2. 中国水产科学研究院黄海水产研究所 农业农村部水产品质量安全检测与评价重点实验室 山东 青岛 266071;
3. 青岛海洋科学与技术试点国家实验室 山东 青岛 266071
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, National Key Laboratory of Testing and Evaluation for Aquatic Product Safety and Quality, Ministry of Agriculture and Rural Affairs, Qingdao 266071, China;
3. Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266071, China
麻痹性贝类毒素(Paralytic shellfish toxin, PSTs)因其分布广、毒性强等特征而被认为是目前世界上危害最大的藻神经毒素之一(Fernández-Reiriz et al, 2008)。研究发现,PSTs极易在双壳贝类中大量蓄积,通过复杂的代谢转化生成50余种衍生物(Wiese et al, 2010),并通过食物链传递,导致消费者出现恶心呕吐、肌肉麻痹、呼吸困难甚至窒息等症状,引发人类食物中毒事件(Etheridge, 2010)。因此,国际上针对PSTs建立了800 μg STX eq/kg的广泛适用的限量标准(刘智勇等, 2006)。塔玛亚历山大藻(Alexandrium tamarense)是中国沿海的优势产毒藻之一(Lilly et al, 2010),在渤海、黄海、长江口附近沿海水域和南海部分海湾均有分布(Li et al, 2020a、b; 窦勇等, 2015; 过锋等, 2011)。近年来调查发现,栉孔扇贝(Chlamys farreri)中PSTs检出率、超标率均较高(钱蓓蕾等, 2012; 杜克梅等, 2013; 刘斌等, 2021),且具有毒素蓄积速度快、代谢慢的特点(Bricelj et al, 1998),其内脏团是PST吸收和净化过程中的主要储存库,占总毒性的80%~98% (Estrada et al, 2007a)。
PSTs是一种神经性毒素,其毒性作用为阻断钠离子通道并抑制神经传导。研究表明,双壳贝类暴露于PSTs后,主要的应激反应包括产生大量活性氧(ROS),诱导抗氧化应激(包括酶促和非酶促防御)反应(Sebastian et al, 2011)、细胞内氧化还原稳态失衡(Estrada et al, 2007b)和导致细胞损伤(即脂质过氧化) (Qiu et al, 2013)。丙二醛(MDA)作为脂质过氧化的主要产物之一,其含量变化水平能直接反映PSTs引起的组织细胞膜损伤情况(梁忠秀等, 2014)。另外,超氧化物歧化酶(SOD)、过氧化物酶(POD)常被作为抗氧化水平评价指标,谷胱甘肽过氧化物酶(GSH-Px)是在抗氧化防御中发挥作用的关键酶(Hlaing et al, 2020)。现有研究常用脂质过氧化水平和抗氧化酶的改变来反映生物体的损伤与应激程度(Tsikas, 2017)。也有研究表明,PSTs可造成组织损伤并引发栉孔扇贝基因异常表达(Lian et al, 2019; Hu et al, 2019),但目前仍缺乏关于PSTs引发栉孔扇贝组织损伤的基因表达变化及调控机制的研究。
因此,本研究以一株分离自我国南海海域的产PSTs的塔玛亚历山大藻AT5-3株为实验对象,以目前我国PSTs残留严重的栉孔扇贝为受试动物进行暴露实验,通过在PSTs暴露过程中栉孔扇贝内脏团细胞的超微结构、脂质过氧化关键指标和关键抗氧化酶活性变化等指标探究栉孔扇贝的损伤与生理响应情况,通过对关键节点的转录组分析,解析在损伤和应激过程中的差异富集通路和表达基因。通过科学评价PSTs对栉孔扇贝损伤情况及诱发机体应激的相关机理,以期为建立与完善食品安全风险评价技术提供参考。
1 材料与方法 1.1 实验材料本实验所用产PSTs毒藻为一株分离自中国南海海域的塔玛亚历山大藻AT5-3株,由中国科学院海洋研究所提供。该毒藻在实验室中以温度(20±1) ℃、光照54 µ/(Em2·s)、光暗比12 h︰12 h的条件,使用L1 Medium培养液进行单种培养。选择处于指数生长期,细胞密度为4×104~4.2×104 cells/mL的藻液用于暴露实验。小球藻作为饵料藻同时培养,培养条件同AT5-3株。
本研究所用实验动物为2龄栉孔扇贝,产自山东省青岛市灵山湾养殖海域,正式实验前进行饥饿处理2 d。所用海水取自青岛海域,实验期间水温控制在(16±1) ℃,保持全天不间断充气。
1.2 栉孔扇贝暴露实验随机选取120只栉孔扇贝置于6个养殖筐,设立对照组和实验组。实验共开展20 d,分暴露阶段(0~6 d)和代谢阶段(6~20 d),暴露阶段2次/d定时投喂指数生长期的塔玛亚历山大藻(AT5-3株),投喂量为8×106 cells/(ind.∙d)。代谢阶段投喂等量小球藻。
1.3 毒素含量测定分别于第0、6、14和20天随机取栉孔扇贝5只,蒸馏水冲洗污泥及附着物后,在冰上迅速解剖分离其内脏团,均质后称量(5.00±0.05) g样品于50 mL离心管中,加入5 mL 1%乙酸水溶液,涡旋混合90 s后沸水浴5 min,于流水下迅速冷却至室温,4 500 r/min离心10 min。取上清液1 mL于2 mL离心管中,加5 µL氨水涡旋混匀,10 000 r/min离心5 min。依次用3 mL乙腈,3 mL 20%乙腈水溶液(含1%乙酸)、3 mL 0.1%氨水溶液活化Supelco ENVI-Carb固相萃取柱,加入提取液后用700 µL超纯水淋洗,再用1 mL 75%乙腈水溶液(含0.25%甲酸)洗脱混匀,最后13 000 r/min离心10 min,取上清液于–80 ℃条件下保存待测。
采用外标法定量,用75%乙腈水(含0.25%甲酸)稀释PSTs标准品溶液配制成标准储备液,再将标准储备液稀释为基质标准工作液。用四极杆–线性阱复合型液相色谱质谱联用仪、HILLIC色谱柱进行检测,质谱条件参考张海涛等(2021)的研究。
1.4 透射电镜样品制备与观察分别取对照组(X-C)、实验第6天(X-6)、实验第14天(X-14)栉孔扇贝各3只,在冰上分离其内脏团,将组织切成2 mm×2 mm×1 mm的小块后用2.5%戊二醛溶液固定,超薄切片制备方法参照甄静静等(2018),使用透射电子显微镜(日立H7000)观察切片并拍照。
1.5 生理指标测定样品分别取对照组(C)、实验第1天、实验第3天、实验第6天栉孔扇贝各3只,在冰上分离各组织之后准确称量,随后加入任氏生理盐水,加入比例为重量(g)∶体积(mL)= 1∶9。冰水浴中匀浆,随后4 ℃ 2 500 r/min离心10 min,取上清液转移至新的1.5 mL离心管中,于液氮中保存。按MDA、POD、GSH-Px和SOD试剂盒(南京建成科技有限公司)说明书分别测定其含量及活力。
1.6 转录组分析分别取对照组(X-C)、实验第3天(X-3)、实验第6天(X-6)栉孔扇贝各3只,冰上取其内脏团后使用液氮迅速冷冻。由北京百迈客生物科技有限公司进行RNA提取、cDNA文库构建及高通量测序。使用TRIzol试剂提取内脏团总RNA,浓度用NanoDrop 2000 (ThermoFisher Scientific,美国)检测,完整性用Agilent 2100 (Agilent Technologies,美国)检测。检测合格后进行cDNA文库构建,并使用Illumina HiSeq (Illumina公司,美国)进行测序。
将得到原始序列进行过滤后得到高质量clean reads,并使用Trinity软件进行de novo组装。取每条基因中最长的转录本作为unigenes与KEGG数据库进行序列比对得到注释信息。
以FPKM值衡量基因表达水平,以校正后的P值(Fold change) < 0.05且错误发现率(FDR)≥2作为标准筛选差异表达基因(DEGs),随后用DSEeq2软件对DEGs进行分析。最后在KEGG、GO数据库中对DEGs进行注释和功能富集分析(以P < 0.05为富集标准)以探究DEGs功能及其与代谢物之间的关系。
1.7 基因共表达网络构建在R软件中运行R包,剔除离群值后构建WGCNA基因共表达网络,选择适当β值,构建拓扑重叠矩阵,再绘制基因的层次聚类树,以皮尔逊相关系数(r)为指标选择目标模块进行KEGG注释,随后使用cytoscape软件绘制基因互作网络分析图,以寻找核心调控基因。
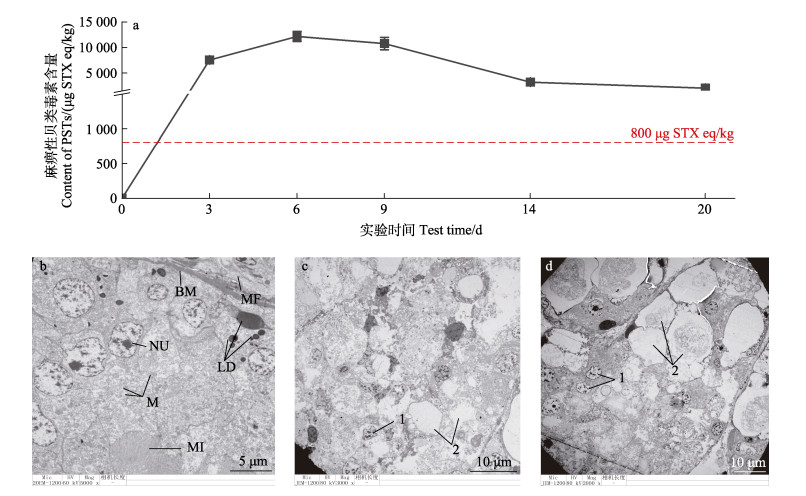
2 结果 2.1 栉孔扇贝毒素蓄积与超微结构损伤PSTs在内脏团中的蓄积含量呈现先升后降的趋势,在实验第6天时达到峰值,最高蓄积含量超出限量标准15倍左右(图 1a)。蓄积速率由快转慢,结束毒素暴露后,PSTs在栉孔扇贝内脏团中缓慢代谢,代谢14 d后PSTs含量仍然超出限量标准,残留率高达62.4%。透射电镜观察发现内脏团随PSTs暴露时间出现明显组织结构损伤,主要包括肠上皮细胞间黏附力下降、部分细胞的细胞膜及线粒体等细胞器消失,并出现了细胞核质固缩、染色质凝聚和细胞空泡化现象(图 1c),实验第14天时虽然毒素含量下降,但组织损伤更为严重(图 1d)。
|
图 1 不同时间栉孔扇贝中PSTs蓄积量和内脏团组织超微结构变化 Fig.1 The accumulation of PSTs and its ultrastructural damage in C. farreri a:PSTs在内脏中的蓄积含量;b:对照组;c:X-6组;d:X-14组。NU:细胞核;M:线粒体;MI:微绒;BM:基底膜;MF:肌纤维;LD:脂滴。1:细胞核质固缩、染色质凝聚;2:细胞空泡化,细胞膜、线粒体等细胞器消失。 a: Accumulation of PSTs in viscera; b: Normal scallops; c: C. farreri exposed for 6 days; d: C. farreri exposed for 14 days; NU: Nucleus; M: Mitochondria; MI: Microvilli; BM: Basement membrane; MF: Muscle fiber; LD: Lipid droplets. 1: Cytoplasmic pyknosis, chromatin condensation; 2: Cell vacuoles and the cell membrane, mitochondria and other organelles disappeared. |
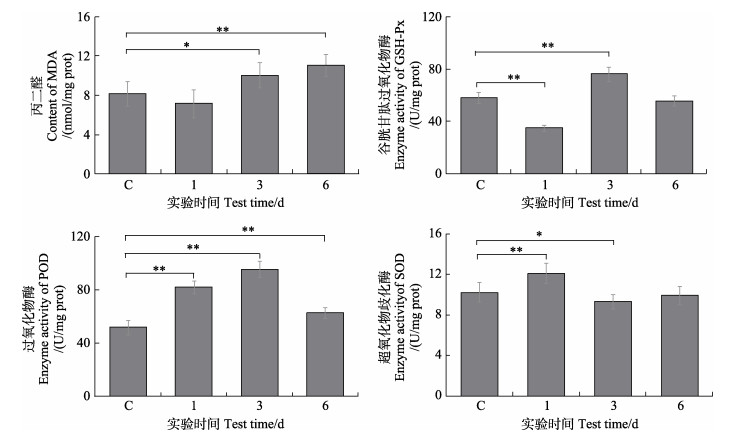
测定MDA、GSH-Px、POD和SOD四项指标用以评估栉孔扇贝的抗氧化应激水平。结果如图 2所示,内脏团中MDA、GSH-Px和POD的应激活跃,SOD则被显著抑制(P < 0.05)。毒素暴露期MDA的含量升高较为显著(P < 0.05),在实验第6天时含量已达到对照组1.38倍。GSH-Px的活力在暴露初期(实验第1天)被显著抑制,随后迅速应激,在实验第3天时达到峰值,但随实验时间的延长其活力再次被抑制。POD活力显著应激,酶活力先上升后下降,活力峰值出现在实验第3天。SOD则呈现与POD相反的活力变化趋势,实验第3天时活力最低,实验第6天时活力有所回升,但仍低于正常水平。
|
图 2 栉孔扇贝内脏团中MDA含量及GSH-Px、POD、SOD酶活力变化 Fig.2 Changes of MDA content and GSH-Px, POD, SOD enzyme activities in visceral mass of C. farreri C为对照组,“*”表示与对照组相比差异显著(P < 0.05),“**”表示与对照组相比差异极显著(P < 0.01),下同。 C is the control group, "*" means significant difference compared with the control group (P < 0.05), "**" means extremely significant difference compared with the control group (P < 0.01), the same below. MDA: Malondialdehyde; GSH-Px: Glutathione peroxidase; POD: Peroxidase; SOD: Superoxide dismutase. |
通过RNA-seq,本研究分别构建了栉孔扇贝对照组(X-C-1、X-C-2、X-C-3)、实验第3天(X-3-1、X-3-2、X3-3)和实验第6天(X-6-1、X-6-2、X-6-3)内脏团的cDNA文库,过滤后获得高质量clean data,Q30均达到了89%以上。用Trinity软件进行拼接组装,共得到包含441 749条Unigenes和624 887个Transcripts,平均长度为562 bp,N50为802 bp。
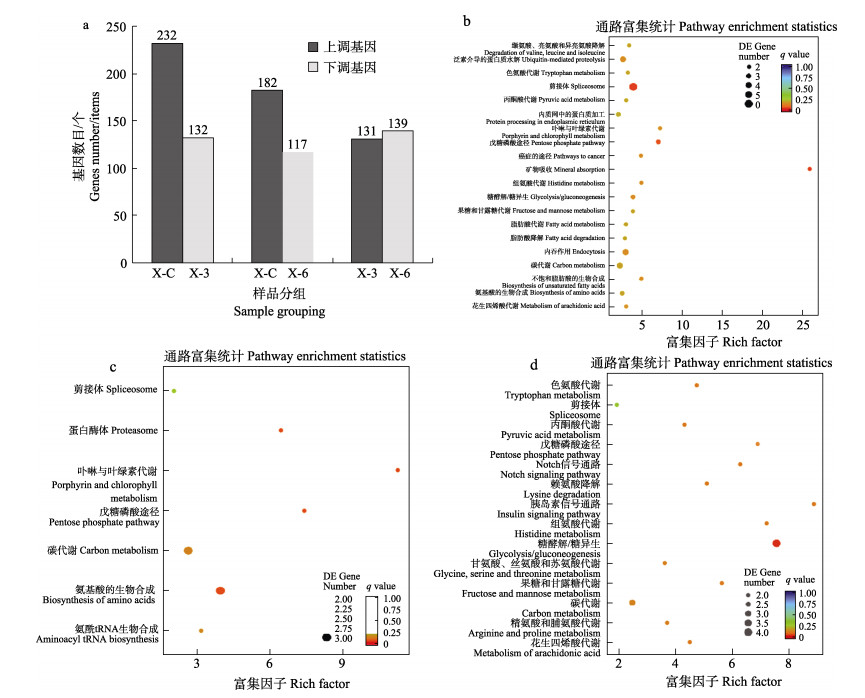
KEGG富集分析共筛选到933个DEGs以表达上调为主,其中,X-3组表达上调最多,而X-6组表达下调居多(图 3)。主要注释在代谢相关通路(占比50%),包括氨基酸代谢及碳水化合物代谢。对照组与实验第3天比较,DEGs主要富集在丙酮酸代谢、糖酵解/糖异生、脂肪酸代谢等通路,实验第3天与第6天相比,DEGs主要富集在丙酮酸代谢、磷酸戊糖途径、糖酵解/糖异生等通路。
|
图 3 栉孔扇贝内脏团差异表达基因数目及KEGG注释结果(Top 20) Fig.3 Number of differentially expressed genes in visceral mass of C. farreri and KEGG annotation a:差异基因数目;b:X-C与X-3的DEGs显著富集结果(KEGG);c:X-C与X-6的DEGs显著富集结果(KEGG);d:X-3与X-6的DEGs显著富集结果(KEGG)。各组别只展示富集最为显著的前20条,不足20条的全部展示。 a: Number of differential genes; b: Significant enrichment results of DEGs of X-C and X-6 (KEGG); c: Significant enrichment result of DEGs of X-C and X-6 (KEGG); d: Significant enrichment result of DEGs of X-3 and X-6 (KEGG). Each group only showed the first 20 items with the most significant enrichment, and less than 20 items were displayed in full. |
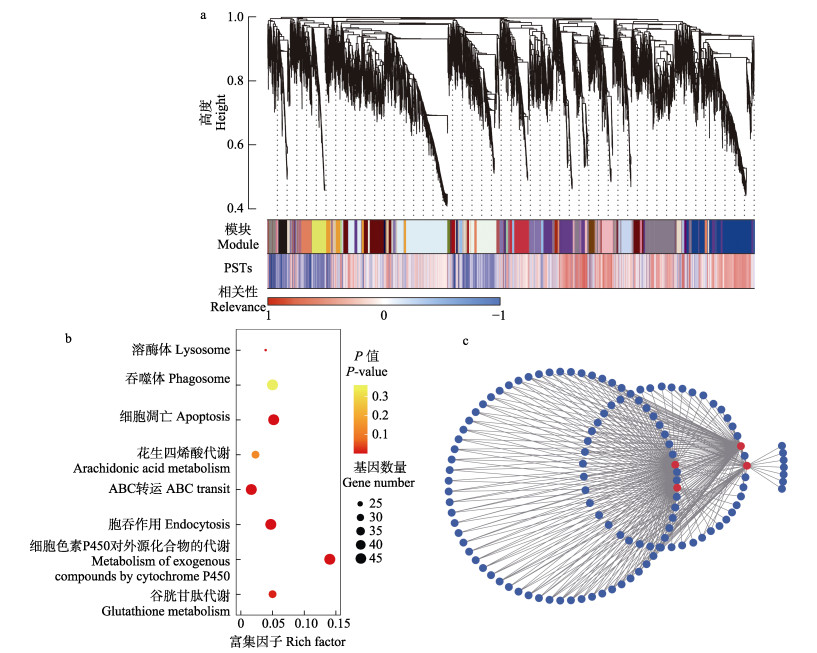
对所有DEGs进行WGCNA分析。结果表明,DEGs被聚类为若干模块(图 4a),其中包括蓝紫色和浅橙色在内的8个模块基因与PSTs蓄积呈显著正相关(r≥0.9),对其基因进行KEGG富集分析(图 4b),结果显示,基因显著富集于细胞吞噬与凋亡(溶酶体、吞噬体、细胞凋亡与胞吞作用)及代谢相关途径(花生四烯酸代谢、ABC转运、细胞色素P450对外源化合物的代谢与谷胱甘肽代谢)。对正相关模块基因进行相互作用分析,发现有242个模块基因相互作用,协同应激PSTs暴露,其中4个高通量差异基因在应激过程中发挥关键调控作用(图 4c)。
|
图 4 差异基因富集与WGCNA分析结果 Fig.4 Differential gene enrichment and WGCNA analysis result a:WGCNA分析差异基因与PSTs蓄积相关性;b:正相关模块基因富集分析;c:正相关模块基因相互作用分析。 a: WGCNA analysis of PSTs exposure correlation; b: Gene enrichment analysis of positive correlation module; c: Gene interaction analysis of positive correlation module. |
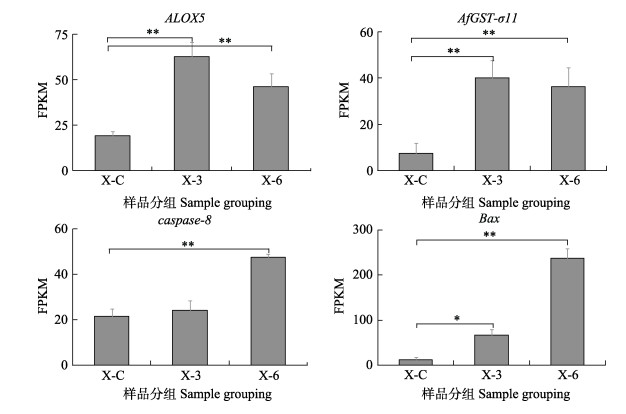
上述4个发挥关键调控作用的基因中被注释为ALOX5、AfGST3-1、caspase-8及Bax4。分别将其在不同时间点的FPKM值为纵坐标作图(图 5)。结果表明,与对照组相比,4种基因的表达量均存在显著差异(P < 0.05),其中,ALOX5、AfGST3-1有相似的表达模式,表达量随实验时间的延长呈先升后降趋势,在实验第3天表达量最高,实验第6天时表达量有所降低,但仍为极显著过表达(P < 0.01)。caspase-8和Bax表达模式相似,表达量随实验时间的延长而逐渐升高,实验第6天表达量最高,均为极显著过表达(P < 0.01)。
|
图 5 关键调控基因在不同时期的表达变化 Fig.5 Expression changes of key regulatory genes in different periods |
现有研究表明,栉孔扇贝暴露PSTs的主要应激包括抗氧化、组织损伤和代谢解毒等反应。上述抗氧化反应可显著降低由于PSTs暴露造成的过氧化损伤(Oyaneder-Terrazas et al, 2022)。本研究观察到栉孔扇贝机体存在短暂的氧化应激反应以消除脂质过氧化,表现为抗氧化酶SOD、POD分别在实验第1、3天时显著应激(P < 0.05)。但在毒素含量最高时抗氧化酶酶活被抑制,组织严重损伤。与本研究相似,Oyaneder-Terrazas等(2022)研究表明,贻贝(Mytilus chilensis)、蛤蜊(Ameghinomya antiqua)等在贝类体内抗氧化酶活性PSTs暴露初期显著升高,暴露末期显著降低且氧化损伤加重。这主要是因为扇贝对PSTs的蓄积具有耐受性强、代谢慢的特点(Oshima et al, 1982; Escobedo-Lozano et al, 2012),栉孔扇贝体内高残留的PSTs诱发的持续性脂质过氧化损伤(Choi et al, 2006),进而成为细胞持续凋亡的诱因之一。
近年来,由PSTs暴露诱发的细胞凋亡现象在牡蛎(Medhioub et al, 2013)、蛤蜊、贻贝(Sokolova et al, 2004a; Mat et al, 2013a; Rolland et al, 2014)等均已报道。本研究中观察到了PSTs对栉孔扇贝内脏团组织造成的损伤,主要表现为线粒体等细胞器消失、细胞核萎缩等,同时观察到染色质凝聚和膜消失(膜损伤)。其中,染色质凝聚及膜损伤被认为是细胞凋亡的标志(Snigirevskaya et al, 2019)。此外,本研究观测到PSTs可显著升高MDA含量(P < 0.05),在毒素含量蓄积最高时,MDA含量极显著升高(P < 0.01),说明PSTs会在栉孔扇贝内脏团中诱发脂质过氧化反应,且脂质过氧化程度可能与PSTs含量正相关。MDA的含量变化是机体脂质过氧化水平的重要表征。赤潮藻所产毒素对贝类造成的脂质过氧化损伤则是触发细胞凋亡的重要原因之一(Ricevuto et al, 2016; De et al, 2017)。最后通过比较WGCNA及KEGG注释结果均表明细胞凋亡与PSTs的蓄积含量呈显著正相关。综上所述,PSTs暴露造成的机体损伤诱发栉孔扇贝细胞凋亡。
半胱氨酸天冬氨酸蛋白酶(caspase)基因家族是引发细胞凋亡的主要执行者,Abi-Khalil等(2017)已证明PSTs可通过caspase基因家族介导的途径诱发牡蛎血细胞发生凋亡。本研究中,caspase基因家族在不同实验时间也存在差异表达,包括凋亡启动相关基因caspse-2/9和caspase-8/10以及凋亡执行基因caspase-3/7,其中caspase-8表达尤为显著,在不同实验时间的表达量存在显著差异(P < 0.01)。caspase-8在细胞凋亡过程中承担分子开关的作用(Calderón-Garcidueñas et al, 2008),有研究表明,将GTX2/3暴露扇贝后,caspase-8的活性增加(Estrada et al, 2014)。且基因caspase-8仅在成年扇贝的消化腺和肾脏中高表达(Wei et al, 2022)。由此进一步推断,凋亡启动基因caspase-8过量表达触发的凋亡机制或是成年栉孔扇贝应激PSTs暴露的特征反应机制。与此同时,基因Bax在控制细胞凋亡的过程中发挥重要作用,可通过增强线粒体膜的通透性,促进促凋亡蛋白的释放(Bock et al, 2019)。Medhioub等(2013)研究发现,暴露于PSTs的牡蛎血细胞中基因Bax显著过表达(P < 0.01)。在本研究中,PSTs造成了栉孔扇贝内脏团组织细胞中基因Bax显著过表达(P < 0.05),且受含量影响在毒素蓄积量最高时(X-6组)表达量极显著升高(P < 0.01)。表明基因Bax可能参与PSTs引发栉孔扇贝内脏团细胞凋亡的调控机制。
栉孔扇贝可激活解毒代谢机制以减轻毒素损伤。贝类的解毒代谢机制主要分为3个阶段。第一阶段对异生物质进行酶修饰,可直接或间接中和PST并将其转化为ROS,细胞色素P450在这一阶段发挥重要作用(Rolland et al, 2014);第二阶段为带电物质与包括谷胱甘肽在内的极性化合物的结合(Fabioux et al, 2015);第三阶段依赖于膜转运蛋白介导的毒素外排,包括多药耐药蛋白(MRP)和ATP结合转运蛋白盒家族成员(ABC)(Wang et al, 2021)。Freitas等(2020)研究表明,暴露于PSTs的贻贝、鸟蛤(Galbuliformes)、竹蛏(Solen strictus)中谷胱甘肽代谢通路被激活。本研究的酶活结果显示,PSTs暴露后GSH-Px活力显著升高(P < 0.05),转录组结果表明,谷胱甘肽代谢通路被显著富集。此外,WGCNA结果显示,细胞色素P450对外源化合物的代谢、谷胱甘肽代谢及ABC转运通路均与PSTs暴露显著正相关,表明PSTs的暴露已激起栉孔扇贝体内谷胱甘肽的解毒应激。说明PSTs激活了栉孔扇贝毒素代谢机制。Hlaing等(2020)研究表明,PSTs可引起栉孔扇贝中CfGST3相关基因的特异性表达,该亚家族在抵御毒素损伤时可发挥多项功能。但本研究表明,持续高浓度的毒素暴露仍将造成组织损伤,由此可知,栉孔扇贝机体的抗氧化及解毒代谢能力有限,无法保护栉孔扇贝机体免受脂质过氧化损伤。
4 结论与展望PSTs在栉孔扇贝体内具有高蓄积,高残留的特征,诱发栉孔扇贝机体持续的抗氧化应激与组织损伤。细胞凋亡可能是栉孔扇贝应激PSTs暴露的防御手段之一。栉孔扇贝暴露于PSTs产毒藻AT5-3后,机体受到脂质过氧化损伤后诱发由caspase-8特征性启动的细胞凋亡程序,促进了栉孔扇贝机体对PSTs的内源性代谢,以维护自身内环境平衡。为保护自身,栉孔扇贝机体抗氧化酶系统及解毒代谢机制快速应激,但效果有限,无法消除高残留PSTs诱发的持续性损伤。这为以后更加深入地研究PSTs对栉孔扇贝的潜在毒性以及栉孔扇贝的免疫分子机制提供了基础。
ABI-KHALIL C, FINKELSTEIN D S, CONEJERO G, et al. The paralytic shellfish toxin, saxitoxin, enters the cytoplasm and induces apoptosis of oyster immune cells through a caspasedependent pathway. Aquatic Toxicology, 2017, 190(1): 133-141 |
BOCK F J, TAIT SWG. Mitochondria as multifaceted regulators of cell death. Nature Reviews Molecular Cell Biology, 2020, 21(2): 85-100 DOI:10.1038/s41580-019-0173-8 |
BRICELJ V M, SHUMWAY S E. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Reviews in Fisheries Science, 1998, 6(4): 315-383 DOI:10.1080/10641269891314294 |
CALDERON-GARCIDUENAS L, SOLT A C, CARLOS H R, et al. Apoptosis: A review of programmed cell death. Toxicologic Pathology, 2008, 36(2): 289-310 DOI:10.1177/0192623307313011 |
CHOI N M, YEUNG L W, SIU W H, et al. Relationships between tissue concentrations of paralytic shellfish toxins and antioxidative responses of clams, Ruditapes philippinarum. Marine Pollution Bulletin, 2006, 52(5): 572-578 DOI:10.1016/j.marpolbul.2006.01.009 |
DOU Y, GAO J W, SHI X T, et al. Outbreak frequency and factors influencing red tides in nearshore waters of the South China Sea from 2000 to 2013. Journal of Hydroecology, 2015, 36(3): 31-37 [窦勇, 高金伟, 时晓婷, 等. 2000-2013年中国南部近海赤潮发生规律及影响因素研究. 水生态学杂志, 2015, 36(3): 31-37 DOI:10.15928/j.1674-3075.2015.03.005] |
DU K M, LEI F, WU N, et al. The pattern of paralytic shellfish poisoning in shellfish cultured in East China Sea and South China Sea. Journal of Jinan University (Natural Science & Medicine Edition), 2013, 34(3): 343-346 [杜克梅, 雷芳, 吴霓, 等. 我国东海和南海近岸海域麻痹性贝类毒素污染状况. 暨南大学学报(自然科学与医学版), 2013, 34(3): 343-346] |
ESCOBEDO-LOZANO A Y, ESTRADA N, ASCENCIO F, et al. Accumulation, biotransformation, histopathology and paralysis in the Pacific Calico Scallop Argopecten ventricosus by the paralyzing toxins of the dinoflagellate Gymnodinium catenatum. Marine Drugs, 2012, 10(4): 1044-1065 |
ESTRADA N A, LAGOS N, GARCÍA C, et al. Effects of the toxic dinoflagellate Gymnodinium catenatum on uptake and fate of paralytic shellfish poisons in the Pacific giant lions-paw scallop Nodipecten subnodosus. Marine Biology, 2007a, 151(4): 1205-1214 DOI:10.1007/s00227-006-0568-x |
ESTRADA N, ASCENCIO F, SHOSHANI L, et al. Apoptosis of hemocytes from lions-paw scallop Nodipecten subnodosus induced with paralyzing shellfish poison from Gymnodinium catenatum. Immunobiology, 2014, 219(12): 964-974 DOI:10.1016/j.imbio.2014.07.006 |
ESTRADA N, DE JESÚS REMERO M, CAMPA-CÓRDOVA A, et al. Effects of the toxic dinoflagellate, Gymnodinium catenatum on hydrolytic and antioxidant enzymes, in tissues of the giant lions-paw scallop Nodipecten subnodosus. Comparative Biochemistry and Physiology, Part C, 2007b, 146(4): 502-510 |
ETHERIDGE S M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon, 2010, 56(2): 108-122 DOI:10.1016/j.toxicon.2009.12.013 |
FABIOUX C, SULISTIYANI Y, HABERKORN H, et al. Exposure to toxic Alexandrium minutum activates the detoxifying and antioxidant systems in gills of the oyster Crassostrea gigas. Harmful Algae, 2015, 48(9): 55-62 |
FERNÁNDEZ-REIRIZ M J, NAVARRO J M, CONTRERAS AM, et al. Trophic interactions between the toxic dinoflagellate Alexandrium catenella and Mytilus chilensis: Feeding and digestive behaviour to long-term exposure. Aquatic Toxicology, 2008, 87(4): 245-251 DOI:10.1016/j.aquatox.2008.02.011 |
FREITAS J S, TERESA F B, DE ALMEDIA E A, et al. Influence of temperature on the antioxidant responses and lipid peroxidation of two species of tadpoles (Rhinella schneideri and Physalaemus nattereri) exposed to the herbicide sulfentrazone (Boral 500SC(R)). Comparative Biochemistry and Physiology Toxicology and Pharmacology: CBP, 2017, 197: 32-44 DOI:10.1016/j.cbpc.2017.04.005 |
FREITAS R, MARQUES F, MARCHI L D, et al. Biochemical performance of mussels, cockles and razor shells contaminated by paralytic shellfish toxins. Environmental Research, 2020, 188: 109846 DOI:10.1016/j.envres.2020.109846 |
GUO F, CUI Y, CHEN B J, et al. Analysis of toxic and harmful substances in shellfish of Jiaozhou Bay. Progress in Fishery Sciences, 2011, 32(6): 115-120 [过锋, 崔毅, 陈碧鹃, 等. 胶州湾贝类体内有毒有害物质污染状况分析. 渔业科学进展, 2011, 32(6): 115-120] |
HLAING S M M, LOU J, CHENG J, et al. Tissue-biased and species-specific regulation of glutathione peroxidase (GPx)genes in scallops exposed to toxic dinoflagellates. Toxins(Basel), 2020, 13(1): 21 |
HU B, LI M, YU X, et al. Diverse expression regulation of Hsp70 genes in scallops after exposure to toxic Alexandrium dinoflagellates. Chemosphere, 2019, 234: 62-69 DOI:10.1016/j.chemosphere.2019.06.034 |
LI D, GENG H X, YU R C, et al. Distribution of Alexandrium pacificum cysts in the area adjacent to the Changjiang River estuary, China. Marine Pollution Bulletin, 2020b, 156: 111206 DOI:10.1016/j.marpolbul.2020.111206 |
LI D, YU R C, GENG H X, et al. Resting cysts of Alexandrium catenella and A. pacificum (Dinophyceae) in the Bohai and Yellow Seas, China: Abundance, distribution and implications for toxic algal blooms-ScienceDirect. Harmful Algae, 2020a, 93(2): 101794 |
LIAN S S, ZHAO L, XUN X G, et al. Genome-wide identification and characterization of SODs in Zhikong scallop reveals gene expansion and regulation divergence after toxic dinoflagellate exposure. Marine Drugs, 2019, 17(12): 700 DOI:10.3390/md17120700 |
LIANG Z X, LI J, REN H, et al. Toxic dinoflagellate Alexandrium tamarense induces oxidative stress and up-regulae Caspase gene (Fc Casp) expression in gills of Chinese shrimp, Fenneropenaeus chinensis. Journal of Fishery Sciences of China, 2014, 21(1): 8 [梁忠秀, 李健, 任海, 等. 塔玛亚历山大藻对中国明对虾鳃组织的氧化胁迫和对 Caspase基因(FcCasp)表达的影响. 中国水产科学, 2014, 21(1): 8] |
LILLY E L, HALANYCH K M, ANDERSON D M. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae). Journal of Phycology, 2010, 43(6): 1329-1338 |
LIU B, ZHAO H Q, LIU B, et al. Investigation and analysis of paralytic shellfish poison pollution in retail shellfish in Hebei province from 2018 to 2020. Journal of Food Safety and Quality, 2021, 12(5): 7 [刘斌, 赵慧琴, 刘波, 等. 2018-2020年河北省市售贝类中麻痹性贝类毒素污染状况调查分析. 食品安全质量检测学报, 2021, 12(5): 7] |
LIU Z Y, JI R. Comparison of criteria for paralytic shellfish poisons in shellfish aquatic products in various countries. China Tropical Medicine, 2006, 6(1): 3 [刘智勇, 计融. 各国贝类水产品中麻痹性贝类毒素限量标准的比对. 中国热带医学, 2006, 6(1): 3] |
MAT A M, HABERKORN H, BOURDINEAUD J P, et al. Genetic and genotoxic impacts in the oyster Crassostrea gigas exposed to the harmful alga Alexandrium minutum. Aquatic Toxicology, 2013, 140/141: 458-465 DOI:10.1016/j.aquatox.2013.07.008 |
MEDHIOUB W, RAMONDENC S, VANHOVE A S, et al. Exposure to the neurotoxic dinoflagellate, Alexandrium catenella, induces apoptosis of the hemocytes of the oyster, Crassostrea gigas. Marine Drugs, 2013, 11(12): 4799-4814 DOI:10.3390/md11124799 |
OSHIMA Y, YASUMOTOR T, KODAMA M, et al. Features of paralytic shellfish poison occurring in Tohoku District. Nsugaf, 1982, 48(4): 525-530 DOI:10.2331/suisan.48.525 |
OYANEDER-TERRAZAS J, FIGUEROA D, ARANEDA O F, et al. Saxitoxin group toxins accumulation induces antioxidant responses in tissues of Mytilus chilensis, Ameghinomya antiqua, and Concholepas concholepas during a bloom of Alexandrium pacificum. Antioxidants (Basel), 2022, 11(2): 392 DOI:10.3390/antiox11020392 |
QIAN B L, XU J, WANG Y, et al. Investigation and evaluation of paralytic shellfish poison in shellfish collected from aquatic product wholesale market in Shanghai. Journal of Food Safety and Quality, 2012(2): 89-92 [钱蓓蕾, 徐捷, 王媛, 等. 上海市售贝类产品中麻痹性贝类毒素污染状况调查及其评价. 食品安全质量检测学报, 2012(2): 89-92 DOI:10.19812/j.cnki.jfsq11-5956/ts.2012.02.004] |
QIU J B, MA F F, FAN H, et al. Effects of feeding Alexandrium tamarense, a paralytic shellfish toxin producer, on antioxidant enzymes in scallops (Patinopecten yessoensis)and mussels (Mytilus galloprovincialis). Aquaculture, 2013, 396 |
RICEVUTO E, LANZONI I, FATTORINI D, et al. Arsenic speciation and susceptibility to oxidative stress in the fanworm Sabella spallanzanii (Gmelin)(Annelida, Sabellidae)under naturally acidified conditions: An in situ transplant experiment in a Mediterranean CO2 vent system. Science of the Total Environment, 2016, 544: 765-773 DOI:10.1016/j.scitotenv.2015.11.154 |
ROLLAND J L, MEDHIOU B, VERGNES A, et al. A Feedback mechanism to control apoptosis occurs in the digestive gland of the oyster Crassostrea gigas exposed to the paralytic shellfish toxins producer Alexandrium catenella. Marine Drugs, 2014, 12(9): 5035-5054 DOI:10.3390/md12095035 |
SEBASTIAN S E, BRENA B M, LUQUET C M, et al. Microcystin accumulation and antioxidant responses in the freshwater clam Diplodon chilensis patagonicus upon subchronic exposure to toxic Microcystis aeruginosa. Ecotoxicology and Environmental Safety, 2011, 74(5): 1188-1194 DOI:10.1016/j.ecoenv.2011.03.012 |
SNIGIREVSKAYA E S, KOMISSARCHIKY Y. Ultrastructural traits of apoptosis. Cell Biology International, 2019, 43(7): 728-738 DOI:10.1002/cbin.11148 |
SOKOLOVA I M, EVANS S, HUGHES F M. Cadmium-induced apoptosis in oyster hemocytes involves disturbance of cellular energy balance but no mitochondrial permeability transition. Journal of Experimental Biology, 2004a, 207(19): 3369-3380 DOI:10.1242/jeb.01152 |
SOKOLOVA I M. Apoptosis in molluscan immune defense. Invertebrate Survival Journal, 2009b, 6(1): 49-58 |
TSIKAS D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Analytical Biochemistry, 2017, 524: 13-30 DOI:10.1016/j.ab.2016.10.021 |
WANG H, LIU S, XUN X, et al. Toxin-and species-dependent regulation of ATP-binding cassette (ABC) transporters in scallops after exposure to paralytic shellfish toxin-producing dinoflagellates. Aquatic Toxicology, 2021, 230: 105697 DOI:10.1016/j.aquatox.2020.105697 |
WEI Z C, DING W, LI M, et al. The caspase homologues in scallop Chlamys farreri and their expression responses to toxic dinoflagellates exposure. Toxins (Basel), 2022, 14(2): 108 DOI:10.3390/toxins14020108 |
WIESE M, D'AGOSTINO P M, MIHALI T K, et al. Neurotoxic alkaloids: Saxitoxin and its analogs. Marine Drugs, 2010, 8(7): 2185-2211 DOI:10.3390/md8072185 |
ZHANG H T, WU H Y, ZHENG G C, et al. Accumulation and transformation of paralytic shellfish toxin in mussel Mytilus galloprovinciali s exposed to Alexandrium catenella. Progress in Fishery Sciences, 2023, 44(1): 181-190 [张海涛, 吴海燕, 郑关超, 等. 链状亚历山大藻暴露下紫贻贝体内麻痹性贝毒蓄积转化规律. 渔业科学进展, 2023, 44(1): 181-190] |
ZHEN J J, YE F Y, WANG D, et al. Effect of cadmium on ultrastructure of gill epithelial cells in clam Meretrix meretrix. Fisheries Science, 2018, 37(4): 469-474 [甄静静, 叶方源, 王都, 等. 镉离子对文蛤鳃上皮细胞超微结构的影响. 水产科学, 2018, 37(4): 469-474] |



