2. 中国水产科学研究院黄海水产研究所 山东 青岛 266071
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
鱼粉和鱼油是饲料中重要的蛋白源和脂肪源,随着全球水产养殖规模的不断扩大,鱼粉鱼油供应不足已成为水产养殖业重要的限制因素。为了饲料工业的可持续发展,养殖鱼类和甲壳动物商品饲料中的鱼粉和鱼油的比例在逐年降低,而碳水化合物(糖类)的含量在不断升高(Tacon et al, 2008; 李琳等, 2023)。作为三大营养素之一,糖类可为机体提供能量,价格远远低于蛋白和脂肪。淀粉是由葡萄糖分子聚合而成的高分子碳水化合物,是水产饲料中最常用的糖类,可替代部分鱼粉和鱼油,降低饲料成本。因此,充分挖掘糖类的营养功能对水产养殖具有重要意义。溶解氧(DO)是指溶解在水环境中的分子态氧,是水生生物获取O2的主要形式,其含量受到水温和盐度等诸多环境因子的综合影响,对水生生物的生存和水体生态环境的平衡具有重要意义(徐贺等, 2016)。低氧是指水环境中DO浓度低于2 mg/L的现象(Rabalais et al, 2002)。水产养殖中,低氧是一种比较普遍且危害性大的应激,影响水生动物的行为、摄食、消化、生长、繁殖力、免疫力、抗氧化能力和代谢模式(Zhu et al, 2013)。低氧可分为急性低氧和慢性低氧,极端天气、供电障碍和活体长途运输等因素会使水环境的溶氧量突然降低,导致水生动物机体出现剧烈的应激反应;而水体环境污染、气候变暖和高密度养殖等因素会使水环境的溶氧量长期保持在较低状态,会对机体的生理功能造成长远的影响(阮雯等, 2020)。尤其是急性低氧会导致养殖鱼类在短时间内大量死亡,给水产养殖业造成严重的经济损失(Ma et al, 2021),因此,寻找提高鱼类对急性低氧耐受性的方法至关重要。
研究表明,在低氧条件下,线粒体的氧化磷酸化过程被抑制,导致机体能量缺乏,从而激活无氧糖酵解途径,分解葡萄糖生成乳酸和ATP,为鱼体提供能量(Polymeropoulos et al, 2017; Cuninghame et al, 2017; 曹梅等, 2021)。在急性低氧条件下,尼罗罗非鱼(Oreochromis niloticus)肝脏的糖原含量显著降低,而血液中的葡萄糖、乳酸和血红蛋白含量显著升高,在斑马鱼(Danio rerio)和大口黑鲈(Micropterus salmoides)中也发现了类似的结果(Li et al, 2018; Ma et al, 2021; Sun et al, 2020)。同时,急性低氧诱导了大口黑鲈鳃、心脏、肝脏和大脑中ampkα、hif-1α (hypoxia inducible factor 1 subunit alpha a)和ldha (lactate dehydrogenase A4)等基因的显著升高(Yang et al, 2019),以上结果表明,低氧促进糖代谢从有氧转为无氧途径。低氧诱导因子(hypoxia inducible factor,HIF)是细胞响应低氧胁迫中最关键的转录因子。HIF由α和β两个亚基组成,其中,α亚基(HIF1α、HIF2α和HIF3α)为氧依赖性,在O2中易分解;而β亚基(HIFβ/ARNT)在O2中可以稳定存在(Zhu et al, 2016)。目前,大部分研究都只是描述了鱼类在急性低氧条件下的生理代谢变化,激活了HIF1α介导的无氧糖酵解通路来提供能量,但很少有研究通过调控饲料营养成分来改变其对急性低氧的耐受性。在斑马鱼的研究中发现,与对照饲料组相比,高碳水化合物饲料(高糖饲料)可以显著促进肝糖原积累,激活hif3α和糖酵解通路,提高斑马鱼对急性低氧的耐受性(Ma et al, 2021)。但与杂食性鱼类不同,肉食性鱼类对饲料碳水化合物的吸收和代谢能力有限,不能很好地利用糖类进行供能(丁斐斐等, 2022)。与大分子的淀粉不同,糊精是淀粉经低度水解之后形成的中间衍生物,其分子质量处于淀粉和葡萄糖之间,粘合性好,更容易被消化和吸收(Alvira, 2022)。鱼类在急性低氧条件下主要利用糖来提供能量,因此,我们提出假设,在饲料中使用易消化的糖类是一种提高鱼类对急性低氧耐受性的有效方法。
红鳍东方鲀(Takifugu rubripes)俗称河鲀,主要分布于北太平洋西部,在中国常见于东海和黄渤海区域。因其味道鲜美、营养价值高,深受日韩消费者的喜爱,是中国海水养殖鱼类的特色品种之一。据统计,2021年中国海水和淡水河鲀鱼的总产量在3万t左右(农业农村部渔业渔政管理局等, 2022)。红鳍东方鲀主要以高密度的工厂化养殖为主,水体DO的维持主要依靠水交换和人工增氧,其鳃盖退化,鳃孔小,很容易受到急性低氧的威胁。因此,本研究分别设置玉米淀粉(对照)和糊精(玉米淀粉水解物) 2个处理组,比较2种糖源对红鳍东方鲀生长和低氧耐性的影响,为提高鱼类对急性低氧的耐受性和水产养殖的健康发展提供理论依据。
1 材料与方法 1.1 实验设计与饲料制作实验饲料依据红鳍东方鲀幼鱼的基本营养需求设计,主要以鱼粉和大豆浓缩蛋白为蛋白源,鱼油为脂肪源,玉米淀粉或糊精为糖源,配制糖水平为23%的2种实验饲料,分别记为Cor和Dex,饲料成分及营养组成见表 1。实验饲料按照实验室标准程序进行制作,使用小型单螺杆挤压机制得直径约为3 mm的颗粒状饲料,60 ℃烘干12 h后置于–20 ℃冷库中保存、备用。
|
|
表 1 实验饲料配方及常规营养组成(%干物质) Tab.1 Ingredients and proximate compositions of the experimental diets (% dry matter basis) |
实验所用的红鳍东方鲀幼鱼购于河北省唐山海都水产食品有限公司,养殖实验在山东省烟台市黄海水产有限公司进行。养殖实验开始前,先用商业饲料进行为期30 d的暂养,并在此期间对红鳍东方鲀幼鱼进行剪牙,以防止红鳍东方鲀幼鱼相互残食,提高养殖的成活率。选择体表无外伤、规格均一、平均初始体重为(19.11±0.5) g的红鳍东方鲀幼鱼120尾,随机分配到6个鱼缸中(200 L),每个实验组设置3个平行缸,每缸20尾鱼,在室内海水流水系统中饲养8周。每天投喂2次(07:00和18:00),投喂量为每缸鱼体重的3%~4%。养殖过程中DO、温度、盐度和pH分别保持为(6.5±0.5) mg/L、(25.0±1.0) ℃、27.5±2.5和7.5±0.5。
1.3 急性低氧应激实验和样品采集56 d养殖结束后,将所有实验鱼禁食12 h,每缸随机选取10尾大小均匀的鱼(30条/处理组)进行急性低氧应激实验。将实验鱼转移到定制的低氧设备中,随着鱼的呼吸作用和时间的推移,水体DO从6.5 mg/L持续降低至1.0 mg/L,每隔2 h观察并记录各组实验鱼在急性低氧条件下的死亡数量,计算存活率。
采样分为常氧采样和低氧采样,禁食12 h后,每个处理挑选12条鱼(4条/每缸),其中,6条放入低氧装置中,DO保持在(2.5±0.5) mg/L;另外6条继续保持在常氧状态[(6.5±0.5) mg/L],6 h后同时采样,收集血清、肝脏和肌肉,液氮速冻后,于–80 ℃冰箱中保存。同时,每个处理组随机选取6尾实验鱼,测定形体指标和全鱼营养成分。
1.4 全鱼粗成分和生化指标测定饲料和全鱼样品水分测定采用105 ℃直接干燥法在烘箱中干燥24 h,以计算水分和干物质含量;使用凯氏定氮法(N×6.25)测定粗蛋白含量;使用索氏抽提法(Foss-Tecator,Hoganas,瑞典)通过石油醚提取和测定粗脂肪含量。粗灰分采用马弗炉(Thermo F6000型)高温灼烧法测定,550 ℃下加热8 h,计算粗灰分含量。
血清、肝脏和肌肉中丙酮酸、乳酸、甘油三酯(TG)、总蛋白(TP)以及肝脏和肌肉中的糖原、血清中葡萄糖的含量均使用商业试剂盒(南京建城生物工程研究所)测定。所有测定步骤均严格按照相应试剂盒的说明书进行,使用酶标仪(Tecan infinite M200,瑞士)读取吸光度。
1.5 RNA提取与基因表达测定使用RNAiso Plus试剂盒(TaKaRa,大连)提取肝脏和肌肉中的RNA。RNA的纯度和浓度采用Titertek Berthold Colibri光谱仪(Colibri,德国)检测。所有RNA样品的260 nm/280 nm的吸光度比值均在1.9~2.0范围内,表明提取的RNA样品的纯度较高,可以满足后续分析。使用Evo M-MLV RT Mix试剂盒(艾科瑞生物,中国)合成第一链cDNA。实时荧光定量PCR的内参基因(rpl19和β-actin)和目标基因(表 2)的引物在NCBI网站设计,由擎科生物科技有限公司合成。实时荧光定量PCR反应体系为10 μL,包括5 μL 2×SYBR混合物、1 μL cDNA(20 ng)、0.3 μL上游引物(10 μmol/L)、0.3 μL下游引物(10 μmol/L)和3.4 μL无酶无菌水。在Roche LightCycler 96实时荧光定量PCR仪(Roche,瑞士)中执行以下程序:95 ℃ 30 s;94 ℃ 5 s,60 ℃ 30 s循环40次,并执行熔解曲线以确保PCR结束时扩增产物的特异性。引物的扩增效率均在90%~110%之间,根据方程式E=10(−1/斜率)–1,计算扩增效率(Ma et al, 2021),并使用2–ΔΔCt方法计算目标基因的相对表达量(Livak et al, 2001)。
|
|
表 2 PCR引物序列 Tab.2 Primers sequences of PCR |
本研究所有数据均使用SPSS 25.0软件(IBM公司,美国)进行独立样本T检验分析,结果以平均值±标准误(Mean±SE)表示,P < 0.05为差异显著。
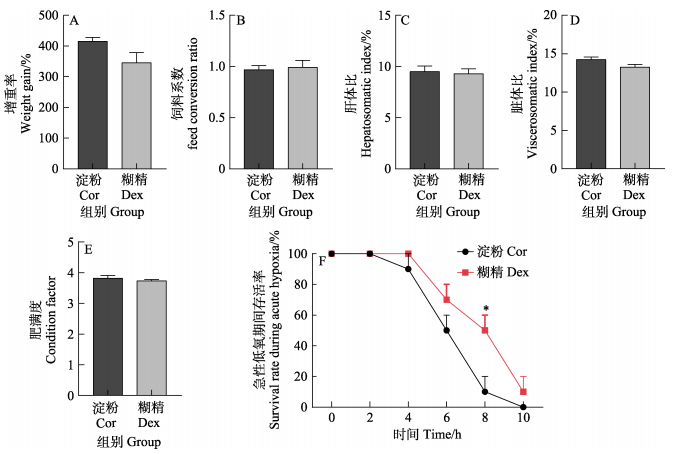
2 结果 2.1 淀粉和糊精对红鳍东方鲀生长和低氧耐受性的影响8周的养殖实验结束后,玉米淀粉组(Cor)和糊精组(Dex)的增重率、饲料系数、肝体比、脏体比和肥满度均无显著差异,但糊精组的增重率有降低的趋势(P > 0.05)。当水体DO降低至2 mg/L以下时,红鳍东方鲀开始出现死亡。随着低氧时间的延长,糊精组在低氧条件下的存活率均高于淀粉组,在低氧8 h出现显著性差异(P < 0.05)。以上结果说明,与淀粉组相比,糊精不会影响红鳍东方鲀的生长,但可提高其对急性低氧的耐受性。
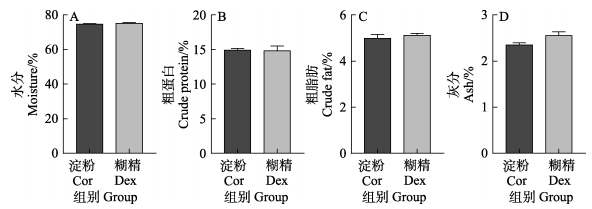
2.2 淀粉和糊精对鱼体营养成分的影响如图 2所示,与淀粉组相比,糊精组红鳍东方鲀全鱼的水分、粗蛋白、粗脂肪和灰分含量均无显著差异(P > 0.05)。说明与淀粉组相比,糊精对红鳍东方鲀鱼体营养成分无显著影响。
|
图 1 淀粉和糊精对红鳍东方鲀生长和低氧耐受性的影响 Fig.1 Effects of starch and dextrin on growth and hypoxia tolerance *代表淀粉组和糊精组间存在显著性差异,P < 0.05。 * means significant difference between starch group and dextrin group, P < 0.05. |
|
图 2 淀粉和糊精对鱼体成分的影响 Fig.2 Effects of starch and dextrin on body composition |
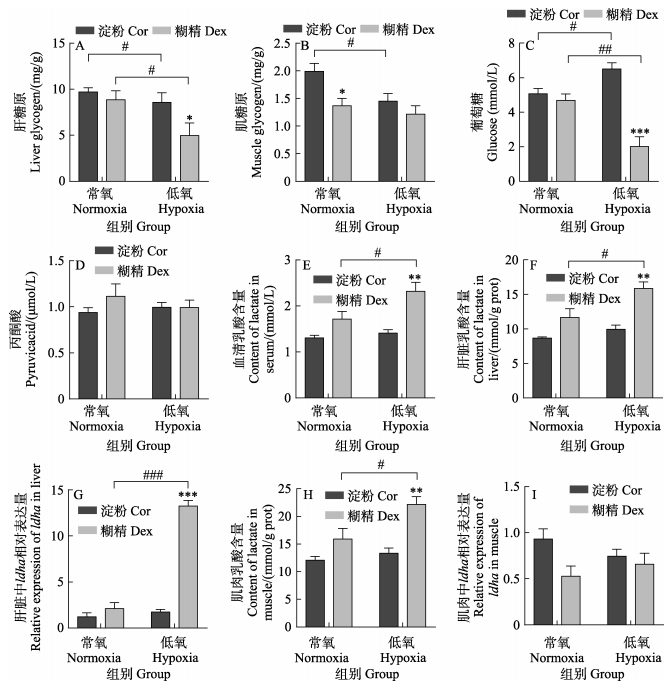
如图 3所示,在常氧条件下,淀粉组和糊精组肝脏和肌肉中的乳酸含量、ldha基因表达量、血清的葡萄糖、丙酮酸和乳酸含量均无显著差异(P > 0.05),肝糖原含量也无显著差异(P > 0.05),肌糖原略低于淀粉组(P < 0.05)。在低氧条件下,糊精组的肝糖原和血清葡萄糖含量显著低于淀粉组(P < 0.05),但血清、肝脏和肌肉的乳酸含量、肝脏的ldha基因表达量均显著高于淀粉组(P < 0.05)。与常氧组相比,淀粉组和糊精组的肝糖原含量在低氧后均显著降低(P < 0.05),说明低氧条件下红鳍东方鲀主要通过分解糖来提供能量。与常氧糊精组相比,低氧糊精组的血清葡萄糖含量显著降低(P < 0.05),同时,血清、肝脏和肌肉的乳酸含量、肝脏ldha基因表达量均显著升高(P < 0.05),而这些指标在低氧前后的淀粉组间却无显著性差异。以上结果表明,急性低氧条件下,糊精组可以更好地分解糖原,激活无氧糖酵解来提供能量。
|
图 3 淀粉和糊精对葡萄糖代谢的影响 Fig.3 Effects of starch and dextrin on glucose metabolism *、**、***代表同一O2水平下的淀粉组和糊精组间存在显著性差异,显著性水平分别为P < 0.05、P < 0.01、P < 0.001;#、##、###代表同一糖源下的常氧组和低氧组间存在显著性差异,显著性水平分别为P < 0.05、P < 0.01、P < 0.001。下同。 *, **, *** mean significant differences between starch group and dextrin group at the same oxygen levels, the significance levels are P < 0.05, P < 0.01, and P < 0.001, respectively; #, ##, ### mean significant differences between normoxia group and hypoxia group at the same carbohydrate sources, the significance levels are P < 0.05, P < 0.01, and P < 0.001, respectively. The same below. |
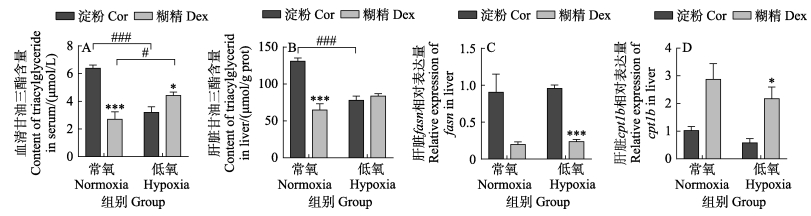
在常氧条件下,糊精组血清和肝脏的甘油三酯含量显著低于淀粉组(P < 0.05,图 4A、B),急性低氧条件下,糊精组血清中甘油三酯含量显著上升(P < 0.05),且显著高于淀粉组(P < 0.05),但淀粉组血清和肝脏的甘油三酯含量在低氧后均显著下降(P < 0.05)。此外,不论是在常氧还是低氧条件下,糊精组肝脏中与脂合成相关的fasn (fatty acid synthase)基因表达量均低于淀粉组(P < 0.05,图 4C),脂分解相关的cpt1b (carnitine palmitoyltransferase 1B)基因表达量均高于淀粉组(P < 0.05,图 4D)。以上结果说明,在急性低氧条件下,淀粉组促进了脂肪的分解,加剧了O2的消耗,而糊精组抑制了脂肪的分解,降低了鱼体O2的需求。
|
图 4 淀粉和糊精对脂质代谢的影响 Fig.4 Effects of starch and dextrin on lipid metabolism |
如图 5所示,在常氧条件下,糊精组血清和肌肉的可溶性总蛋白含量与淀粉组无显著差异(P > 0.05,图 5A、C),但糊精组肝脏的可溶性总蛋白含量和mtor(mechanistic target of rapamycin kinase)基因表达量显著高于淀粉组(P < 0.05,图 5B、E)。在常氧条件下,糊精组和淀粉组肌肉的可溶性总蛋白含量无差异,但低氧后,糊精组肌肉的可溶性总蛋白含量显著低于淀粉组(P < 0.05)。与常氧组相比,糊精组在低氧后的肌肉可溶性总蛋白含量、肝脏akt1 (v-akt murine thymoma viral oncogene homolog 1)(图 5D)和mtor基因表达量均显著性降低。与之相反,淀粉组肝脏的mtor基因表达在低氧后却显著升高。以上结果表明,在急性低氧条件下,糊精组会抑制肝脏中蛋白的合成。
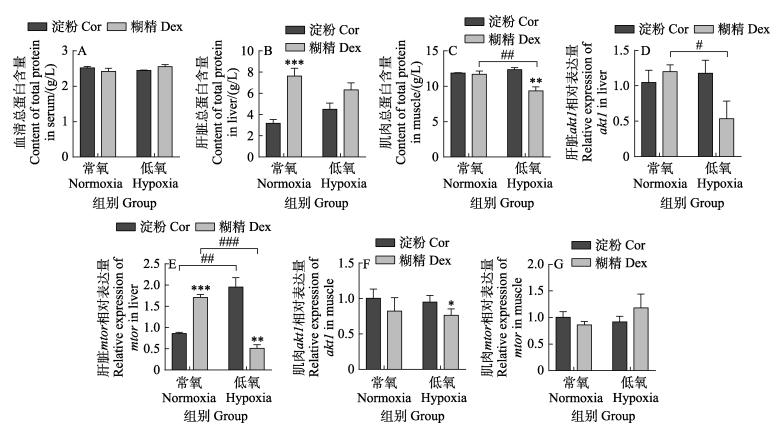
|
图 5 淀粉和糊精对蛋白质代谢的影响 Fig.5 Effects of starch and dextrin on protein metabolism |
红鳍东方鲀肝脏和肌肉中的hif-1α、hif-2α (endothelial PAS domain protein 1b)、hif-3α (hypoxia inducible factor 1 subunit alpha, like)以及HIF信号通路下游靶基因vegfa (vascular endothelial growth factor A)基因表达情况见图 6。结果显示,在常氧条件下,与淀粉组相比,糊精组显著提高了肝脏hif-3α的表达量,抑制了肌肉hif-1α的表达量(P < 0.05)。在低氧条件下,糊精组肝脏中的hif-1α和hif-3α、肌肉中的hif-1α和vegfa基因表达量均显著高于淀粉组(P < 0.05)。与常氧组相比,低氧条件下的糊精组肝脏和肌肉的hif-1α的表达量均显著升高(P < 0.05),但在淀粉组低氧前后无显著差异(P > 0.05)。此外,与常氧组相比,低氧条件下的淀粉组和糊精组的肝脏hif-2α表达量显著降低(P < 0.05),hif-3α和vegfa的表达量均显著升高(P < 0.05)。上述数据表明,糊精组在低氧条件下可以更好地更激活HIF介导的低氧信号通路,促进红鳍东方鲀对低氧的适应。

|
图 6 淀粉和糊精对HIF信号通路的影响 Fig.6 Effects of starch and dextrin on HIF signal pathway |
糖类(如淀粉)是水产饲料中重要且廉价的能量来源,添加适宜水平的糖类可以起到节约饲料中蛋白质和脂肪的效果。但与哺乳动物不同,鱼类对淀粉的消化和利用能力有限,尤其是肉食性鱼类,摄入高糖饲料会造成持续高血糖和代谢紊乱等(Moon, 2001)。与高分子的淀粉不同,糊精是淀粉经水解之后形成的中间衍生物,其分子质量处于淀粉和葡萄糖之间,更容易被消化和吸收(Mao et al, 2021)。研究表明,随着饲料中糊精水平的增加,牙鲆(Paralichthys olivaceus)的特定生长率和饲料效率也随之增加,同时,全鱼和肝脏的脂质含量也显著降低(Lee et al, 2003)。在暗纹东方鲀(Takifugu obscurus)幼鱼中,在饲料中添加25%的糊精作为糖源可使其特定生长率和饲料效率达到最高,但更高水平的糊精则会抑制生长(刘襄河等, 2013)。在饲料中添加25%的糊精作为饲料糖源对乌克兰鳞鲤(Cyprinus carpio)生长和糖代谢起到促进作用(高妍等, 2015)。此外,在鲤鱼(Cyprinus carpio)饲料中分别添加0、7.5%和15%的糊精对其生长未产生显著影响(韩依笑等, 2021)。在本研究中,在饲料中添加23%的糊精替代淀粉对红鳍东方鲀的生长性能和鱼体成分均无显著影响。以上研究说明,不同鱼类对饲料中糊精的利用能力存在差异,糊精替代淀粉不会影响红鳍东方鲀的生长性能。
3.2 淀粉和糊精对红鳍东方鲀糖酵解通路的影响低氧是鱼类养殖过程中常见的环境应激之一,鱼类可以通过激活无氧糖酵解来适应急性低氧(Ma et al, 2021)。研究表明,饲喂高糖饲料可激活鱼类肝脏中的己糖激酶(HK)、葡萄糖激酶(GK)、磷酸果糖激酶(PFK)、丙酮酸激酶(PK)等糖酵解关键酶的活性和基因的表达(Enes et al, 2006; Fernández et al, 2007; 张世亮等, 2012)。在大菱鲆(Scophthalmus maximus)饲料中添加15%的糊精可显著提高肝脏GK和PK等糖酵解相关酶的活性(聂琴等, 2013)。研究发现,急性低氧显著降低了银曼龙鱼(Trichogaster microlepis)和大口黑鲈肝脏的糖原含量,增加了血清和肝脏中乳酸的含量(Huang et al, 2015; Yang et al, 2019)。当哺乳动物低氧时,无氧糖酵解被激活以产生能量并减少O2消耗,此途径受到HIF信号通路的调节(Kim et al, 2006),hif-1α通常响应急性低氧反应,而hif-2α则在慢性低氧过程中发挥重要作用(Holmquist-Mengelbier et al, 2006)。在低氧条件下,细胞质中积累的Hif1α入核与Hifβ形成二聚体,并与含有低氧反应元件(HRE)的靶基因的启动子区域结合,激活糖酵解(glut、hk、pfk和ldh)、红细胞生成(epo)和血管生成(vegfa)等相关靶基因的表达,从而促进鱼类对低氧的适应(Zhu et al, 2013。在麦瑞加拉鲮鱼(Cirrhinus mrigala)中,急性低氧增加了鳃中hif-1α的mRNA表达,也提高了肝脏和肌肉中无氧糖酵解相关酶(如HK、LDH和G6pase)的活性(Varghese et al, 2018)。在斑马鱼中,高糖饲料促进了hif-3α的基因表达,激活了无氧糖酵解途径,提高了斑马鱼对急性低氧的耐受性(Ma et al, 2020)。在本研究中也出现了类似的结果,在低氧条件下,糊精组的肝脏糖原和血清葡萄糖含量显著低于淀粉组,但血清、肝脏和肌肉的乳酸含量、肝脏的ldha基因表达量均显著高于淀粉组。同时,糊精组肝脏中hif-1α和hif-3α、肌肉中hif-1α和vegfa的基因表达量均显著高于淀粉组。以上结果表明,在急性低氧条件下,糊精可更好地激活HIF介导的无氧糖酵解通路,促进了糖原的分解,生成乳酸和ATP,为鱼体提供更多的能量,从而增强其对急性低氧的耐受能力。
3.3 淀粉和糊精对红鳍东方鲀脂肪和蛋白代谢的影响目前,大多数的鱼类低氧研究都聚焦于糖代谢,却忽略了低氧条件下营养素对脂肪和蛋白代谢的影响。Sun等(2020)研究发现,急性低氧8 h后,大口黑鲈肝脏中甘油三酯和非酯化脂肪酸的含量均显著升高,而在复氧12 h后,糖异生和脂肪氧化利用显著增加。在大菱鲆中,急性低氧增加了肝脏中hif-2α、hif-3α和脂质合成相关基因的表达量和甘油三酯的含量,同时,脂质分解和蛋白质合成受到抑制,从而降低了耗氧率(Ma et al, 2023)。小鼠在低氧条件下缺乏能量,激活了ampk基因,并抑制了mtor基因表达和蛋白质合成(Cassidy et al, 2019)。同样,在亚马逊慈鲷(Astronotus ocellatus)和鲫鱼(Carassius carassius)中也发现,急性低氧抑制了肝脏和肌肉中的蛋白质合成速率(Cassidy et al, 2018; Smith et al, 1996)。在本研究中,与常氧组相比,在低氧条件下淀粉组促进了血清和肝脏中脂肪的分解和mtor基因的表达量,而在低氧后糊精组降低了脂肪的分解、肌肉可溶性总蛋白含量和肝脏akt1和mtor基因的表达量。因此,在饲料中添加糊精抑制了红鳍东方鲀鱼体脂肪的分解和蛋白的合成,减少了O2的消耗,提高了对急性低氧的耐受性。
4 结论急性低氧会造成养殖鱼类大量死亡。本研究发现,用易消化的糊精替代饲料中的淀粉不会影响红鳍东方鲀的生长性能,但激活了HIF信号通路介导的无氧糖酵解来为鱼体提供更多的能量,同时还可以抑制脂肪的分解和蛋白质的合成,减少O2的消耗,从而提高红鳍东方鲀对急性低氧的耐受性。本研究结果从营养学角度提出了提高鱼类对急性低氧耐受性的有效方法,为水产养殖业的健康发展提供了重要指导。
ALVIRA E. Theoretical study of structures and charge distributions of 2-, 3-and 6-hydroxypropyl-β-cyclodextrin with different degrees of substitution. Journal of Biomolecular Structure and Dynamics, 2022, 1-9 |
Bureau of Fisheries Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China society of Fisheries. China fishery statistical yearbook 2022. Beijing: China Agriculture Press, 2022 [农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2022中国渔业统计年鉴. 北京: 中国农业出版社, 2022]
|
CAO M, WANG X Q, QIN C X, et al. Transcriptome analysis of Palaemon carincauda subjected to hypoxic stress. Progress in Fishery Sciences, 2021, 42(2): 112-123 [曹梅, 王兴强, 秦传新, 等. 脊尾白虾对低氧响应的转录组学分析. 渔业科学进展, 2021, 42(2): 112-123 DOI:10.19663/j.issn2095-9869.20190924001] |
CASSIDY A A, DRIEDZIC W R, CAMPOS D, et al. Protein synthesis is lowered by 4EBP1 and eIF2-α signaling while protein degradation may be maintained in fasting, hypoxic Amazonian cichlids Astronotus ocellatus. Journal of Experimental Biology, 2018, 221(2): jeb167601 |
CASSIDY A A, LAMARRE S G. Activation of oxygen-responsive pathways is associated with altered protein metabolism in Arctic char exposed to hypoxia. Journal of Experimental Biology, 2019, 222(22): jeb203901 |
CUNINGHAME S, JACKSON R, LEES S J, et al. Two common variants of human papillomavirus type 16 E6 differentially deregulate sugar metabolism and hypoxia signalling in permissive human keratinocytes. Journal of General Virology, 2017, 98(9): 2310-2319 DOI:10.1099/jgv.0.000905 |
DING F F, ZHOU N N, ZHANG L, et al. Effects of sodium cholate on growth and glucose metabolism of largemouth bass (Micropterus salmoides). Acta Hydrobiologica Sinica, 2022, 46(8): 1177-1186 [丁斐斐, 周楠楠, 张乐, 等. 饲料中添加胆酸钠对大口黑鲈生长及糖代谢的影响. 水生生物学报, 2022, 46(8): 1177-1186] |
ENES P, PANSERAT S, KAUSHIK S, et al. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 2006, 143(1): 89-96 DOI:10.1016/j.cbpa.2005.10.027 |
FERNÁNDEZ F, MIQUEL A G, CÓRDOBA M, et al. Effects of diets with distinct protein-to-carbohydrate ratios on nutrient digestibility, growth performance, body composition and liver intermediary enzyme activities in gilthead sea bream (Sparus aurata, L.) fingerlings. Journal of Experimental Marine Biology and Ecology, 2007, 343(1): 1-10 DOI:10.1016/j.jembe.2006.10.057 |
GAO Y, LI J H, FANG Z Z, et al. Effects of dietary dextrin level on growth and carbohydrate metabolism of ukraine scaly carp (Cyprinus carpio). Chinese Journal of Animal Nutrition, 2015, 27(5): 1401-1410 [高妍, 李静辉, 方珍珍, 等. 饲料中糊精水平对乌克兰鳞鲤生长及糖代谢的影响. 动物营养学报, 2015, 27(5): 1401-1410 DOI:10.3969/j.issn.1006-267x.2015.05.010] |
HAN Y X, TANG Y, GUO L, et al. Effects of low nitrogen feed with different dextrin content on growth and physiological and biochemical indexes of common carp (Cyprinus carpio). Journal of Tianjin Agricultural University, 2021, 28(1): 34–39, 44 [韩依笑, 汤雨, 郭立, 等. 不同糊精含量低氮饲料对鲤生长及生理生化指标的影响. 天津农学院学报, 2021, 28(1): 34–39, 44 DOI:10.19640/j.cnki.jtau.2021.01.007] |
HOLMQUIST-MENGELBIER L, FREDLUND E, LÖFSTEDT T, et al. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell, 2006, 10(5): 413-423 DOI:10.1016/j.ccr.2006.08.026 |
HUANG C Y, LIN H C, LIN C H, et al. Effects of hypoxia on ionic regulation, glycogen utilization and antioxidative ability in the gills and liver of the aquatic air-breathing fish Trichogaster microlepis. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 2015, 179: 25-24 DOI:10.1016/j.cbpa.2014.09.001 |
KIM J, TCHERNYSHYOV I, SEMENZA G L, et al. HIF-1- mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism, 2006, 3(3): 177-185 DOI:10.1016/j.cmet.2006.02.002 |
LEE S M, KIM K D, LALL S P. Utilization of glucose, maltose, dextrin and cellulose by juvenile flounder (Paralichthys olivaceus). Aquaculture, 2003, 221: 427-438 DOI:10.1016/S0044-8486(03)00061-9 |
LI L, ZHANG F R, LIU C L, et al. Composition analysis of farmed and wild Yadong trout (Salmo trutta fario). Progress in Fishery Sciences, 2023, 44(2): 77-86 [李琳, 张斐然, 刘长琳, 等. 养殖和野生亚东鲑机体成分比较分析. 渔业科学进展, 2023, 44(2): 77-86] |
LI M X, WANG X D, QI C L, et al. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture, 2018, 495: 187-195 DOI:10.1016/j.aquaculture.2018.05.031 |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 2001, 25(4): 402-408 DOI:10.1006/meth.2001.1262 |
LU X H, YE C X, ZHENG L M, et al. Effect of dietary dextrin levels on growth, activities of digestive enzyme and blood biochemical indices of juvenile obscure puffer (Takifugu obscurus). Journal of Fisheries of China, 2013, 37(9): 1359-1368 [刘襄河, 叶超霞, 郑丽勉, 等. 饲料糊精水平对暗纹东方鲀幼鱼生长, 消化酶活性和血液生化指标的影响. 水产学报, 2013, 37(9): 1359-1368] |
MA Q, HU C T, YUE J, et al. High-carbohydrate diet promotes the adaptation to acute hypoxia in zebrafish. Fish Physiology and Biochemistry, 2020, 46(2): 665-679 DOI:10.1007/s10695-019-00742-2 |
MA Q, WANG X, LI L Y, et al. High protein intake promotes the adaptation to chronic hypoxia in zebrafish (Danio rerio). Aquaculture, 2021, 535: 736356 DOI:10.1016/j.aquaculture.2021.736356 |
MA Q, XU H, WEI Y, et al. Effects of acute hypoxia on nutrient metabolism and physiological function in turbot, Scophthalmus maximus. Fish Physiology and Biochemistry, 2023 DOI:10.1007/s10695-022-01154-5 |
MAO H, CHEN Z, LI J, et al. Structural comparisons of pyrodextrins during thermal degradation process: The role of hydrochloric acid. Food Chemistry, 2021, 349: 129174 DOI:10.1016/j.foodchem.2021.129174 |
MOON T W. Glucose intolerance in teleost fish: Fact or fiction?. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2001, 129(2/3): 243-249 |
NIE Q, MIAO H J, MIAO SHU Y, et al. Effects of dietary carbohydrate sources and levels on the activities of carbohydrate metabolic enzymes in turbot. Acta Hydrobiologica Sinica, 2013, 37(3): 425-433 [聂琴, 苗惠君, 苗淑彦, 等. 不同糖源及糖水平对大菱鲆糖代谢酶活性的影响. 水生生物学报, 2013, 37(3): 425-433] |
POLYMEROPOULOS E T, ELLIOTT N G, FRAPPELL P B. Hypoxic acclimation leads to metabolic compensation after reoxygenation in Atlantic salmon yolk-sac alevins. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 2017, 213: 28-35 DOI:10.1016/j.cbpa.2017.08.011 |
RABALAIS N N, TURNER R E, WISEMAN JR W J. Gulf of Mexico hypoxia, aka "The dead zone". Annual Review of Ecology and Systematics, 2002, 33(1): 235-263 DOI:10.1146/annurev.ecolsys.33.010802.150513 |
RUAN W, JI W W, ZHENG L, et al. On hypoxia stress in fish and its nutritional regulation and response. Marine Fisheries, 2020, 42(6): 751-761 [阮雯, 纪炜炜, 郑亮, 等. 鱼类低氧胁迫及营养调控和应对研究进展. 海洋渔业, 2020, 42(6): 751-761 DOI:10.3969/j.issn.1004-2490.2020.06.011] |
SMITH R W, HOULIHAN D F, NILSSON G E, et al. Tissue- specific changes in protein synthesis rates in vivo during anoxia in crucian carp. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology, 1996, 271(4): R897-R904 DOI:10.1152/ajpregu.1996.271.4.R897 |
SUN J L, ZHAO L L, WU H, et al. Acute hypoxia changes the mode of glucose and lipid utilization in the liver of the largemouth bass (Micropterus salmoides). Science of the Total Environment, 2020, 713: 135157 DOI:10.1016/j.scitotenv.2019.135157 |
TACON A G J, METIAN M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture, 2008, 285(1/2/3/4): 146-158 |
VARGHESE T, PAL A K, MISHAL P, et al. Physiological and molecular responses of a bottom dwelling carp, Cirrhinus mrigala to short-term environmental hypoxia. Turkish Journal of Fisheries and Aquatic Sciences, 2018, 18(3): 483-490 |
XU H, CHEN X M, WANG G Q, et al. Research progress of hypoxia on aquaculture. Feed Industry, 2016, 37(2): 33-37 [徐贺, 陈秀梅, 王桂芹, 等. 低氧胁迫在水产养殖中的研究进展. 饲料工业, 2016, 37(2): 33-37] |
YANG S, WU H, HE K, et al. Response of AMP-activated protein kinase and lactate metabolism of largemouth bass (Micropterus salmoides) under acute hypoxic stress. Science of the Total Environment, 2019, 666: 1071-1079 DOI:10.1016/j.scitotenv.2019.02.236 |
ZHANG S L, AI Q H, XU W, et al. Effects of dietary carbohydrate to lipid ratio on growth, feed utilization, plasma glucose and glycolytic enzyme activities of juvenile darkbarbel catfish, Pelteobagrus vachelli. Acta Hydrobiologica Sinica, 2012, 36(3): 466-473 [张世亮, 艾庆辉, 徐玮, 等. 饲料中糖/脂肪比例对瓦氏黄颡鱼生长、饲料利用、血糖水平和肝脏糖酵解酶活力的影响. 水生生物学报, 2012, 36(3): 466-473] |
ZHU C X, ZHU Q, WANG C, et al. Hostile takeover: manipulation of HIF-1 signaling in pathogen-associated cancers. International Journal of Oncology, 2016, 49(4): 1269-1276 |
ZHU C D, WANG Z H, YAN B. Strategies for hypoxia adaptation in fish species: A review. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 2013, 183(8): 1005-1013 |



