, 美国)测定RNA浓度,当A260 nm/A280 nm在1.8~2.0之间,RNA可用于后续实验。使用PrimeScript™ RT reagent kit with gDNA eraser (TaKaRa, 日本)合成cDNA第一链,按照说明书的操作步骤进行,合成的cDNA模板保存于–20 ℃备用。
1.3 基因克隆根据NCBI数据库中预测的npy序列(XP_023282438.1)设计特异性引物(表 1)。基因克隆以脑cDNA为模板,PCR体系(50 μL)包含25 μL rTaq酶、ddH2O 21 μL、上下游引物各1 μL和cDNA模板2 μL。PCR扩增条件:95 ℃预变性5 min;95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸30 s,38个循环;最后72 ℃再延伸5 min,4 ℃保存。
|
|
表 1 本研究所用引物 Tab.1 Primers used in this study |
将符合目的基因大小的条带用Steady Pure DNA凝胶回收试剂盒(艾科瑞生物)进行胶回收,将胶回收产物与pEASY-T1 Simple载体、Trans1-T1 Phage Resistant感受态细胞(TransGene Biotech, 北京)进行连接转化,37 ℃培养箱中培养12 h,挑取阳性克隆产物送至生工生物工程(上海)股份有限公司测序。
1.4 基因定量表达分析基因定量表达分析使用TB Green Premix Ex TaqTM Ⅱ试剂盒(TaKaRa, 日本),仪器为Lightcycler480ⅡReal-time PCR仪(Roche, 瑞士)。PCR体系(10 μL): TB Green Premix Ex Taq Ⅱ 5 μL,上、下游引物(10 μmol/L)各0.4 μL,ddH2O 3.2 μL,cDNA模板1 μL。PCR扩增条件:95 ℃ 30 s;95 ℃ 5 s,60 ℃ 20 s,共40个循环,反应结束后进行熔解曲线分析以验证产物特异性。所有目的基因和内参基因的标准曲线相关系数(r2)和扩增效率(E):0.99 < (r2) < 0.999,0.9 < E < 1.1。18S为内参基因,基因相对表达量参照2–ΔΔCt法计算(Livak et al, 2001)。
1.5 结果分析使用NCBI数据库(https://www.ncbi.nlm.nih.gov/)查找预测的黄条鰤npy序列,利用软件SignalP5.0 Server (http://www.cbs.dtu.dk/services/SignalP/)分析黄条鰤npy基因的信号肽,通过Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/)进行序列比对和同源性分析,利用软件ExPASy (http://web.expasy.org/ compute_pi/)分析成熟蛋白的分子量和等电点,采用SWISS-MODEL自动化蛋白质建模服务器ProModⅡ程序估计三级蛋白质结构(https://www.expasy.org/ swissmod/SWISS-MODEL.html)。通过MEGA 7.0软件,以邻接法Neighbor-Joining (NJ)构建系统进化树。
实验数据以平均值±标准误(Mean±SE)表示。采用SPSS 26.0统计软件进行T检验、单因素方差分析(one-way ANOVA)和Duncan多重比较,显著性水平P设为0.05,当P < 0.05时认为差异显著,当P < 0.01时认为差异极显著。
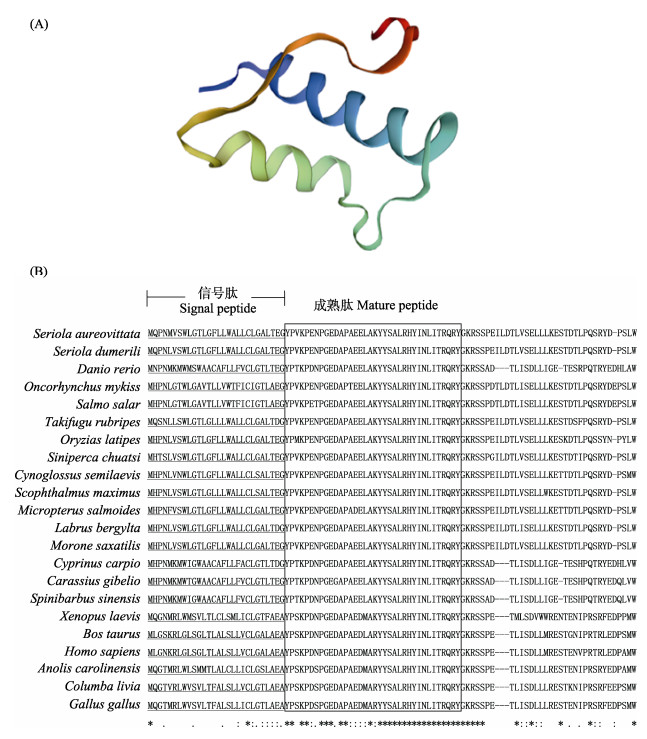
2 结果 2.1 黄条鰤npy cDNA序列克隆、组织分布和系统进化分析黄条鰤npy基因的ORF序列长度为300 bp,编码99个氨基酸,其中,包括28个氨基酸的信号肽,36个氨基酸的成熟肽,32个氨基酸的C端未知功能肽段和1个GKR蛋白水解位点(图 1),预测的蛋白质分子量为11.24 kDa,等电点为5.02。

|
图 1 黄条鰤npy基因ORF序列和推导的氨基酸序列
Fig.1 Sequence of open reading frame and deduced amino acid of S. aureovittata npy gene
起始密码子加框,终止密码子用星号表示。 推定的信号肽加下划线,水解位点用方框表示。 成熟肽标以下划线和粗体字母表示。 The start codon is boxed, and the stop codon is indicated by asterisk. Putative signal peptides are underlined, and proteolytic site is framed. Mature peptides are underlined and indicated in bold letters. |
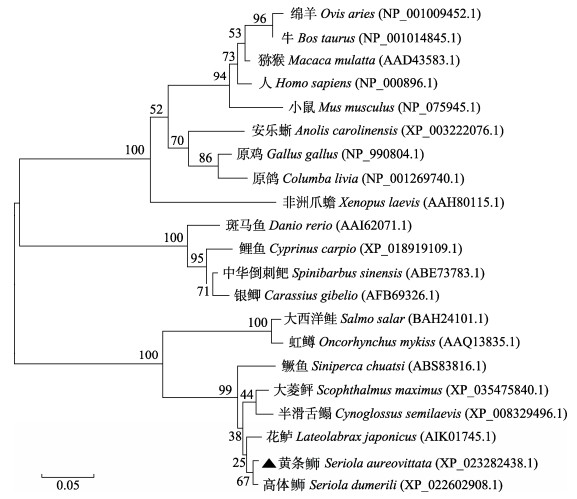
黄条鰤与斑马鱼(Danio rerio)、大西洋鲑等物种的NPY氨基酸序列比对结果见表 2。黄条鰤npy与高体鰤(Seriola dumerili) (99.0%)、条纹鲈鱼(Morone saxatilis) (98.0%)、大口黑鲈(Micropterus salmoides) (96.0%)、大菱鲆(Scophthalmus maximus) (94.9%)的npy表现出高度的同源性,其次是鳜鱼(Siniperca chuatsi)和半滑舌鳎(Cynoglossus semilaevis) (93.9%)、青鳉(Oryzias latipes) (92.9%)。此外,与其他鱼相比,序列相似度也较高(66.3%~80.8%)。npy基因序列的三级结构由2个螺旋区组成,即α螺旋和富含脯氨酸的螺旋区。2个螺旋区是互逆平行的,都有两性电离的特点,且靠疏水键维持稳定(图 2A)。与其他脊椎动物相比较,黄条鰤npy信号肽和成熟肽的结构非常保守,都包含28个氨基酸的信号肽、36个氨基酸的成熟肽、3个脯氨酸和2个酪氨酸残基(Pro2/5/8和Tyr20/27) (图 2B)。系统进化分析表明,黄条鰤npy与高体鰤聚为一个小的分支,且与鲈形目(Perciformes)、鲽形目(Pleuronectiformes)的鱼类聚为一个大的分支(图 3)。
|
|
表 2 黄条鰤与其他脊椎动物npy基因氨基酸序列的同源性分析/% Tab.2 Homology analysis of amino acid sequences of npy gene of S. aureovittata and other vertebrates /% |
|
图 2 黄条鰤NPY的分子特征 Fig.2 Molecular characterization of S. aureovittata NPY A:预测的黄条鰤NPY三级结构;B:黄条鰤npy基因编码的氨基酸序列与其他脊椎动物的比对分析。连字符(–)指示在一些序列中引入的间隙以最大化对齐,相同的序列用星号(*)表示,保守氨基酸用点号(.)表示,高度保守的氨基酸用冒号(: )表示,推定的信号肽标以下划线表示,NPY成熟肽用方框表示。 A: The predicted tertiary structure of NPY in S. aureovittata; B: Alignment of the amino acid sequences of the npy gene between S. aureovittata and other vertebrates. Gaps introduced in some sequences to maximize the alignment are indicated by hyphens. Identical sequences are indicated by asterisks. Dots denote conserved amino acids, and colons indicate highly conserved amino acids. Putative signal peptides are underlined, the mature NPY peptide sequences are boxed. |
|
图 3 黄条鰤npy基因与其他脊椎动物的NJ系统进化树 Fig.3 The phylogenetic tree of npy gene of S. aureovittata and other vertebrates using neighbor-joining method |
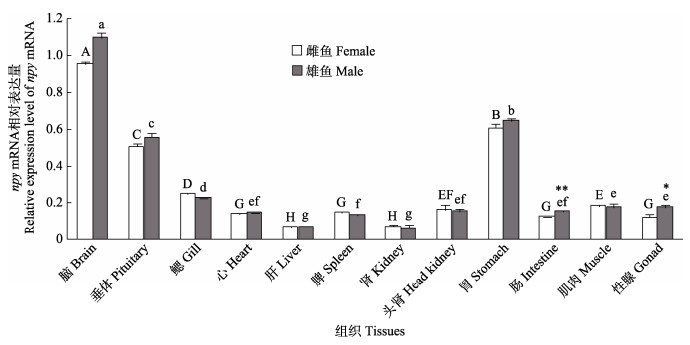
npy在黄条鰤脑、垂体和胃中表达量较高,其他组织少量表达。通过对雌、雄黄条鰤npy的组织表达特性比较发现,在雌鱼和雄鱼的性腺组织中,npy mRNA表达水平呈现显著性差异(P < 0.05),在肠组织中存在极显著性差异(P < 0.01) (图 4)。
|
图 4 黄条鰤npy mRNA的组织表达分布特征 Fig.4 Spatial expression pattern of npy mRNA of S. aureovittata 柱上方相同字母表示差异不显著(P > 0.05),不同小写字母表示雄鱼各个组织之间存在显著性差异(P < 0.05),不同大写字母表示雌鱼各个组织之间存在显著性差异(P < 0.05)。*表示雌鱼和雄鱼在同一组织内存在显著差异(P < 0.05),**表示存在极显著性差异(P < 0.01)。 Above the same column, the same letters indicate no significant difference (P > 0.05), while with different lowercase letters mean significant difference between the various tissues of male fish (P < 0.05), and different uppercase letters mean significant difference between the various tissues of female fish (P < 0.05). * indicates significant difference within the same tissue between female and male fish (P < 0.05), and ** indicates highly significant difference within the same tissue between female and male fish (P < 0.01). |
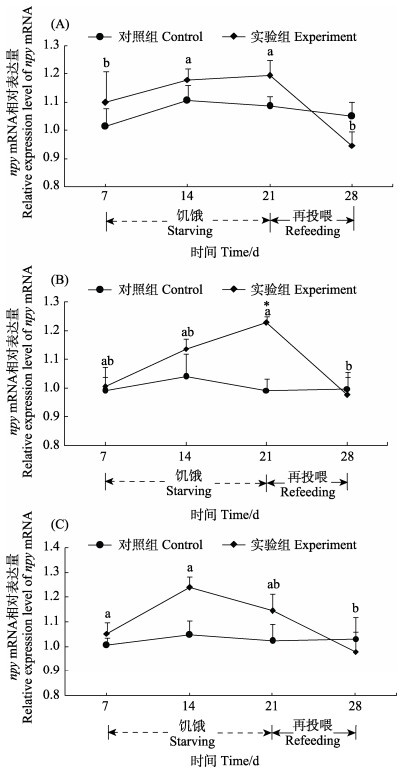
与对照组相比,饥饿组黄条鰤脑、垂体和胃组织中npy mRNA上调表达,恢复投喂后npy mRNA表达水平下降。脑组织中,随着饥饿时间的延长,实验组npy mRNA表达水平有上升的趋势,恢复投喂7 d后恢复到对照组水平(图 5A)。垂体组织中,实验组npy mRNA变化趋势与脑相似,特别是在饥饿21 d,与对照组相比,实验组npy显著高表达(P < 0.05) (图 5B)。实验组胃组织中npy mRNA水平在饥饿14 d时略高于对照组,恢复投喂7 d后略低于对照组(图 5C)。
|
图 5 黄条鰤的脑(A)、垂体(B)和胃(C)中npy mRNA在饥饿再投喂条件下的相对表达 Fig.5 Relative expression of npy mRNA in the brain (A), pituitary (B) and stomach (C) of S. aureovittata after starving and refeeding 不同字母表示组内有显著性差异(P < 0.05),*表示在同一个时间点,对照组和实验组之间存在显著性差异(P < 0.05)。 Different letters indicate significant difference within the group (P < 0.05), and * indicates significant difference between control and experimental groups fish at one given time point (P < 0.05). |
黄条鰤npy基因编码99个氨基酸,这与大口黑鲈(刘浩等, 2016)、牙鲆(Paralichthys olivaceus) (Wang et al, 2015)一致,而银鲫npy基因编码96个氨基酸(Lei et al, 2019),大西洋鲑npy基因编码100个氨基酸(Murashita et al, 2009)。黄条鰤成熟肽中的3个脯氨酸和2个酪氨酸残基(Pro2/5/8和Tyr20/27)在脊椎动物中是非常保守的,这些脯氨酸和酪氨酸残基在维持npy家族的构象中具有重要作用(Cerdá-Reverter et al, 2000)。与其他硬骨鱼类相似,黄条鰤npy的成熟肽C端附近也发现13个氨基酸残基(ALRHYINLITRQR),说明这13个氨基酸残基高度保守(Deng et al, 2019; Lei et al, 2019)。在爬行类、两栖类、哺乳类和硬骨鱼类的NPY成熟肽中不含半胱氨酸(C),只在信号肽第21个氨基酸位点上有一个半胱氨酸(C),在重口裂腹鱼(Deng et al, 2019)、银鲫(Lei et al, 2019)也得到相同结果。通过氨基酸比对发现,黄条鰤与条纹鲈鱼、大口黑鲈分别有2个和3个氨基酸差异,与高体鰤仅有1个氨基酸不同,并且与高体鰤npy编码的氨基酸序列一致性高达99%。另外,构建的npy基因的系统进化树显示,黄条鰤与鲈形目、鲽形目其他鱼类聚为一支,亲缘关系较近。
3.2 黄条鰤npy组织分布特征黄条鰤npy mRNA在大部分组织中均有表达,特别是脑、垂体和胃中有较高的表达,这与银鲫(Lei et al, 2019)、重口裂腹鱼(Deng et al, 2019)、斑马鱼(Kaniganti et al, 2021)的结果一致。本研究还发现,黄条鰤雌鱼和雄鱼肠组织和性腺中npy mRNA的表达有显著性差异,表明npy基因在黄条鰤的组织表达模式具有明显的性别二态性,这与黄条鰤hsp70基因的组织表达模式一致(方璐等, 2023),提示npy基因在雌、雄性腺发育、消化等生理过程中发挥重要的差异化调控作用,具体的功能差异及可能的作用途径有待深入研究确证。目前,在其他硬骨鱼中尚未见npy基因的性别二态性相关报道,根据这一特性,可以在产卵前针对性地对雌、雄亲鱼分别进行营养强化,或可为黄条鰤亲鱼提供差异化强化培育的思路。
3.3 黄条鰤NPY对饥饿再投喂的应答特性摄食调控是由中枢和外周系统协同完成。已有研究证实,NPY行使生理功能主要是通过NPY受体的直接或间接作用来实现。斑马鱼室旁核中的NPY与NPY Y1、NPY Y2或NPY Y5受体结合引发传出信号,激活PKC通路,增加食欲和摄食量(Yokobori et al, 2012)。对大西洋鲑的研究表明,弓状核中的NPY神经元还可以对进入的外周信号如瘦素、胰岛素等因子做出快速反应,信号向下传递到各个组织,刺激胃等消化系统分泌npy mRNA (Kalananthan et al, 2020);同时上调促摄食因子(agrp和ghrelin),下调抑摄食因子(pomc和leptin) (Opazo et al, 2019; Zhang et al, 2020),从而实现对生物体的摄食调控。
本研究中,黄条鰤在饥饿期间,npy mRNA呈现为上调表达,恢复投喂7 d后,npy mRNA表达水平恢复到对照组水平,这与银鲫(Lei et al, 2019)、重口裂腹鱼(Deng et al, 2019)的研究结果相似,表明与其他硬骨鱼一样,黄条鰤npy也参与了摄食调控。
本研究中,饥饿期间,黄条鰤脑和垂体npy mRNA表达量上升,其原因可能是饥饿刺激神经中枢发出促食信号,从而启动了npy mRNA的表达,从而达到增强食欲的作用(Yousefvand et al, 2021);且在恢复投喂7 d后,神经中枢发出饱食信号,使npy mRNA表达量略低于对照组。本研究还发现,黄条鰤饥饿21 d,脑组织中npy mRNA的表达量升高(P > 0.05);Kehoe (2007)等对大西洋鳕(Gadus morhua)研究发现,饥饿7 d,其脑组织中npy mRNA表达变化同本结果一致;然而,Deng (2019)研究发现,重口裂腹鱼饥饿5 d时,其脑组织中npy mRNA出现显著变化。上述研究表明,npy基因在禁食初期出现显著应答的时间不同,这可能是由于物种特异性和环境差异造成。另外,本研究仅检测了脑、垂体和胃组织中npy基因的表达调控特性,未对npy信号通路上其他相关基因如leptin、ghrelin、npyr、pomc、gh和igf等摄食与生长调控因子进行检测及互作关系进行分析,因此,未能进一步揭示npy基因对黄条鰤摄食调控作用的信号通路,下一步本实验室将深入开展相关研究,揭示npy调控黄条鰤摄食作用的信号通路。
胃是动物重要的消化器官,在摄食调控中扮演了重要角色(Abdalla, 2017)。本研究中,与脑、垂体组织一样,黄条鰤胃组织中npy mRNA也存在着明显的变化,表明胃中的npy基因对饥饿再投喂产生了应答,也参与了黄条鰤的摄食调节。Kalananthan等(2020)研究发现,大西洋鲑胃的扩张与肠壁上的内分泌因子和受体之间的相互作用调节着肽激素的分泌,这些肽激素将胃和肠道的饱腹程度以及营养含量传递给中枢系统。本研究中,饥饿再投喂诱导黄条鰤脑、垂体和胃中npy mRNA表达的规律一致,均呈现上升再下降的变化趋势,说明脑、垂体和胃中npy对于饥饿的响应机制是一致的。关于npy基因的调控方式仍需进一步研究,以探明npy基因在黄条鰤饥饿再投喂中的机制,进一步优化黄条鰤的摄食投喂策略。
综上,本研究首次克隆了黄条鰤npy基因的ORF序列并对其结构和表达模式进行了研究。npy转录本主要在脑中显著高表达,其次是垂体和胃,这与其他硬骨鱼类似。饥饿再投喂实验表明,黄条鰤NPY是具有显著促食欲的内分泌因子,其促摄食的分子调控机制值得进一步研究。
ABDALLA M M I. Central and peripheral control of food intake. Endocrine Regulations, 2017, 51(1): 52-70 DOI:10.1515/enr-2017-0006 |
ASSAN D, WANG Y, MUSTAPHA U F, et al. Neuropeptide Y in spotted scat (Scatophagus argus), characterization and functional analysis towards feed intake regulation. Fishes, 2022, 7(3): 111 DOI:10.3390/fishes7030111 |
BERTUCCI J I, BLANCO A M, SUNDARRAJAN L, et al. Nutrient regulation of endocrine factors influencing feeding and growth in fish. Frontiers in Endocrinology, 2019, 10: 83 DOI:10.3389/fendo.2019.00083 |
BOOTH M A, PIROZZI I. The digestible histidine requirement of juvenile yellowtail kingfish Seriola lalandi. Aquaculture, 2022, 548: 737543 DOI:10.1016/j.aquaculture.2021.737543 |
CERDÁ-REVERTER J M, MARTINEZ-RODRIGUEZ G, ZANUY S, et al. Molecular evolution of the neuropeptide Y (NPY) family of peptides: Cloning of three NPY-related peptides from the sea bass (Dicentrarchus labrax). Regulatory Peptides, 2000, 95(1/2/3): 25-34 |
DENG X, LEI L, YUAN D, et al. Cloning, expression profiling, and effects of fasting status on neuropeptide Y in Schizothorax davidi. Journal of Food Biochemistry, 2019, 43(7): e12892 |
FANG L, XU Y J, CUI A J, et al. Molecular cloning and temporal expression pattern of hsp70 gene during the early life stages of Seriola lalandi. Progress in Fishery Sciences, 2023, 44(4): 55-63 [方璐, 徐永江, 崔爱君, 等. 黄条鰤hsp70基因克隆及其在早期生长发育过程中的表达调控特性.. 渔业科学进展, 2023, 44(4): 55-63] |
HORIO N, LIBERLES S D. Hunger enhances food-odour attraction through a neuropeptide Y spotlight. Nature, 2021, 592(7853): 262-266 DOI:10.1038/s41586-021-03299-4 |
KAH O, PONTET A, DANGER J M, et al. Characterization, cerebral distribution and gonadotropin release activity of neuropeptide Y (NPY) in the goldfish. Fish Physiology and Biochemistry, 1989, 7(1): 69-76 |
KALANANTHAN T, MURASHITA K, RØNNESTAD I, et al. Hypothalamic agrp and pomc mRNA responses to gastrointestinal fullness and fasting in Atlantic salmon (Salmo salar L).. Frontiers in Physiology, 2020, 11: 61 |
KANIGANTI T, DEOGADE A, MADUSKAR A, et al. Sensitivity of olfactory sensory neurons to food cues is tuned to nutritional states by Neuropeptide Y signaling. Journal of Neurochemistry, 2021, 159(6): 1028-1044 DOI:10.1111/jnc.15488 |
KEHOE A S, VOLKOFF H. Cloning and characterization of neuropeptide Y (NPY) and cocaine and amphetamine regulated transcript (CART) in Atlantic cod (Gadus morhua). Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 2007, 146(3): 451-460 DOI:10.1016/j.cbpa.2006.12.026 |
LEI L, DENG X, YUAN D, et al. Molecular cloning and function characterization in feeding of neuropeptide Y in Gibel carp, Carassius auratus gibelio. Pakistan Journal of Zoology, 2019, 51(3): 1017-1025 |
LIU H, BAI J J, LI S J. Cloning and analysis of DNA and cDNA sequence in NPY gene of Northern and Florida subspecies of largemouth bass. Biotechnology Bulletin, 2016, 32(5): 99-106 [刘浩, 白俊杰, 李胜杰. 大口黑鲈北方亚种和佛罗里达亚种NPY基因的DNA和cDNA克隆及序列分析. 生物技术通报, 2016, 32(5): 99-106] |
LIU X Z, XU Y J, LI R, et al. Analysis and evaluation of nutritional composition of the muscle of yellowtail kingfish (Seriola aureovittata). Progress in Fishery Sciences, 2017, 38(1): 128-135 [柳学周, 徐永江, 李荣, 等. 黄条鰤(Seriola aureovittata)肌肉营养组成分析与评价. 渔业科学进展, 2017, 38(1): 128-135] |
LIVAK K J, SCHMITTGEN T. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods, 2001, 25(4): 402-408 DOI:10.1006/meth.2001.1262 |
MURASHITA K, KUROKAWA T, EBBESSON L O E, et al. Characterization, tissue distribution, and regulation of agouti-related protein (AgRP), cocaine-and amphetamine- regulated transcript (CART) and neuropeptide Y (NPY) in Atlantic salmon (Salmo salar). General and Comparative Endocrinology, 2009, 162(2): 160-171 DOI:10.1016/j.ygcen.2009.03.015 |
OPAZO R, PLAZA-PARROCHIA F, CARDO G R, et al. Fasting upregulates npy, agrp, and ghsr without increasing ghrelin levels in zebrafish (Danio rerio) larvae. Frontiers in Physiology, 2019, 9: 1901 DOI:10.3389/fphys.2018.01901 |
SOENGAS J L, CERDÁ-REVERTER J M, DELGADO M J. Central regulation of food intake in fish: An evolutionary perspective. Journal of Molecular Endocrinology, 2018, 60(4): R171-R199 DOI:10.1530/JME-17-0320 |
TATEMOTO K, CARLQUIST M, MUTT V. Neuropeptide Y: A novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature, 1982, 296(5858): 659-660 DOI:10.1038/296659a0 |
TOLÅS I, KALANANTHAN T, GOMES A S, et al. Regional expression of npy mRNA paralogs in the brain of Atlantic salmon (Salmo salar, L) and response to fasting.. Frontiers in Physiology, 2021, 12: 720639 |
VOLKOFF H, RØNNESTAD I. Effects of temperature on feeding and digestive processes in fish. Temperature, 2020, 7(4): 307-320 DOI:10.1080/23328940.2020.1765950 |
WANG B, WANG K J, CUI A J, et al. LPXRFa down-regulates brain reproductive genes in yellowtail kingfish (Seriola lalandi). Aquaculture, 2021, 545: 737251 DOI:10.1016/j.aquaculture.2021.737251 |
WANG Q, TAN X, DU S, et al. Characterization, tissue distribution, and expression of neuropeptide Y in olive flounder Paralichthys olivaceus. Chinese Journal of Oceanology and Limnology, 2015, 33(3): 553-558 DOI:10.1007/s00343-015-4090-1 |
XU Y J, ZHANG Z G, LIU X Z, et al. Morphometric characteristics of the embryonic and postembryonic development of yellowtail kingfish, Seriola aureovittata. Journal of Fishery Sciences of China, 2019, 26(1): 172-182 [徐永江, 张正荣, 柳学周, 等. 黄条鰤早期生长发育特征. 中国水产科学, 2019, 26(1): 172-182] |
YOKOBORI E, AZUMA M, NISHIGUCHI R, et al. Neuropeptide Y stimulates food intake in the zebrafish, Danio rerio. Journal of Neuroendocrinology, 2012, 24(5): 766-773 |
YOUSEFVAND S, HAMIDI F. The role of ventromedial hypothalamus receptors in the central regulation of food intake. International Journal of Peptide Research and Therapeutics, 2021, 27(1): 689-702 |
ZHANG L, REED F, HERZOG H. Leptin signalling on arcuate NPY neurones controls adiposity independent of energy balance or diet composition. Journal of Neuroendocrinology, 2020, 32(9): e12898 |



