2. 农业农村部水产品质量安全检测与评价重点实验室 中国水产科学研究院黄海水产研究所 山东 青岛 266071;
3. 唐山市水产技术推广站 河北 唐山 063004
2. Key Laboratory of Testing and Evaluation for Aquatic Product Safety and Quality, Ministry of Agriculture and Rural Affairs; Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China;
3. Tangshan Aquatic Product Technology Extension Station, Tangshan 063004, China
贝类由于滤食海洋有毒藻类导致毒素在体内累积,贝类毒素可直接影响海洋生物的生命活动,威胁海洋生态系统稳定,且毒素可以经食物链传递到贝类以及鱼类等生物体内,危及人类健康和经济安全(Doucette et al, 2006; Munday et al, 2013)。依据食源性中毒症状可以将贝类毒素分为麻痹性贝类毒素(paralytic shellfish poisoning, PSP)、腹泻性贝类毒素(diarrhetic shellfish poisoning, DSP)、神经性贝类毒素(neurotoxic shellfish poisoning, NSP)和记忆缺失性贝类毒素(amnesic shellfish poisoning, ASP)四大类(Visciano et al, 2016; Daranas et al, 2001)。目前的研究主要集中在PSP和DSP。PSP是目前分布最广、危害最大的一种藻毒素,而根据近年来对中国沿海部分海区的调查显示,双壳贝类已经广泛受到DSP的污染(Liu et al, 2017; Li et al, 2014)。
世界多国发生贝类毒素中毒事件。2002年7月,在靠近麦哲伦海峡的巴塔哥尼亚峡湾的两位渔民食用贻贝(Mytilus edulis)导致麻痹性贝类毒素中毒死亡(García et al, 2004)。2011年,3人因食用采自美国华盛顿州塞基姆湾州立公园的贻贝而出现腹泻性贝类中毒症状。对贝类DSP的监测显示,这些毒素在多种贝类中广泛存在且浓度高于安全值(Trainer et al, 2013)。2016年4月,秦皇岛市发生了一起PSP引起的海产品中毒事件(Ding et al, 2017)。2019年5月,唐山市沿海地区陆续出现9例因食用贻贝引起的疑似食源性疾病事件。目前,针对贝类毒素的防控技术及脱除方法研究较少且较难应用于实际生产加工。因此,需要对贝类毒素进行严格的监测管理,从而保护消费者的健康。用于贝类毒素的检测方法主要有小鼠生物法(Turrell et al, 2007)、酶联免疫法(Hu et al, 2013; Sassolas et al, 2013)、高效液相色谱–串联质谱法(Li et al, 2014; Wang et al, 2015)、亲水性色谱串联质谱法(Boundy et al, 2015)和高效液相色谱–荧光检测器法(Lian et al, 2017)等。液相色谱–串联质谱法(LC-MS/MS)能够对单个组分分别定性和定量分析,可取代小鼠生物法,是国际社会监测贝类毒素的重点方法(Cho et al, 2013; Mattarozzi et al, 2016; Berre et al, 2015)。
目前,我国关于贝类毒素检测的相关研究主要集中在预警、调查分析等方面。2011年6月—2012年4月期间对北黄海(獐子岛附近)扇贝中PSP含量检测发现,PSP含量在6—10月明显增加,平均含量在258~432 μg/kg之间(Wu et al, 2018)。有研究对2006年4月—2007年3月东海南麂岛的虾夷扇贝(Patinopecten yessoensis)和贻贝中PSP的检测发现,4月和5月期间,贝类样本的毒素浓度范围为689~ 963 μg STXeq/kg,2006年6—12月未检测到PSP,2007年1—3月毒素含量为189~408 μg STXeq/kg (Jiang et al, 2014)。渤海是半封闭的内海,然而,人类活动和富营养化导致了海洋环境的恶化,进而增加有害藻华的发生(Peng, 2015)。2006—2008年对渤海贝类产品的调查表明,约54%的贝类受到大田软海绵酸(Okadaic acid, OA)类毒素(OA和DTXs)的污染(Liu et al, 2017)。2013—2014年渤海莱山、莱州、汉沽、秦皇岛和葫芦岛5个代表性海水养殖区的大部分贝类样本中检测到PSP,含量在0~27.6 nmol/g之间(Liu et al, 2017)。2016年4月,秦皇岛市发生了一起PSP引起的海产品中毒事件,检测结果显示,致病贻贝体内含有高浓度PSP (约10 758 µg STXeq/kg),包括GTX 1/4和GTX 2/3及其代谢产物(Ding et al, 2017)。
唐山沿海地区是我国重要的贝类养殖区。迄今为止,在唐山贝类养殖区缺少对DSP和PSP的系统性监测,且关于贝类毒素对人类健康影响的风险评估研究较少。本研究通过高效液相色谱–串联质谱(HPLC- MS/MS)法,对2019年10月—2020年9月从渤海湾唐山海域采集的四角蛤(Mactra veneriformis)、菲律宾蛤仔(Ruditapes philippinarum)、脉红螺(Rapana venosa)、牡蛎(Crassostrea gigas)、青蛤(Cyclina sinensis)、文蛤(Meretrix meretrix)和硬壳蛤(Mercenaria mercenaria) 7种代表性的经济贝类中5种腹泻性贝类毒素和14种麻痹性贝类毒素组成成分和含量进行分析。采用风险熵值法和点评估方法对贝类的食用安全性进行风险评估,以期查明贝类中毒素的情况,防止食用贝类中毒事件的再次发生,为加强该区域贝类食用管理、有效保障贝类食用安全提供科学依据。
1 材料与方法 1.1 采样区域贝类样品采集地点在河北唐山市乐亭县姜各庄镇南部浅海养殖区(39°44.672′N, 119°12.764′E)和曹妃甸区柳赞镇十里海养殖区(39°15.473′N, 118°68.662′E)。
1.2 样品采集与处理样品采集时间为2019年10月—2020年9月。其中,2020年4—9月每月4次,其他月份每月2次。主要采集的贝类品种为四角蛤、菲律宾蛤仔、脉红螺、牡蛎、青蛤、文蛤和硬壳蛤7种,每种贝类的样本量为34份,且每份样品的重量约为3 kg。运输过程0~4 ℃保存,用自来水清洗表面泥沙后撬壳取全部可食性软体组织,用匀浆机将贝类软体组织均质混匀,于–20 ℃冰箱中保存,待测。
1.3 检测方法 1.3.1 腹泻性贝类毒素测试方法前处理:准确称取2 g (精确至0.01 g)样品于50 mL离心管中,加入4.5 mL甲醇,涡旋混匀,超声提取10 min,4 500 r/min离心5 min,移出上清液于15 mL离心管中。残渣中加入4.5 mL甲醇重复提取一次,合并提取液,用水定容至10 mL。准确吸取提取液1 mL加入到125 μL 2.5 mol/L NaOH溶液,混匀后,于76 ℃下温育40 min,冷至室温,加入125 μL 2.5 mol/L HCl溶液并混匀,所得水解液用0.22 μm滤膜过滤,供液相色谱–串联质谱测定。
仪器条件:色谱柱:XB-C18柱(100.0 mm×2.1 mm,2.6 μm);流动相:A为水(含5 mmol/L甲酸铵,0.1%甲酸)溶液,B为乙腈,梯度洗脱程序见表 1;流速:0.30 mL/min;进样量:10 μL;柱温:35 ℃。质谱离子源:电喷雾离子源;检测方式:多反应检测模式;离子源温度:350 ℃;鞘气电压:40 Arb;辅助气压力:10 Arb;喷雾电压:正离子为3 500 V,负离子为3 000 V;OA、DTX 1、DTX 2、YTX和AZA 1母离子、子离子和碰撞能量见表 2。
|
|
表 1 腹泻性贝类毒素梯度洗脱程序 Tab.1 Gradient elution procedure of diarrhetic shellfish poisoning (DSP) |
|
|
表 2 5种腹泻性贝类毒素的母离子、子离子和碰撞能量 Tab.2 Precursor ions, product ions and collision energy for five diarrhetic shellfish poisoning (DSP) |
前处理:准确称取5 g (精确至0.01 g)样品于50 mL离心管中,加入5 mL 0.5%甲酸溶液,涡旋混匀,超声提取10 min,4 500 r/min离心10 min,移出上清液至15 mL离心管中,残渣中再加入4.0 mL 0.5%甲酸溶液重复提取2次,合并上清液,用0.5%甲酸溶液定容至15 mL。取6 mL提取液至15 mL离心管,加入6 mL乙酸乙酯,涡旋混合30 s,4 500 r/min离心10 min,弃去上层液。再向提取液中加入6 mL三氯甲烷,涡旋混合30 s,4 500 r/min离心10 min;取3 mL上层溶液,加入已活化的HLB固相萃取柱中,收集流出液至10 mL离心管中,再加1 mL 0.5%甲酸溶液至固相萃取柱中,收集流出液。向流出液中加入乙腈定容至8 mL,混匀后放置5 min,10 000 r/min离心10 min,取1 mL上清液至1.5 mL离心管中,10 000 r/min离心10 min,用0.22 μm滤膜过滤,供液相色谱–串联质谱测定。
仪器条件:色谱柱:TSK gel Amide-80柱(2.0 mm× 15.0 cm,3.0 μm);流动相:A为水(含5 mmol/L甲酸铵,0.1%甲酸)溶液,B为乙腈,梯度洗脱程序见表 3;流速:0.30 mL/min,进样量:10 μL,柱温:35 ℃。质谱离子源:电喷雾离子源;检测方式:多反应检测模式;离子源温度:320℃;鞘气电压:40 Arb;辅助气压力:10 Arb;喷雾电压:正离子为4 000 V,负离子为3 500 V;14种麻痹性贝类毒素母离子、子离子和碰撞能量见表 4。
|
|
表 3 麻痹性贝类毒素梯度洗脱程序 Tab.3 Gradient elution procedure of paralytic shellfish poisoning (PSP) |
|
|
表 4 14种麻痹性贝类毒素母离子、子离子和碰撞能量 Tab.4 Precursor ions, product ions and collision energy of 14 paralytic shellfish poisoning (PSP) |
腹泻性贝类毒素标准曲线的线性范围为5~200 ng/mL,相关系数在0.995以上;方法检出限为5 μg/kg (S/N > 3),选用空白牡蛎样品进行3个不同浓度的加标实验(20、50和100 μg/kg,n=6),回收率均在70%~120%之间。麻痹性贝类毒素标准曲线的线性范围为4~200 ng/mL,相关系数在0.995以上;方法检出限为10~20 μg/kg (S/N > 3),其中,STX、NEO、dcSTX、dcNEO、GTX 5为20.0 µg/kg,GTX 1/4、GTX 2/3/6、dcGTX 2/3、C 1/2为10 µg/kg,选用空白牡蛎样品进行3个不同浓度的加标实验(50、100和200 μg/kg,n=6),回收率均在70%~120%之间。
2 结果与讨论 2.1 渤海海域唐山贝类养殖区贝类样品污染状况分析 2.1.1 贝类样品毒素成分及含量分析2019年10月—2020年9月唐山贝类养殖区7种贝类样品中均未检出DSP。检出的PSP中STX、GTX 1、GTX 2、dcGTX 3毒素含量变化范围分别为0~57.39、0~537.95、0~183.59和0~135.29 μg/kg (图 1),其中,GTX 1检出率和含量皆较高;NEO、dcSTX、GTX 4、GTX 3、GTX 5、GTX 6、dcNEO、dcGTX 2和C 1/2未检出。2008—2009年中国北方沿海采集的有毒虾夷扇贝和紫石房蛤(Saxidomus purpuratus)样品中存在C 1、GTX 1/4和GTX 2/3,其中C 1毒素含量最高(Li et al, 2012),与本研究结果具有一定差异。有研究发现,在温度、盐度、光照及营养等适宜的条件下,贝类体内毒素之间会发生化学转化或酶促转化,贝类摄食有毒藻后,部分C毒素在体内可转化成GTX毒素,故贝类体内毒素种类的差异性可能与其内在转化有关(Wu et al, 2018; Escobedo-Lozano et al, 2012)。我国近岸水体中的太平洋亚历山大藻(Alexandrium pacificum)和链状亚历山大藻(A. catenella)含有较高比例的C毒素,GTX 1/4和GTX 2/3比例也较高,微小亚历山大藻(A. minutum)则通常只含有GTX 1/4和GTX 2/3,C和STX毒素极少见(Liu et al, 2022),奥氏亚历山大藻(A. ostenfeldii)产自渤海并生产STX和NEO(Gu et al, 2022),链状裸甲藻(Gymnodinium catenatum)和太平洋亚历山大藻的细胞也存在于渤海(Gao et al, 2015, Liuet al, 2017)。本研究检测得到,唐山贝类养殖区贝类PSP成分为STX、GTX 1、GTX 2和dcGTX 3,与现有研究报道的渤海海域产生PSP的藻类中毒素组分具有一定的差异。Liu等(2017)研究发现,贝类毒素与在同一地点采集的浮游植物样品中的毒素组分有较大不同,浮游植物样品中以C 1/2为主,贝类样品中以NEO、STX和GTX 1/4等强效毒素为主,这与本研究结果具有一致性。2019年唐山沿海地区疑似出现赤潮,对疑似赤潮地区的浮游植物调查显示,亚历山大藻属的浓度处于较高水平(表 5) (马国臣, 2020),这与本研究中2019—2020年渤海海域唐山贝类养殖区7种经济贝类PSP含量较高的结果一致。
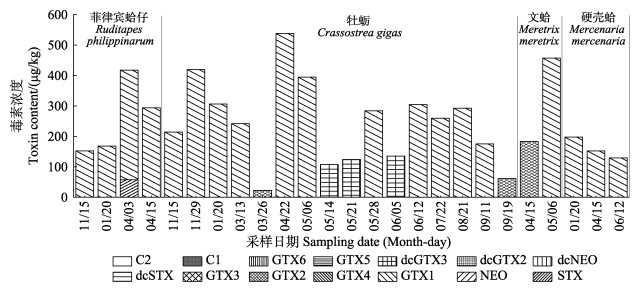
|
图 1 阳性贝类样品中麻痹性贝类毒素组分比较 Fig.1 Comparison of paralytic shellfish toxin components in positive shellfish samples |
|
|
表 5 2019年渤海赤潮地区浮游植物调查(马国臣, 2020) Tab.5 Phytoplankton monitoring results of coastal red tide survey in Bohai Sea in 2019 (Ma, 2020) |
7种贝类样品中菲律宾蛤仔、牡蛎、文蛤和硬壳蛤的检出率分别为11.76%、47.06%、5.90%和8.82%;四角蛤、海螺和青蛤均未检出(图 2),说明牡蛎对PSP吸收蓄积能力较强。利用公式1,通过毒性当量因子(toxicity equivalency factors, TEF)(表 6)计算贝类样品中PSP的总量,得到菲律宾蛤仔、牡蛎、文蛤和硬壳蛤阳性样品中PSP最高含量分别为414.26、532.57、452.77和195.46 μg STXeq/kg,即牡蛎 > 文蛤 > 菲律宾蛤仔 > 硬壳蛤(图 3)。Turner等(2015)研究发现,不同品种贝类的检出率及含量差异是由于贝类对有毒藻类滤食、吸收、转化以及积累毒素的能力不同,贝类毒素含量与贝类对有毒藻的摄食行为、对有毒藻毒素的吸收和代谢能力等因素有关,同时也与海水中有毒微藻的种类、毒性、丰度及接触有毒藻的时间长短等有关(Chen et al, 2013; Bricelj et al, 2005)。毒素毒力值(STXeq)计算公式如下:
| $ \text{STX}_{\text{eq}} ({\mathtt{μ}}\text{g}/\text{kg})=X_{n}×r_{n}$ | (1) |
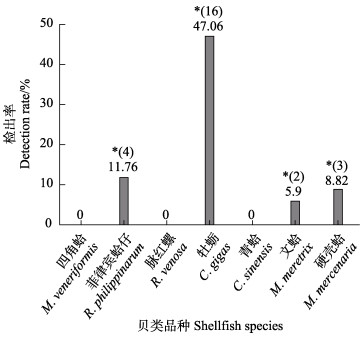
|
图 2 7种贝类样品中麻痹性贝类毒素的检出率(n=34,*表示阳性样品数) Fig.2 Detection rate of paralytic shellfish toxins in seven shellfish species (n=34, * indicating the number of positive samples) |
|
|
表 6 麻痹性贝类毒素毒性当量因子 Tab.6 Toxicity equivalence factors of paralytic shellfish poisoning toxins |

|
图 3 7种阳性贝类样品中麻痹性贝类毒素毒力值比较(虚线表示PSP的监管标准) Fig.3 Comparison of toxicity values of paralytic shellfish toxin in the seven positive shellfish samples (Dotted line indicates the PSP regulatory standard level) |
式中,Xn代表不同种类PSP的含量(μg/kg);rn代表毒性因子。
2.1.3 贝类毒素季节变化特征大部分阳性贝类样品集中出现在4—6月(图 4),与现有报道渤海湾PSP主要在4、5月显示出高含量的结论一致(Ding et al, 2017)。据报道,2019年5月,唐山市沿海地区陆续出现9例因食用贻贝引起的疑似食源性疾病事件,唐山市疾控中心对曹妃甸区及丰南、乐亭等周边沿海地区生产和销售的贻贝等双壳贝类进行DSP和PSP监测,检测结果显示,3份贻贝中GTX 4毒素含量超过检出限(20.0 μg/kg),分别为94、53和50 μg STXeq/kg,毒性较低。Ding等(2017)对2016年5月发生在秦皇岛的一起疑似PSP中毒事件后的现场贻贝样品进行了检测,结果显示,GTX 1/4和GTX 2/3有检出,未检测到C 1/2、C 3/4、GTX 5/6、dcGTX 2/3、dcGTX 1/4、dcSTX和dcNEO。2014—2016年期间对江苏省沿海一带(启东、如东、东台、大丰及赣榆县)的PSP进行的全年监测中,同样发现5月PSP浓度最高(Wang et al, 2019)。毒素浓度的季节变化可能与海水中有毒微藻的种类、毒性和丰度以及贝类暴露于有毒藻类的持续时间有关,而浮游藻类群落与水环境密切相关,受诱发赤潮的环境因素(如温度、酸碱度和营养物质)影响(David et al, 2009),其中4—6月的水温(20 ℃左右)对塔玛亚历山大藻的生长最为适宜(Liu et al, 2020)。在过去的20年里,人们对采自渤海的浮游植物的污染状况进行了调查,从5个海水养殖区采集的20个浮游植物样本中,有13个样品中检测到毒素,其中6月和9月采集的样品中毒素含量(以nmol/L海水计)远高于11月和12月采集的样品,这反映了有毒藻类可能在春季和秋季增殖(Liu et al, 2017)。此外,浮游植物PSP的季节变化规律与渤海赤潮的发生相对应,赤潮多发生在5月和10月(Zhao et al, 2005)。

|
图 4 7种贝类阳性样品中麻痹性贝类毒素总量比较 Fig.4 Total amount of paralytic shellfish toxins in positive samples from seven shellfish species samples |
贝类中PSP污染问题已经是全球性问题,多个国家近海贝类中均检出多种PSP,含量较高,污染严重(Goya et al, 2020; Numano et al, 2019)。但世界各地贝类中检出的PSP组分及含量存在较大差异,地域性差异明显。Goya等(2020)对1980—2012年在阿根廷5个沿海区域采集的贝类样品进行了检测,结果显示,毒素分布以GTX 1/4和GTX 2/3为主,其次是C 1/2、STX和dcGTX 2/3。本研究检测得到渤海湾唐山海域PSP总量最高浓度为532.57 μg STXeq/kg,其中GTX 1含量最高,而大亚湾贝类中PSP浓度最高水平为14, 015 μg STXeq/kg (Wang et al, 2011),二者结果差异较大,后者PSP浓度远高于渤海湾唐山海域,据报道,在南海大鹏湾和大亚湾、东海长江口附近的沿海水域和黄海北部出现了太平洋亚历山大藻和链状亚历山大藻等浮游植物的大量繁殖(Wang et al, 2009; Gao et al, 2015),这可能是导致当地贝类蓄积毒素的主要原因之一。对比研究发现,来自不同海域的贝类毒素谱、含量及季节变化均不同,不同海域PSP优势藻种的差异是造成PSP污染地域性差异的主要原因之一(Finnis et al, 2017; Wang et al, 2020)。
2.2 渤海海域唐山贝类养殖区贝类食用安全风险评估根据国内外研究及生态风险评估方法(Kalf et al, 1997; Tongo et al, 2017),本研究分别采用风险熵值法(risk quotients,RQ)和点评估法进行贝类食用安全的风险评估。其中,风险熵值法具有可操作性强和结论明确的特点。点评估模型是一种膳食暴露评估方法,所得结果的代表性与适用性取决于评估假设的前提和数据的充分性。
2.2.1 风险熵值法根据实际监测到或由模型估算出的样品中贝类毒素浓度与判断贝毒的毒性数据进行比较(最大允许限量),计算出贝毒暴露风险熵(公式2),国际通用安全阈值为RQ=1。限量标准参照欧盟规定的800 μg STXeq/kg对PSP进行风险评估,通过计算得到渤海海域唐山贝类养殖区采集到的贝类样品中PSP的RQ值均小于1 (图 5),表明该时间段内此区域中贝类存在较小的食用风险。由于全年的贝类样品中均未检出DSP,表明DSP不是主要污染毒素,不存在潜在食用安全风险。
| $ \text{RQ}=\frac{总毒素含量}{限量标准含量} $ | (2) |

|
图 5 不同季节贝类食用安全风险 Fig.5 Safety risks of shellfish consumption in different seasons |
采用点评估模型对渤海湾唐山海域贝类食用安全进行风险评估。通过计算每人每天单位体质量毒素摄入量(daily toxin intake,DTI)(公式3),将DTI与急性毒性参考剂量(acute reference doses,ARfD)比较,根据公式4,计算暴露风险指数(exposure risk index,ERI):若ERI≤1,表明暴露风险可接受;若ERI > 1,表明暴露风险超过限度,需要启动风险管理程序。毒素ARfD值参照欧洲食品安全局(European Food Safety Authority,EFSA),STX毒素组的ARfD值为0.5 μg STXeq/kg b.w,设定成年人体质量60 kg (EFSA, 2009)。根据本次抽检样品中麻痹性贝类毒素的含量计算贝类一次性安全食用量(表 7)。结果显示,4—6月贝类富集毒素含量较高,建议减少贝类一次性食用量。
| $ \begin{array}{l}毒素摄入量(\text{DTI})=\\ \frac{毒素含量({\mathtt{μ}}\text{gSTX}\text{eq/kg})\times 食用量(\text{kg/d})}{体质量(\text{kg})}\end{array} $ | (3) |
| $ 暴露风险指数\text{(ERI})=\text{DTI}/\text{ARfD} $ | (4) |
|
|
表 7 推荐贝类一次性安全摄入量 Tab.7 Recommended safe single intake of shellfish |
由于贝类不同组织中累积的毒素水平可能存在很大差异,因此,对样品的每个组织分别进行了分析。结果表明,在贝类所有组织中内脏团的贝类毒素的含量最高,这与Wong等(2009)的研究结果一致。根据公式3和4计算不同贝类组织的一次性安全食用量(表 8)。结果表明,在贝类毒素积累量较大的季节,建议减少贝类内脏团的一次性食用量。
|
|
表 8 不同贝类组织推荐一次性安全摄入量 Tab.8 Recommended safe single intake of different shellfish tissues |
本研究采用高效液相色谱–串联质谱(HPLC-MS/MS)法测试了渤海海域唐山贝类养殖区2019年10月—2020年9月间7种贝类样品中贝类毒素的组分和含量,并采用风险熵值法和点评估法进行食用安全风险评估。结果表明,全年的贝类样品中均未检出DSP,表明DSP不是主要污染毒素,不存在潜在食用安全风险。PSP检出成分包括STX、GTX 1、GTX 2和dcGTX 3,主要成分为GTX 1;未检出NEO、dcSTX、GTX 3、GTX 4、GTX 5、GTX 6、dcNEO、dcGTX 2和C 1/2。PSP主要集中在4—6月检出,贝类毒素组成及成分差异与贝样的采样地点、时间等综合因素有关。此区域贝类中的毒素含量均低于欧盟限量标准(800 μg STXeq/kg),安全风险评估结果表明,该地区7种贝类不存在食用安全风险,根据膳食暴露评估模型给出建议一次性摄入量。然而,根据我国沿海贝类毒素污染情况调查分析,后期将在更广空间范围内进行长期持续监测,以保障贝类养殖业的发展和消费者健康。
BERRE M L, KILCOYNE M, KANE M. Generation of a panel of high affinity antibodies and development of a biosensor- based immunoassay for the detection of okadaic acid in shellfish. Toxicon, 2015, 103: 169-175 DOI:10.1016/j.toxicon.2015.06.030 |
BOUNDY M J, SELWOOD A I, HARWOOD D T, et al. Development of a sensitive and selective liquid chromatography-mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. Journal of Chromatography A, 2015, 1387: 1-12 DOI:10.1016/j.chroma.2015.01.086 |
BRICELJ V M, CONNELL L, KONOKI K, et al. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature, 2005, 434(7034): 763-767 DOI:10.1038/nature03415 |
CHEN J H, YU R C, GAO Y, et al. Tracing the origin of paralytic shellfish toxins in scallop Patinopecten yessoensis in the northern Yellow Sea. Food Additives and Contaminants, 2013, 30(11): 1933-1945 DOI:10.1080/19440049.2013.838644 |
CHO Y K, OZEKI R, YOTSU-YAMASHITA M, et al. Single- cell analysis of paralytic shellfish toxins in Alexandrium tamarense by HPLC with post-column fluorescent derivatization. Harmful Algae, 2013, 25: 47-53 DOI:10.1016/j.hal.2013.02.005 |
DARANAS A H, NORTE M, FERNÁNDEZ J J. Toxic marine microalgae. Toxicon, 2001, 39: 1101-1132 DOI:10.1016/S0041-0101(00)00255-5 |
DAVID C P C, MARIA Y Y S, SIRINGAN F P, et al. Coastal pollution due to increasing nutrient flux in aquaculture sites. Environmental Geology, 2009, 58(2): 447-454 DOI:10.1007/s00254-008-1516-5 |
DING L, QIU J B, LI A F. Proposed biotransformation pathways for new metabolites of paralytic shellfish toxins based on field and experimental mussel samples. Journal of Agricultural and Food Chemistry, 2017, 65(27): 5494-5502 DOI:10.1021/acs.jafc.7b02101 |
DOUCETTE G, MANEIRO I, RIVEIRO I, et al. Phycotoxin pathways in aquatic food webs: Transfer, accumulation and degradation. Ecology of Harmful Algae, 2006, 189: 283-295 |
EFSA. Scientific opinion of the panel on contaminants in the food chain on a request from the European commission on marine biotoxins in shellfish-summary on regulated marine biotoxins. EFSA Journal, 2009, 1306: 1-23 |
ESCOBEDO-LOZANO A Y, ESTRADA N, ASCENCIO F, et al. Accumulation, biotransformation, histopathology and paralysis in the pacific calico scallop Argopecten ventricosus by the paralyzing toxins of the dinoflagellate Gymnodinium catenatum. Marine Drugs, 2012, 10: 1044-1065 DOI:10.3390/md10051044 |
FINNIS S, KRSTIC N, MCINTYRE L, et al. Spatiotemporal patterns of paralytic shellfish toxins and their relationships with environmental variables in British Columbia, Canada from 2002 to 2012. Environmental Research, 2017, 156: 190-200 DOI:10.1016/j.envres.2017.03.012 |
GAO Y, YU R C, CHEN J H, et al. Distribution of Alexandrium fundyense and A. pacificum (Dinophyceae) in the Yellow Sea and Bohai Sea. Marine Pollution Bulletin, 2015, 96: 210-219 DOI:10.1016/j.marpolbul.2015.05.025 |
GARCI´A C, BRAVO M, LAGOS M, et al. Paralytic shellfish poisoning: Post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon, 2004, 43(2): 149-158 DOI:10.1016/j.toxicon.2003.11.018 |
GOYA A B, TARNOVIUS S, HATFIELD R G, et al. Paralytic shellfish toxins and associated toxin profiles in bivalve mollusc shellfish from Argentina. Harmful Algae, 2020, 99(101910): 1-17 |
GU H F, WU Y R, LÜ S H, et al. Emerging harmful algal bloom species over the last four decades in China. Harmful Algae, 2022, 111: 1-10 |
HU L, LIU J, WANG Q, et al. Development of an immunochromatographic strip test for the rapid detection of okadaic acid in shellfish sample. Journal of Applied Phycology, 2013, 25(4): 1091-1099 DOI:10.1007/s10811-012-9949-3 |
JIANG T, XU Y, LI Y, et al. Seasonal dynamics of Alexandrium tamarense and occurrence of paralytic shellfish poisoning toxins in bivalves in Nanji Islands, East China Sea. Marine and Freshwater Research, 2014, 65(4): 350-358 DOI:10.1071/MF13001 |
KALF D F, CROMMENTUIJN T, PLASSCHE E. Environmental quality objectives for 10 polycyclic aromatic hydrocarbons (PAHs). Ecotoxicology and Environmental Safety, 1997, 36(1): 89-97 DOI:10.1006/eesa.1996.1495 |
LI A, MA J, CAO J, et al. Analysis of paralytic shellfish toxins and their metabolites in shellfish from the North Yellow Sea of China. Food Additives and Contaminants, 2012, 29: 1455-1464 DOI:10.1080/19440049.2012.699005 |
LI X, LI Z Y, CHEN J H, et al. Detection, occurrence and monthly variations of typical lipophilic marine toxins associated with diarrhetic shellfish poisoning in the coastal seawater of Qingdao City, China. Chemosphere, 2014, 111: 560-567 DOI:10.1016/j.chemosphere.2014.05.006 |
LIAN Z, WANG J. Selective isolation of gonyautoxins 1, 4 from the dinoflagellate Alexandrium minutum based on molecularly imprinted solid-phase extraction. Marine Pollution Bulletin, 2017, 122(1/2): 500-504 |
LIU M, KROCK B, YU R, et al. Co-occurrence of Alexandrium minutum (Dinophyceae) ribotypes from the Chinese and Malaysian coastal waters and their toxin production. Harmful Algae, 2022, 115: 102238 DOI:10.1016/j.hal.2022.102238 |
LIU Y, DAI L, CHEN Z F, et al. Spatiotemporal variation of paralytic shellfish toxins in the sea area adjacent to the Changjiang River estuary. Environmental Pollution, 2020, 259: 1-8 |
LIU Y, YU R C, KONG F Z, et al. Lipophilic marine toxins discovered in the Bohai Sea using high performance liquid chromatography coupled with tandem mass spectrometry. Chemosphere, 2017, 183: 380-388 DOI:10.1016/j.chemosphere.2017.05.073 |
LIU Y, YU R C, KONG F Z, et al. Paralytic shellfish toxins in phytoplankton and shellfish samples collected from the Bohai Sea, China. Marine Pollution Bulletin, 2017, 115(1/2): 324-331 |
MA G C. Tracking and analysis of red tide in Tangshan coastal area in 2019, China. Hebei Fisheries, 2020(6): 41-45 [马国臣. 2019年唐山沿海赤潮跟踪调查及分析. 河北渔业, 2020(6): 41-45 DOI:10.3969/j.issn.1004-6755.2020.06.012] |
MATTAROZZI M, MILIOLI M, BIANCHI F, et al. Optimization of a rapid QuEChERS sample treatment method for HILIC-MS2 analysis of paralytic shellfish poisoning (PSP) toxins in mussels. Food Control, 2016, 60: 138-145 DOI:10.1016/j.foodcont.2015.07.027 |
MUNDAY R, REEVE J. Risk assessment of shellfish toxins. Toxins, 2013, 5(11): 2109-2137 DOI:10.3390/toxins5112109 |
NUMANO S, KUDO Y, CHO Y, et al. Temporal variation of the profile and concentrations of paralytic shellfish toxins and tetrodotoxin in the scallop, Patinopecten yessoensis, cultured in a bay of East Japan. Marine Drugs, 2019, 17: 2-17 |
PENG S. The nutrient, total petroleum hydrocarbon and heavy metal contents in the seawater of Bohai Bay, China: Temporal–spatial variations, sources, pollution statuses, and ecological risks. Marine Pollution Bulletin, 2015, 95(1): 445-451 DOI:10.1016/j.marpolbul.2015.03.032 |
SASSOLAS A, CATANANTE G L, HAYAT A, et al. Improvement of the efficiency and simplification of ELISA tests for rapid and ultrasensitive detection of okadaic acid in shellfish. Food Control, 2013, 30: 144-149 DOI:10.1016/j.foodcont.2012.05.028 |
TONGO I, EZEMONYE L, AKPEH K. Distribution, characterization, and human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in Ovia River, Southern Nigeria. Environmental Monitoring and Assessment, 2017, 189: 247 DOI:10.1007/s10661-017-5931-5 |
TRAINER V, MOORE L, BILL B, et al. Diarrhetic shellfish toxins and other lipophilic toxins of human health concern in Washington State. Marine Drugs, 2013, 11: 1815-1835 DOI:10.3390/md11061815 |
TURNER A D, MCNABB P S, HARWOOD D T, et al. Single- laboratory validation of a multitoxin ultra-performance LC-Hydrophilic interaction LC-MS/MS method for quantitation of paralytic shellfish toxins in bivalve shellfish. Journal of AOAC International, 2015, 98: 609-621 DOI:10.5740/jaoacint.14-275 |
TURRELL E A, STOBO L. A comparison of the mouse bioassay with liquid chromatography-mass spectrometry for the detection of lipophilic toxins in shellfish from Scottish waters. Toxicon-Oxford, 2007, 50(3): 442-447 DOI:10.1016/j.toxicon.2007.04.002 |
VISCIANO P, SCHIRONE M, BERTI M, et al. Marine biotoxins: Occurrence, toxicity, regulatory limits and reference methods. Frontiers in Microbiology, 2016, 7: 1-10 |
WANG H, HU Z, SHANG L, et al. Toxicity comparison among four strains of Margalefidinium polykrikoides from China, Malaysia, and USA (belonging to two ribotypes) and possible implications. Journal of Experimental Marine Biology and Ecology, 2020, 524: 151293 DOI:10.1016/j.jembe.2019.151293 |
WANG J H, WU J Y. Occurrence and potential risks of harmful algal blooms in the East China Sea. Science of the Total Environment, 2009, 407: 4012-4021 DOI:10.1016/j.scitotenv.2009.02.040 |
WANG X Z, WU H, CHENG Y, et al. Multi-year assessment of paralytic shellfish toxins in hard clam species along the coastline of Jiangsu Province, China. Acta Oceanologica Sinica, 2019, 38: 24-33 |
WANG Z H, NIE X P, JIANG S J, et al. Source and profile of paralytic shellfish poisoning toxins in shellfish in Daya Bay, South China Sea. Marine Environmental Research, 2011, 72(1/2): 53-59 |
WANG Z, BROADWATER M H, RAMSDELL J S. Analysis of diarrhetic shellfish poisoning toxins and pectenotoxin-2 in the bottlenose dolphin (Tursiops truncatus) by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 2015, 1416: 22-30 DOI:10.1016/j.chroma.2015.08.066 |
WONG C K, HUNG P, LEE K L H, et al. Effect of steam cooking on distribution of paralytic shellfish toxins in different tissue compartments of scallops Patinopecten yessoensis. Food Chemistry, 2009, 114(1): 72-80 DOI:10.1016/j.foodchem.2008.09.018 |
WU H Y, LUAN Q S, GUO M M, et al. Phycotoxins in scallops (Patinopecten yessoensis) in relation to source, composition and temporal variation of phytoplankton and cysts in North Yellow Sea, China. Marine Pollution Bulletin, 2018, 135: 1198-1204 DOI:10.1016/j.marpolbul.2018.08.045 |
ZHAO L, WEI H. The influence of physical factors on the variation of phytoplankton and nutrients in the Bohai Sea. Journal of Oceanography, 2005, 61: 335-342 DOI:10.1007/s10872-005-0044-0 |



