2. 广东泽和诚生物技术有限公司 广东 广州 510225
2. Guangdong Zehecheng Biotechnology Co., Ltd., Guangzhou 510225, China
花鲈(Lateolabrax maculatus)具有适应性广、肉鲜味美、营养丰富与生长快速等优质特性,2021年养殖产量已高达19.91万t (胡鹏莉等, 2019; 农业农村部渔业渔政管理局等, 2022)。然而,日益增加的需求和有限的海洋资源供应之间的矛盾,导致近年来鱼粉价格飞涨,成为制约花鲈产业发展的因素之一。因此,为了满足未来发展需求,寻找替代鱼粉的可持续优质蛋白质源已成为当下花鲈养殖业的迫切任务。
黄粉虫(Tenebrio molitor)具有蛋白含量高(47%~ 54%)、氨基酸组成均衡以及免疫活性物质丰富等优势(Lee et al, 1995; Ng et al, 2001; 阮华波, 2012; Kumar et al, 2019),是一种易于大量获得的优质昆虫蛋白源,有望成为饲料中鱼粉的理想替代物(Ghaly et al, 2009; Iaconisi et al, 2017)。已有研究表明,当黄粉虫粉替代鱼粉比例低于16%时,能够促进岩鱼(Sebastes schlegeli)幼鱼的生长(Khosravi et al, 2018)。当黄粉虫粉替代鱼粉比例较低(20%~30%)时,对虹鳟(Oncorhynchus mykiss)(Gasco et al, 2014; Belforti et al, 2016)及金头鲷(Sparus aurata) (Piccolo et al, 2017)的生长性能没有负面影响,并且可以增强丁鳜(Tinca tinca)和虹鳟肠道的抗氧化能力(Hidalgo et al, 2022; Henry et al, 2018)。但当饲料中黄粉虫粉替代鱼粉的比例过高时,会出现抑制鱼类营养物质消化吸收能力(Belforti et al, 2016; Piccolo et al, 2017)和抗氧化能力(Sánchez-Muros et al, 2016; Song et al, 2018),甚至降低鱼类生长性能(Gasco et al, 2016; Hidalgo et al, 2022)等问题。此外,黄粉虫粉脂肪含量最高可达34%,导致在饲料制作过程中无法长期保存,为了兼顾加工工艺和高蛋白需求,往往对黄粉虫粉进行脱脂处理(Henry et al, 2015)。研究表明,脱脂黄粉虫粉在虹鳟饲料中替代鱼粉水平可高达100%(Rema et al, 2019),并且替代65%水平的鱼粉可以提高真鲷(Pargus major)的末增重(Ido et al, 2019)。同时,脱脂黄粉虫粉替代鱼粉改变了欧洲鲈(Perca fluviatilis)肠道菌群多样性(Tran et al, 2022)。
本研究旨在探究饲料中脱脂黄粉虫粉替代鱼粉对花鲈生长性能、免疫反应及肠道健康的影响,评估花鲈饲料中脱脂黄粉虫粉替代鱼粉的适宜比例,为脱脂黄粉虫粉在花鲈饲料中的应用提供理论参考。
1 材料与方法 1.1 实验饲料本研究根据花鲈的营养需求,以鱼粉和豆粕为主要蛋白源,以鱼油和豆油为主要脂肪源配制蛋白水平为44%、脂肪水平为12%的基础饲料(表 1)。以基础饲料为对照组(FM),分别用脱脂黄粉虫粉(TM,广东泽和诚生物科技有限公司提供)替代基础饲料中5% (5% TM)、10% (10% TM)、15% (15% TM)和20% (20% TM)的鱼粉配制成5组等氮等脂的实验饲料。饲料原料经粉碎后,过80目筛,采用逐级扩大法充分混匀,加水搅拌后,通过双螺旋杆饲料挤条机制成粒径为2.5 mm的颗粒料,于55 ℃烘箱中烘干5 h,–20 ℃冰箱储存待用。
|
|
表 1 实验饲料基础配方与主要营养组分(%干物质基础) Tab.1 Composition and approximate composition of experimental diets (% DM basis) |
实验所用花鲈鱼苗购自福建漳州惠丰养殖场。正式实验前,花鲈苗暂养于1 000 L的养殖桶中,使用FM组饲料驯养2周。待鱼苗适应实验条件后,挑选300尾体格健壮、规格均一的花鲈(2.83±0.02) g,随机分配到15个500 L的淡水养殖桶中,每桶20尾鱼,共5组,每组3个重复。养殖期间,每日饱食投喂2次(08:00和17:00),投喂结束后通过虹吸将污物排出,随后更换1/3淡水以确保水质良好。从养殖的第5周开始收集粪便,在每次投喂30 min后,使用虹吸管从缸底部收取粪便到过滤袋上,保存于–20 ℃冰箱, 用于测定营养物质表观消化率。养殖期间,记录每桶花鲈的摄食总量和死鱼数量并称重,保持水温26~27 ℃,溶解氧≥6.0 mg/L,氨氮 < 0.1 mg/L,pH 7.5~ 8.5。养殖实验持续9周。
1.2 样品采集养殖实验结束后,停食24 h,丁香酚麻醉(1∶10 000)后称量并计数。每桶随机取3尾鱼置于自封袋,–20 ℃保存,用于全鱼体组成分析;每桶随机取出10尾鱼称重后,尾静脉取血,于1.5 mL离心管中静置12 h后,2 500 r/min离心10 min,收集血清,–80 ℃保存,用于血清溶菌酶(LZM)和二胺氧化酶(DAO)活性的测定。解剖分离其肝脏、腹脂并分别称重,以计算肝脏指数和腹脂率。随后,每桶随机取2尾鱼用于肠道消化酶活性的测定;每桶取2尾鱼用于肠道炎性因子以及紧密连接蛋白相关基因表达量的测定;每桶随机取2尾鱼,分离肠道,去除表面脂肪,取2 cm中肠置于盛有4%甲醛固定液的5 mL冻存管中,4 ℃保存,通过HE染色法,制作肠道组织切片,用于肠道组织形态学分析;最后,每桶随机取2尾鱼的肠道,用于肠道菌群16S rRNA的分析。
1.3 指标测定 1.3.1 生长性能、形体指标的相关计算公式如下| $ \text{存活率(SR,%)}=末尾数/初尾数×100\%;$ |
| $ \text{增重率(WGR,%)=[末均质量(g)–初均质量(g)]/初均质量(g)×100%};$ |
| $ \text{特定生长率(SGR,%/d)=100%×[ln末均质量(g) – ln初均质量(g)]/养殖天数;} $ |
| $ \text{蛋白质沉积率(PDR,%)=[末均重(g)×末全鱼粗蛋白含量(%)–初均重(g)×初全鱼粗蛋白含量(%)]/[投喂量(g)×饲料粗蛋白含量(%)]×100%;} $ |
| $ \text{饲料系数(FCR)=投喂量/[末均质量(g)–初均质量(g)];} $ |
| $ \text{摄食率(FR,%/d)=100% × 投喂量(g)/[末均质量(g)/2+初均质量(g)/2]/养殖天数;} $ |
| $ \text{肝体比(HSI,%)=肝脏质量(g)/体质量(g)×100%;} $ |
| $ \text{脏体比(VSI,%)=内脏质量(g)/体质量(g)×100%;} $ |
| $ \text{肥满度(CF)=体质量(g)/体长}^{3}(\text{cm}^{3});$ |
| $ \text{腹脂率(AFR,%)=腹部脂肪质量(g)/体质量(g)×100% } $ |
花鲈全鱼体组成中水分、粗脂肪、粗灰分的测定参考AOAC(2005)的方法,水分采用105 ℃烘箱干燥法(DHG-9123A,宁波江南仪器厂)测定,粗脂肪采用索氏抽提法(以乙醚为溶剂)测定,粗灰分采用550 ℃马弗炉灼烧法测定,粗蛋白采用杜马斯燃烧定氮法(Rapid N Ⅲ氮/蛋白质分析仪,Elementar公司,德国)测定。
1.3.3 血清与组织生化指标血清溶菌酶、二胺氧化酶、肠道消化酶(包括脂肪酶和蛋白酶)活性的测定均按照试剂盒说明书进行(南京建成生物工程研究所)。肠道组织匀浆的蛋白质浓度采用BCA法(索莱宝生物技术有限公司)进行测定。
1.3.4 肠道组织形态学分析参照李潇等(2019)的实验方法,将甲醛固定12 h的肠道组织样品经酒精逐级脱水、二甲苯透明、透蜡、包埋后,6 µm切片,HE染色法染色(n=6)。肠道组织形态学分析参考Baeverfjord等(1996)和Bonaldo等(2011)的研究。使用正置显微镜(Leica DM5500B, 德国)拍摄切片、观察并测量肠道形态学数据,包括肠绒毛高度(villus height, VH)、绒毛宽度(villus width, VW)和肌层厚度(muscular thickness, MT)。
1.3.5 肠道微生物多样性分析肠道微生物多样性分析参考Chen等(2021)和Zhang等(2021)的方法。对微生物V3~V4区16S rRNA基因进行PCR扩增,使用的引物为341F (CCTACGGGNGGCWGCAG)和806R (GGACTACHVGGGTATCTAAT)。使用Illumina平台进行测序(PE250)。使用FASTP 0.18.0进一步过滤原始读数。使用FLASH 1.2.11对原始序列进行双向读取后,使用UPARSE 9.2.64根据97%的相似度进行OTUs聚类,并对物种丰度、α-多样性进行计算,分析各组间差异。
1.3.6 肠道炎症与紧密连接蛋白因子相对表达量肠道炎性因子、紧密连接蛋白相关基因相对表达量使用qPCR法进行检测。具体方法如下:
总RNA提取方法参照Zhang等(2018),使用艾科瑞生物科技公司的总RNA提取试剂盒提取肠道组织的总RNA。反转录及实时荧光定量PCR按照TaKaRa RR047A试剂盒说明书将检测合格的RNA反转录为cDNA第一条链。使用SYBR®Green Master Mix (AceQ®qPCR,南京诺唯赞生物科技有限公司)试剂在Thermal cycler (ABI StepOne PlusTM)实时荧光定量PCR操作系统进行扩增,测定花鲈肠道待测基因的相对表达量。以β-actin作为内参基因,数据结果采用2–ΔΔCT法分析。
根据NCBI数据库中的花鲈炎性因子基因和紧密连接蛋白基因的序列设计引物,引物由北京擎科生物科技股份有限公司合成,使用Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/)设计引物,通过基因的扩增显示不同处理之间的差异,扩增效率为95%~105%,引物序列详见表 2。
|
|
表 2 花鲈肠道实时荧光定量引物序列 Tab.2 Nucleotide sequences of the primers used for real-time PCR of the intestine of L. maculatus |
通过SPSS 26.0统计软件的Kolmogorov Smirnov和Levene's检验来分别检验所有数据的方差正态分布和齐次性假设。采用单因素方差分析(one-way ANOVA)来确定主因素对所分析数据的影响,然后,采用Tukey's检验法进行多重比较,确定各组之间的差异,差异显著水平为P < 0.05。所有数据均以平均值±标准误(Mean±SE)的形式来表示,每组设3个生物学重复。使用GraphPad Prism 8.0软件作图。
2 结果 2.1 脱脂黄粉虫粉替代鱼粉对花鲈生长性能的影响由表 3可知,随着脱脂黄粉虫粉替代比例的增加,花鲈增重率和蛋白质沉积率出现先升高后降低的趋势,在5% TM组达到最大值,20% TM组的增重率和蛋白质沉积率均显著低于FM组和5% TM组(P < 0.05)。与对照组相比,各替代组的摄食率、饲料效率、存活率、肝体比和腹脂率均无显著差异(P > 0.05)。脏体比在FM组和5% TM组显著低于20% TM组(P < 0.05)。脱脂黄粉虫粉替代水平与花鲈增重率的关系用二次回归模型表示为Y=–1.584x2+9.232 8x+1 892,R2= 0.956 6,此时脱脂黄粉虫粉最适替代量为7.31%(图 1)。
|
|
表 3 脱脂黄粉虫粉替代鱼粉对花鲈生长性能的影响 Tab.3 Effect of replacing fish meal with defatted mealworm meal (TM) on growth performance of L. maculatus |

|
图 1 脱脂黄粉虫粉替代水平与花鲈增重率的关系 Fig.1 Relationship between TM replacement level and weight gain rate of L. maculatus |
脱脂黄粉虫粉替代鱼粉对花鲈全鱼体组成的影响如表 4所示。各组花鲈全鱼体组成中的水分、粗蛋白、粗脂肪和灰分含量均无显著性差异(P > 0.05)。
|
|
表 4 脱脂黄粉虫粉替代鱼粉对花鲈全鱼体组成的影响(%湿重) Tab.4 Effect of replacing fish meal with defatted TM on whole-body composition of L. maculatus (% wet weight basis) |
由图 2可知,随着脱脂黄粉虫粉替代鱼粉比例的增加,花鲈血清溶菌酶活性呈先升高后降低的趋势,其在5% TM组出现最大值,但与FM组相比,各水平替代组溶菌酶活性均无显著性差异(P > 0.05)。二胺氧化酶的活性随着脱脂黄粉虫粉替代鱼粉比例的增加,呈先升高后降低的趋势,但各组间无显著差异(P > 0.05)。
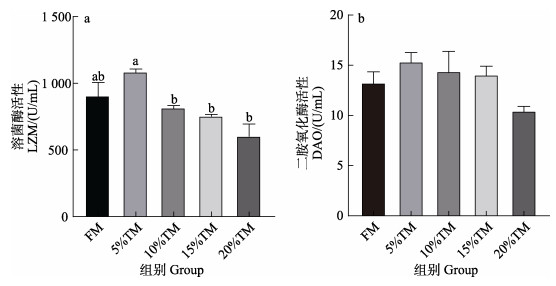
|
图 2 脱脂黄粉虫粉替代鱼粉对花鲈血清生化指标的影响 Fig.2 Effect of replacing fish meal with TM on serum biochemical indexes of L. maculatus 不同字母表示差异显著(P < 0.05)。下同。 The significant differences are shown with different letters (P < 0.05). The same below. |
如图 3所示,FM组和5%TM组的花鲈肠道绒毛结构完整,排列整齐伸向肠腔,肠道肌层和粘膜下层发育良好,随着替代比例的增加,花鲈肠道出现绒毛排列不均匀、不紧密等现象。如表 5所示,随着替代比例的增加,绒毛宽度、绒毛高度和肌层厚度均呈先升高后降低的趋势,其中,5% TM组显著高于其他组(P < 0.05),20% TM组绒毛宽度和肌层厚度均显著低于其他组,而绒毛高度与FM和15% TM组没有显著差异,但显著低于5% TM和10% TM组(P < 0.05)。

|
图 3 脱脂黄粉虫粉替代鱼粉对花鲈肠道组织形态的影响 Fig.3 Effect of dietary TM on intestine tissue morphology of L. maculatus a: FM; b: 5% TM; c: 10% TM; d: 15% TM; e: 20% TM |
|
|
表 5 脱脂黄粉虫粉替代鱼粉对花鲈肠道组织形态的影响 Tab.5 Effects of replacing fish meal with TM on the morphological parameters of the intestine of L. maculatus |
由图 4可知,与FM组相比,5%~15% TM组IL-1β相对表达量显著下调(P < 0.05),20% TM组无显著性差异(P > 0.05);随着脱脂黄粉虫粉替代比例的增加,IL-4相对表达量总体呈先下降后上升的趋势,在10% TM组达最低(P < 0.05),在20% TM组显著高于其他组(P < 0.05);由图 4可知,各替代组肠道紧密连接蛋白基因ZO-1和Ocln的相对表达量与FM组无显著性差异(P > 0.05),而ZO-1的相对表达量在5% TM组达到最高值(P < 0.05),而Ocln的相对表达量则在10% TM组达到最高值(P < 0.05)。

|
图 4 脱脂黄粉虫粉替代鱼粉对花鲈肠道炎性因子和紧密连接蛋白相关基因相对表达量的影响 Fig.4 Effect of replacing fish meal with TM on the relative expression of genes related to inflammatory cytokine and tight junction proteins of the intestine of L. maculatus |
由图 5可知,脱脂黄粉虫粉替代不同比例的鱼粉后,花鲈肠道脂肪酶和蛋白酶活性在各实验组均未见显著差异(P > 0.05)。
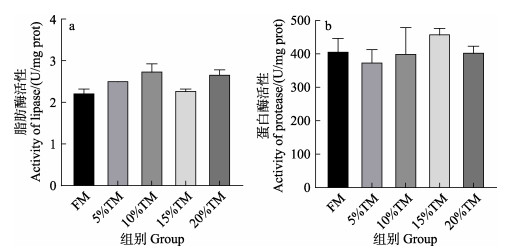
|
图 5 脱脂黄粉虫粉替代鱼粉对花鲈肠道消化酶活性的影响 Fig.5 Effect of replacing fish meal with TM on the activities of digestive enzymes of the intestine of L. maculatus |
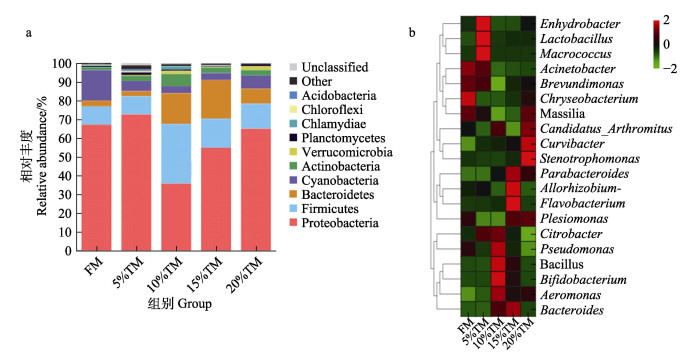
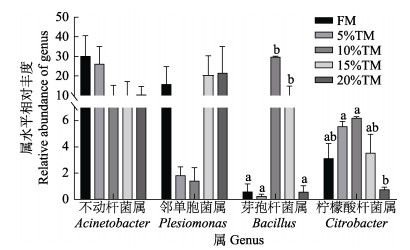
由表 6可知,脱脂黄粉虫粉替代鱼粉后对花鲈肠道菌群多样性指数无显著影响(P > 0.05)。基于门水平,各实验组花鲈肠道的主要优势菌群的结果如图 6a所示,主要包括变形菌门(Proteobacteria)、厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、蓝藻门(Cyanobacteria)和放线菌门(Actinobacteria)。其中,变形菌门、拟杆菌门和放线菌门的相对丰度在各实验组间无显著差异(P > 0.05),而10% TM组的厚壁菌门丰度显著高于其他各组(P < 0.05),其他组间无显著差异(P > 0.05);各组花鲈肠道中属水平主要优势种群有不动杆菌属(Acinetobacter)、邻单胞菌属(Plesiomonas)、栖水菌属(Enhydrobacter)、芽孢杆菌属(Bacillus)和柠檬酸杆菌属(Citrobacter)。其中,10%和15% TM组芽孢杆菌属相对丰度显著高于其他各组(P < 0.05) (图 7);各组间柠檬酸杆菌属相对丰度总体呈先上升后下降的趋势,5% TM、10% TM组显著高于20% TM组(P < 0.05);不动杆菌属相对丰度有随着黄粉虫粉替代比例增加而降低的趋势,当替代水平为5%和10%时邻单胞菌属的相对丰度有下降的趋势(图 7)。
|
|
表 6 脱脂黄粉虫粉替代鱼粉对花鲈肠道菌群多样性的影响 Tab.6 Effect of replacing fish meal with TM on the microbial diversity indexes of the intestine of L. maculatus |
|
图 6 脱脂黄粉虫粉替代鱼粉对花鲈肠道微生物相对丰度的影响
Fig.6 Effect of replacing fish meal with TM on the relative abundance of microbial community of the intestine of L. maculatus.
a: 门水平; b: 属水平 属水平图中每一行代表一个物种,每列代表一个分组。颜色代表物种丰度,颜色越接近绿色丰度越低,越接近红色丰度越高。图例为对应物种丰度数值。 a: Phylum level; b: Genus level Each row in the heat map represents a species, and each column represents a subgroup. The color represents the species abundance. The closer the color is to green, the lower the abundance, and the closer the color is to red, the higher the abundance. The legend shows the abundance values of the corresponding species. |
|
图 7 脱脂黄粉虫粉替代鱼粉对花鲈肠道微生物属水平差异物种相对丰度的影响 Fig.7 Effect of replacing fish meal with TM on the relative abundance of differential species of the intestine of L. maculatus |
本研究中,随着脱脂黄粉虫粉替代水平的增加,花鲈生长性能呈现先升高后降低的趋势,在5% TM替代组增重率最高,替代水平为5%~15%的鱼粉对花鲈生长性能无负面影响,但替代水平达到20%时不利于花鲈的生长。以增重率为评价指标,利用二次回归模型计算得出花鲈饲料中脱脂黄粉虫粉最适替代水平为7.31%。与本研究结果相似,随着黄粉虫粉替代饲料中鱼粉水平(0~20%)的升高,鳜鱼(Siniperca scherzeri)的增重率出现升高的趋势,随后,随着替代水平的增加(20%~30%)增重率出现下降趋势(Sankian et al, 2018)。脱脂黄粉虫粉替代饲料45%的鱼粉会导致大黄鱼(Larimichthys crocea)增重率显著下降(Zhang et al, 2022)。但也有研究表明,在饲料中脱脂黄粉虫粉替代20%~65%的鱼粉而不会对真鲷的增重率造成影响(Ido et al, 2019),并且在虹鳟的饲料中脱脂黄粉虫粉替代鱼粉水平可高达100% (Rema et al, 2019),造成上述差异可能是由不同鱼类对黄粉虫粉利用能力的差异导致的。肝体比是评价鱼体健康的重要指标,较低的肝体比反映鱼体可以正常调节自身肝功能并避免过度负荷,从而降低肝病或脂肪肝发生的风险(陆游等, 2018)。本研究中,随着替代比例的增加,各实验组肝体比无明显差异,表明脱脂黄粉虫粉替代20%鱼粉不会对花鲈肝脏造成损伤。溶菌酶(LZM)是鱼类非特异性免疫反应的重要指标(Cronin et al, 2001)。溶菌酶活性升高,反映机体吞噬细胞活力增强。本研究表明,随着脱脂黄粉虫粉替代水平的增加,溶菌酶活性呈先上升后下降的趋势,在5%替代组达到最高值,说明适当摄食脱脂黄粉虫粉可能激活溶菌酶活性,而脱脂黄粉虫粉替代量较高时,会对鱼体免疫产生负面效应。Zhang等(2022)也发现,脱脂黄粉虫粉替代15%的鱼粉时,大黄鱼血清溶菌酶活性显著上升,但随着替代比例的增加呈下降趋势。本研究条件下,花鲈饲料中脱脂黄粉虫粉替代比例为20%时,花鲈摄食量下降,脏体比和腹脂率上升,蛋白质沉积率下降,最终降低花鲈的生长性能。
3.2 脱脂黄粉虫粉对花鲈肠道健康的影响饲料中的营养物质主要通过胃肠道进行消化吸收,肠黏膜结构在鱼类的生长与营养物质的消化吸收方面具有主导作用(吴桐强等, 2019)。本研究中,脱脂黄粉虫粉替代15%的鱼粉不会影响花鲈肠道的组织形态,然而,当替代水平达到20%时,会导致花鲈肠道肌层厚度显著变薄,绒毛高度和宽度显著降低。之前的研究也证实,黄粉虫粉替代20%鱼粉会导致虹鳟肠道绒毛高度降低(Józefiak et al, 2019),这可能是因为黄粉虫粉含有几丁质等不容易被鱼类消化的成分,这些物质在饲料中过量存在可能会损伤鱼类的肠道粘膜(彭凯等, 2021),影响肠道健康,最终导致鱼类生长性能下降(Karlsen et al, 2017; Shiau et al, 1999)。肠道紧密连接层在肠道细胞水平上起着屏障作用,ZO-1是肠道紧密连接层的结构性蛋白,其表达量上调可以调节肠道上皮细胞旁通透性,并通过结合肌动蛋白来改善肠道紧密连接层的稳态,维持肠道健康(彭凯等, 2021; Lu et al, 2014; Li et al, 2000)。闭合蛋白(Ocln)是肠上皮细胞的跨膜蛋白,研究表明,将肠上皮细胞中的Ocln蛋白敲除后会导致肠细胞的选择透过性失调(Abe et al, 2003)。在本研究中,ZO-1和Ocln的表达量随替代比例的增加呈先升高后降低的趋势,表明花鲈饲料中脱脂黄粉虫粉替代5%~15%的鱼粉能够改善花鲈肠道健康。
肠道不仅作为消化吸收营养物质的主要场所,同时参与了机体应激和免疫调控的过程。肠道细胞分泌的细胞因子,可以刺激细胞活化、增殖和分化,从而调控免疫应答,维持肠道健康。已有研究表明,促炎细胞因子含量的升高和抗炎细胞因子含量的降低能够加剧炎症反应,破坏肠道健康稳态,当二者处于平衡状态时,肠道才能维持健康(Tian et al, 2017; Song et al, 2017)。本研究表明,脱脂黄粉虫粉替代5%~15%的鱼粉会抑制促炎因子IL-1β的转录,而当替代比例为20%时,会诱导促炎因子和抗炎因子IL-4的转录,这表明,脱脂黄粉虫粉替代比例过高可能会促进花鲈肠道炎症发生。
鱼体肠道中的菌群通过直接接触和生成代谢产物等方式与宿主肠道黏膜发生交互作用,影响着宿主肠道的消化吸收、免疫功能和形态结构,对宿主的生长和健康起着重要的作用(Gilbert et al, 2018; Sha et al, 2016; Meng et al, 2021)。本研究表明,脱脂黄粉虫粉替代不同比例的鱼粉后,花鲈肠道微生物的优势菌门为变形菌门、拟杆菌门和厚壁菌门,这与对花鲈(林能锋等, 2021)、草鱼(Ctenopharyngodon idellus) (Wu et al, 2012)和大黄鱼(He et al, 2018)等鱼类肠道微生物的研究结果相似。在门水平上,厚壁菌门是鱼类肠道的有益微生物菌群,可以为鱼体提供外源性消化酶,有促进消化和吸收的功能(Ray et al, 2012)。在本研究条件下,摄食10% TM饲料的花鲈肠道微生物厚壁菌门的相对丰度更高。在属水平上,芽孢杆菌是水产动物肠道中常见的益生菌之一(Hu et al, 2019; Li et al, 2019)。研究表明,在饲料中添加枯草芽孢杆菌能促进珍珠龙胆石斑鱼(Epinephelus lanceolatu♂×Epinephelus fuscoguttatus♀)幼鱼的生长性能,提高抗病力、消化酶和抗氧化酶活性(李军亮等, 2019),也可以改善鲤(Cyprinus carpio)的肠肝健康和菌群稳态(胡娟等, 2021)。本研究发现,10%~15% TM组花鲈肠道中芽孢杆菌属的相对丰度增加,与对西伯利亚鲟(Acipenser baerii)(Jozefiak et al, 2019)的研究结果一致。邻单胞菌属是水产动物研究常见的条件致病菌(刘志刚等, 2015; 邹升等, 2019),本研究结果表明,邻单胞菌属在5%~10%替代水平组有下降的趋势。说明饲料中脱脂黄粉虫粉替代鱼粉改变了花鲈肠道的菌群结构,适量的脱脂黄粉虫粉替代鱼粉(5%~10%)可以提高有益菌丰度、降低有害菌丰度,而高水平脱脂黄粉虫粉替代鱼粉(20%)会导致花鲈肠道有害菌丰度上升、有益菌丰度降低。
4 结论在本研究条件下,脱脂黄粉虫粉替代饲料中5%~ 15%的鱼粉,对花鲈生长性能、免疫能力不会造成负面影响,并且能维护肠道健康。当脱脂黄粉虫粉替代比例为20%时,会导致花鲈肠道组织结构受损,肠道微生物失衡,诱发肠炎,致使免疫力和生长性能下降。经二次曲线回归模型分析,建议花鲈饲料脱脂黄粉虫粉替代鱼粉的水平不高于7.31%。
ABE T, SUGANO E, SAIGO Y, et al. Interleukin-1β and barrier function of retinal pigment epithelial cells (ARPE-19): Aberrant expression of junctional complex molecules. Investigative Ophthalmology and Visual Science, 2003, 44(9): 4097-4104 DOI:10.1167/iovs.02-0867 |
AOAC. Official methods of analysis of AOAC International. 18th ed. Washington, D. C.: Association of Official Analytical Chemists International, 2005: 70-76
|
BAEVERFJORD G, KROGDAHL Å. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. Journal of Fish Diseases, 1996, 19(5): 375-387 DOI:10.1111/j.1365-2761.1996.tb00376.x |
BELFORTI M, GAI F, LUSSIANA C, et al. Tenebrio molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility and chemical composition of fillets. Italian Journal of Animal Science, 2016, 14(4): 670-676 |
BONALDO A, PARMA L, MANDRIOLI L, et al. Increasing dietary plant proteins affects growth performance and ammonia excretion but not digestibility and gut histology in turbot (Psetta maxima) juveniles. Aquaculture, 2011, 318(1/2): 101-108 |
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2022. Beijing: China Agriculture Press, 2022: 1-57 [农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2022中国渔业统计年鉴. 北京: 中国农业出版社, 2022: 1-57]
|
CHEN Z, LI Y Z, PENG Y Y, et al. Effects of antibiotics on hydrolase activity and structure of microbial community during aerobic co-composting of food waste with sewage sludge. Bioresource Technology, 2021, 321: 124506 DOI:10.1016/j.biortech.2020.124506 |
CRONIN M A, CULLOTY S C, MULCAHY M F. Lysozyme activity and protein concentration in the haemolymph of the flat oyster Ostrea edulis (L.). Fish and Shellfish Immunology, 2001, 11(7): 611-622 DOI:10.1006/fsim.2001.0338 |
GASCO L, BELFORTI M, ROTOLO L, et al. Mealworm (Tenebrio molitor) as a potential ingredient in practical diets for rainbow trout (Oncorhynchus mykiss)//Proc. 1st Int. Conf. Insects to Feed the World, Wageningen University, Wageningen, The Netherlands (Abstr.). 2014, 69
|
GASCO L, HENRY M, PICCOLO G, et al. Tenebrio molitor meal in diets for European seabass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Animal Feed Science and Technology, 2016, 220: 34-45 DOI:10.1016/j.anifeedsci.2016.07.003 |
GHALY A E, ALKOAIK F N. The yellow mealworm as a novel source of protein. American Journal of Agricultural and Biological Sciences, 2009, 4(4): 319-331 DOI:10.3844/ajabssp.2009.319.331 |
GILBERT J A, BLASER M J, CAPORASO J G, et al. Current understanding of the human microbiome. Nature Medicine, 2018, 24(4): 392-400 DOI:10.1038/nm.4517 |
HE J, WANG P, FENG J, et al. Effects of replacing fish meal with corn gluten meal on growth, intestinal histology, and intestinal microbiota of large yellow croaker, Larimichthys crocea. Journal of Fishery Sciences of China, 2018, 25(2): 361-372 DOI:10.3724/SP.J.1118.2018.17180 |
HENRY M A, GAI F, ENES P, et al. Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish and Shellfish Immunology, 2018, 83: 308-313 DOI:10.1016/j.fsi.2018.09.040 |
HENRY M, GASCO L, PICCOLO G, et al. Review on the use of insects in the diet of farmed fish: Past and future. Animal Feed Science and Technology, 2015, 203: 1-22 DOI:10.1016/j.anifeedsci.2015.03.001 |
HIDALGO M C, MORALES A E, PULA H J, et al. Oxidative metabolism of gut and innate immune status in skin and blood of tench (Tinca tinca) fed with different insect meals (Hermetia illucens and Tenebrio molitor). Aquaculture, 2022, 558: 738384 DOI:10.1016/j.aquaculture.2022.738384 |
HU J, GAO C C, YAO Y Y, et al. Effects of dietary Bacillus subtilis HGcc-1 on gut and liver health, serum complement and gut microbiota of common carp fingerlings (Cyprinus carpio). Journal of Fisheries of China, 2019, 43(4): 1126-1137 [胡娟, 高辰辰, 药园园, 等. 饲用枯草芽孢杆菌HGcc-1对鲤肠肝健康, 血清补体及肠道菌群的影响. 水产学报, 2019, 43(4): 1126-1137] |
HU P L, WU R, LU K L, et al. Effects of replacing fish meal with composite protein on growth, diet digestibility, and gut health in Japanese seabass (Lateolabrax maculatus). Progress in Fishery Sciences, 2019, 40(6): 56-65 [胡鹏莉, 吴瑞, 鲁康乐, 等. 复合蛋白替代鱼粉对花鲈生长、消化能力和肠道健康的影响. 渔业科学进展, 2019, 40(6): 56-65 DOI:10.19663/j.issn2095-9869.20181214002] |
IACONISI V, MARONO S, PARISI G, et al. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture, 2017, 476: 49-58 DOI:10.1016/j.aquaculture.2017.04.007 |
IDO A, HASHIZUME A, OHTA T, et al. Replacement of fish meal by defatted yellow mealworm (Tenebrio molitor) larvae in diet improves growth performance and disease resistance in red seabream (Pargus major). Animals (Basel), 2019, 9(3): 100 |
JÓZEFIAK A, NOGALES-MÉRIDA S, MIKOŁAJCZAK Z, et al. The utilization of full-fat insect meal in rainbow trout (Oncorhynchus mykiss) nutrition: The effects on growth performance, intestinal microbiota and gastrointestinal tract histomorphology. Annals of Animal Science, 2019, 19(3): 747-765 DOI:10.2478/aoas-2019-0020 |
JOZEFIAK A, NOGALES-MERIDA S, RAWSKI M, et al. Effects of insect diets on the gastrointestinal tract health and growth performance of Siberian sturgeon (Acipenser baerii Brandt, 1869). BMC Veterinary Research, 2019, 15(1): 1-11 DOI:10.1186/s12917-018-1758-8 |
KARLSEN, AMLUND H, BERG A, et al. The effect of dietary chitin on growth and nutrient digestibility in farmed Atlantic cod, Atlantic salmon and Atlantic halibut. Aquaculture Research, 2017, 48(1): 123-133 DOI:10.1111/are.12867 |
KHOSRAVI S, KIM E, LEE Y S, et al. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for juvenile rockfish (Sebastes schlegeli). Entomological Research, 2018, 48(3): 214-221 DOI:10.1111/1748-5967.12306 |
KUMAR R, KAUR N, KAMILYA D. Chitin modulates immunity and resistance of Labeo rohita (Hamilton, 1822) against gill monogeneans. Aquaculture, 2019, 498: 522-527 DOI:10.1016/j.aquaculture.2018.09.013 |
LEE S Y, MOON H J, KURATA S, et al. Purification and cDNA cloning of an antifungal protein from the hemolymph of Holotrichia diomphalia larvae. Biological and Pharmaceutical Bulletin, 1995, 18(8): 1049-1052 DOI:10.1248/bpb.18.1049 |
LI D X, MRSNY R J. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. Journal of Cell Biology, 2000, 148(4): 791-800 DOI:10.1083/jcb.148.4.791 |
LI J L, YANG Q H, TAN B P, et al. Effect of low-fishmeal diet with Bacillus subtilis on growth performance, digestive enzyme activity, antioxidant enzyme activity and its mRNA expression of juveniles Epinephelus lanceolatu ♂ × Epinephelus fuscoguttatus ♀. Journal of Fisheries of China, 2019, 43(4): 1126-1137 [李军亮, 杨奇慧, 谭北平, 等. 低鱼粉饲料添加枯草芽孢杆菌对珍珠龙胆石斑鱼幼鱼生长、消化酶活性、抗氧化酶活性及其mRNA表达的影响. 水产学报, 2019, 43(4): 1126-1137] |
LI X, WANG L, ZHANG C X, et al. Effects of partially replacing fish meal with Brewer's yeast meal on growth, antioxidant capacity, and liver and intestine tissue morphology of bullfrog [Rana (Lithobates) catesbeiana]. Chinese Journal of Animal Nutrition, 2019, 31(4): 1864-1876 [李潇, 王玲, 张春晓, 等. 啤酒酵母粉替代部分鱼粉对牛蛙生长、抗氧化能力以及肝脏和肠道组织形态学的影响. 动物营养学报, 2019, 31(4): 1864-1876] |
LIN N F. Microbial diversity of intestinal contents and mucus in cultured seabass (Lateolabrax maculatus). Journal of Aquaculture, 2021(6): 1-7 [林能锋. 养殖花鲈(Lateolabrax maculatus)肠道菌群的多样性分析. 水产养殖, 2021(6): 1-7 DOI:10.3969/j.issn.1004-2091.2021.06.001] |
LIU Z G, KE X L, LU M X, et al. Identification and pathological observation of a pathogenic Plesiomonas shigelloides strain isolated from cultured tilapia (Oreochromis niloticus). Acta Microbiologica Sinica, 2015, 55(1): 96-106 [刘志刚, 可小丽, 卢迈新, 等. 尼罗罗非鱼致病性类志贺邻单胞菌(Plesiomonas shigelloides)的分离鉴定及其病理学观察. 微生物学报, 2015, 55(1): 96-106] |
LU R Y, YANG W X, HU Y J. The role of epithelial tight junctions involved in pathogen infections. Molecular Biology Reports, 2014, 41(10): 6591-6610 DOI:10.1007/s11033-014-3543-5 |
LU Y, JIN M, YUAN Y, et al. Effects of different lipid sources on growth performance, body composition, the serum biochemical indices, fatty acids composition and antioxidant capacity in juvenile yellow catfish (Pelteobagrus fulvidraco). Journal of Fisheries of China, 2018, 42(7): 1094-1110 [陆游, 金敏, 袁野, 等. 不同脂肪源对黄颡鱼幼鱼生长性能、体成分、血清生化指标、体组织脂肪酸组成及抗氧化能力的影响. 水产学报, 2018, 42(7): 1094-1110] |
MENG X L, WU S K, HU W P, et al. Clostridium butyricum improves immune responses and remodels the intestinal microbiota of common carp (Cyprinus carpio L.). Aquaculture, 2021, 530: 735753 DOI:10.1016/j.aquaculture.2020.735753 |
NG W K, LIEW F L, ANG L P, et al. Potential of mealworm (Tenebrio molitor) as an alternative protein source in practical diets for African catfish, Clarias gariepinus. Aquaculture Research, 2001, 32: 273-280 DOI:10.1046/j.1355-557x.2001.00024.x |
PENG K, XIAO H F, MO W Y, et al. Effects of dietary black soldier fly larvae meal on serum metabolites, antioxidant and immune indexes and intestinal histological appearance of Micropterus salmoides. Chinese Journal of Animal Nutrition, 2021, 33(12): 6964-6972 [彭凯, 萧鸿发, 莫文艳, 等. 饲料中添加黑水虻幼虫粉对加州鲈血清代谢物、抗氧化与免疫指标及肠道组织结构的影响. 动物营养学报, 2021, 33(12): 6964-6972] |
PICCOLO G, IACONISI V, MARONO S, et al. Effect of Tenebrio molitor larvae meal on growth performance, in vivo nutrients digestibility, somatic and marketable indexes of gilthead sea bream (Sparus aurata). Animal Feed Science and Technology, 2017, 226: 12-20 DOI:10.1016/j.anifeedsci.2017.02.007 |
RAY A K, GHOSH K, RINGØ E. Enzyme-producing bacteria isolated from fish gut: A review. Aquaculture Nutrition, 2012, 18(5): 465-492 DOI:10.1111/j.1365-2095.2012.00943.x |
REMA P, SARAVANAN S, ARMENJON B, et al. Graded incorporation of defatted yellow mealworm (Tenebrio molitor) in rainbow trout (Oncorhynchus mykiss) diet improves growth performance and nutrient retention. Animals (Basel), 2019, 9(4): 187 |
RUAN H B. Induction, antibacterial activity, separation and purification of antimicrobial peptides of Tenebrio molitor. Master´s Thesis of Sichuan Agricultural University, 2012 [阮华波. 黄粉虫抗菌肽的诱导、抑菌作用及其分离纯化. 四川农业大学硕士研究生学位论文, 2012]
|
SÁNCHEZ-MUROS M J, DE HARO C, SANZ A, et al. Nutritional evaluation of Tenebrio molitor meal as fish meal substitute for tilapia (Oreochromis niloticus) diet. Aquaculture Nutrition, 2016, 22(5): 943-955 |
SANKIAN Z, KHOSRAVI S, KIM Y O, et al. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters and antioxidant enzyme activities of mandarin fish (Siniperca scherzeri) juveniles. Aquaculture, 2018, 496: 79-87 |
SHA Y J, LIU M, WANG B J, et al. Bacterial population in intestines of Litopenaeus vannamei fed different probiotics or probiotic supernatant. Journal of Microbiology and Biotechnology, 2016, 26(10): 1736-1745 |
SHIAU S Y, YU Y P. Dietary supplementation of chitin and chitosan depresses growth in tilapia, Oreochromis niloticus× O. aureus. Aquaculture, 1999, 179(1/2/3/4): 439-446 |
SONG S G, CHI S Y, TAN B P, et al. Effects of fishmeal replacement by Tenebrio molitor meal on growth performance, antioxidant enzyme activities and disease resistance of the juvenile pearl gentian grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). Aquaculture Research, 2018, 49(6): 2210-2217 |
SONG Z X, JIANG W D, LIU Y, et al. Dietary zinc deficiency reduced growth performance, intestinal immune and physical barrier functions related to NF-κB, TOR, Nrf2, JNK and MLCK signaling pathway of young grass carp (Ctenopharyngodon idella). Fish and Shellfish Immunology, 2017, 66: 497-523 |
TIAN L, ZHOU X Q, JIANG W D, et al. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish and Shellfish Immunology, 2017, 66: 548-563 |
TRAN H Q, PROKEŠOVÁ M, ZARE M, et al. Production performance, nutrient digestibility, serum biochemistry, fillet composition, intestinal microbiota and environmental impacts of European perch (Perca fluviatilis) fed defatted mealworm (Tenebrio molitor). Aquaculture, 2022, 547: 737499 |
WU S G, WANG G T, ANGERT E R, et al. Composition, diversity, and origin of the bacterial community in grass carp intestine. PLoS One, 2012, 7(2): e30440 |
WU T Q, ZHONG L, LIU Z P, et al. Effects of glutamine dipeptide on growth, serum biochemistry, immunity indexes and intestinal morphology of juvenile grass carp (Ctenopharyngodon idellus). Chinese Journal of Animal Nutrition, 2019, 31(8): 3682-3689 [吴桐强, 钟蕾, 刘庄鹏, 等. 谷氨酰胺二肽对草鱼幼鱼生长、血清生化、免疫指标及肠道组织结构的影响. 动物营养学报, 2019, 31(8): 3682-3689] |
ZHANG C X, RAHIMNEJAD S, WANG Y R, et al. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture, 2018, 483: 173-182 |
ZHANG J, DONG Y Z, SONG K, et al. Effects of the replacement of dietary fish meal with defatted yellow mealworm (Tenebrio molitor) on juvenile large yellow croakers (Larimichthys crocea) growth and gut health. Animals (Basel), 2022, 12(19): 2659 |
ZHANG Y P, YANG L H, ZHAO N N, et al. Soluble polysaccharide derived from Laminaria japonica attenuates obesity-related nonalcoholic fatty liver disease associated with gut microbiota regulation. Marine Drugs, 2021, 19(12): 699 |
ZOU S, GONG L, LI D J, et al. Isolation, identification and virulence of pathogenic bacteria in gut of diseased grass carp Ctenopharyngodon idellus. Fisheries Science, 2019, 38(2): 152-162 [邹升, 龚亮, 李东杰, 等. 患病草鱼肠道病原菌的分离鉴定及毒力研究. 水产科学, 2019, 38(2): 152-162] |



