2. 上海海洋大学 水产科学国家级实验教学示范中心 上海 201306
2. National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China
鱼类是变温动物,体温变化取决于外部水环境温度的变化,温度是其行为、生理和分子过程的重要外在因素(Nikoskelainen et al, 2004; 邹华锋等, 2016; 王映等, 2022)。水温的急速下降会引发鱼类强烈的应激反应,可能会导致生长抑制或者死亡(Huang et al, 2017; Foulkes et al, 2007)。在自然界中,从40 ℃热带水域到–1.9 ℃的南极洲都有各种鱼类的广泛分布,其中,南极海域常年水温为–1.9 ℃,为了应对低温刺激,极地鱼类机体调节能量稳态非常关键(Tien et al, 2018)。
瘦素(Leptin)是一种具有多重生物学功能的蛋白质类激素,在调节摄食、能量平衡和生殖过程中发挥重要作用(罗铮等, 2018; Huising et al, 2006; Zhang et al, 2011)。瘦素基因(Lep)首次克隆于肥胖突变型小鼠(Mus musculus),最初命名为肥胖基因,该基因的突变导致小鼠过度肥胖(Zhang et al, 1994)。进一步研究发现,在哺乳动物体内,Lep基因通过对机体的摄食和能量消耗进行调控,从而降低动物的采食量,进而实现降低体重和体脂率的作用(Ahima et al, 2000; Elmquist et al, 2005)。Lep基因还参与调节机体的免疫应答,并对动物胚胎发育和生长繁殖具有重要作用(Khosropour et al, 2017; Pérez-Pérez et al, 2018)。在鱼类中,Kurokawa等(2005)采用比较基因组学方法首次从红鳍东方鲀(Takifugu rubripes)克隆得到了lep基因序列。关于Leptin对温度调控方面的作用在哺乳动物中研究较多,主要包括对能量代谢的调控以及对温度适应性进化影响等(Yang et al, 2008; McGaffin et al, 2009; Kanda et al, 2004; Yu et al, 2014; Toro et al, 2014)。目前,关于Lep在鱼类中作用的报道主要集中在摄食、脂肪能量代谢和繁殖调控等方面,对温度的调控作用研究尚少有报道。
鳞头犬牙南极鱼(Dissostichus mawsoni)是南冰洋海域食物网重要的组成部分,不仅是顶级捕食者,还是鲸类和海豹的主要食物来源,在南冰洋生态系统中占据重要位置。在近3千万年里,鳞头犬牙南极鱼一直生活在南极水域–1.9 ℃寒冷又封闭的低温环境中,是极端环境下温度适应机制研究的极好材料(Hanchet et al, 2015)。与相同水深的其他鱼类相比,鳞头犬牙南极鱼在皮下和肌肉中有广泛的脂质沉积(Chen et al, 2019)。本实验室前期在该鱼中发现,LepB的部分缺失造成其在结构上仅有3个α螺旋,也发现LepA与温度变化密切相关(Wang et al, 2021),据此推测,LepB可能在鳞头犬牙南极鱼低温环境适应中发挥重要作用。本研究克隆鳞头犬牙南极鱼的LepB基因,构建质粒LepB (DMLB)转染ZFL细胞,探究其在极端低温环境适应中的作用,以期为揭示鳞头犬牙南极鱼LeptinB基因在鱼类应对低温胁迫时如何调节机体能量稳态的分子机制提供基础资料。
1 材料与方法 1.1 实验材料鳞头犬牙南极鱼从南极中山站的海冰中钻孔垂钓捕获,将其保存在液氮中运回实验室直至使用。
细胞系为ZFL (ATCC, 美国),质粒为pTOL2- β-actin-EGFP。转染pTOL2-LepB+HIS-EGFP质粒的ZFL细胞命名为DMLB实验组细胞,转染TOL2空载质粒的ZFL细胞命名为对照组TOL2细胞。
1.2 南极鱼LepB基因的克隆及真核表达载体的构建使用Trizol (Invitrogen, 美国)提取鳞头犬牙南极鱼卵巢总RNA,用NanoDrop分光光度计进行浓度检测和质检,用TransScript® First-Strand cDNA Synthesis SuperMix试剂盒(TaKaRa)反转录合成单链cDNA。根据实验室已获取的鳞头犬牙鱼的基因序列,使用Primer Premier 5.0软件设计特异性引物(表 1),进行PCR扩增,以获得该基因的开放阅读框(ORF)。由于缺乏鳞头犬牙南极鱼特异性瘦素的抗体,本研究设计构建了pTOL2-LepB+HIS-EGFP表达载体(图 1),根据鳞头犬牙南极鱼LepB基因编码的氨基酸序列,采用斑马鱼β-actin基因的启动子,选取EcoRⅠ和BamHⅠ的酶切位点作为克隆位点,与实验室保存的真核表达载体pTOL2-bactin-2A-EGFP进行无缝克隆连接,引物见表 1。为了便于检测质粒的表达情况,在C端引入HIS标签。
|
|
表 1 LepB基因的克隆和无缝克隆引物 Tab.1 The primers for LepB cloning and the In-Fusion cloning |

|
图 1 pTOL2-LepB+HIS-EGFP真核表达载体构建图谱 Fig.1 Expression vector map of pTOL2-LepB+HIS-EGFP |
将LepB基因的克隆产物用Qiagen胶回收试剂盒进行回收纯化,用In-Fusion HD cloning kit (TaKaRa)连接,将连接产物转化至大肠杆菌(E coli) DH-5α感受态细胞中,适当稀释后涂布在含100 μg/mL卡那霉素的平板上,PCR验证插入片段,验证合格后将产物送至生工生物工程(上海)股份有限公司测序,阳性克隆菌扩大培养,用质粒提取试剂盒提取质粒(Promega, 美国),提取的质粒保存于–20 ℃冰箱中,用于转染实验。
1.3 ZFL细胞培养与质粒转染、蛋白表达将ZFL培养在含89% DMEM/F12 (含1%谷氨酰胺,HyClone)、10% FBS和1%青霉素–链霉素培养基中,温度为28 ℃,CO2浓度为5% (即最适生长条件)。待细胞的汇合度达到70%~80%时,用polyplus jet Optimus® DNA转染试剂(百奥创新,北京)进行转染。扩增培养后将G418硫酸盐(生工生物,上海)溶解稀释液加入到转染的ZFL细胞中,筛选出转染成功的细胞。7 d时G418浓度为1 000 mg/mL,此后为500 mg/mL,这是大多数未转染细胞死亡的浓度。丢弃G418,并用绿色荧光突出显示成功转染的细胞。
筛选出的LepB细胞和TOL2对照细胞用混合蛋白质裂解液(含10% PMSF)进行蛋白质提取,冰上裂解30 min,4 ℃低温12 000 r/min离心15 min,取上清液分装于离心管中,–80 ℃保存。使用Bradford测量蛋白质浓度,通过SDS-PAGE分离蛋白质(每个样品30 µg),并将其电转移至聚偏二氟乙烯硝酸纤维素膜(Millipore, 德国)。脱脂牛奶封闭后,用His一抗(1∶1000, Abcam, 英国) 4 ℃过夜,山羊抗鼠二抗(1∶500, 杭州)室温孵育2 h,内源性对照为β-肌动蛋白。用增强化学发光检测试剂(Beyotime, 中国)显示蛋白质。
1.4 细胞低温处理形态观察和细胞活力检测将转染空载质粒和LepB重组质粒的细胞进行10 ℃低温胁迫处理后,采用倒置荧光显微镜观察细胞形态并拍照。使用增强型CCK-8试剂盒(碧云天,江苏)检测细胞增殖能力以及细胞毒性,细胞增殖速度产生颜色变化,颜色的深浅与细胞增殖成正比,与细胞毒性成反比。使用酶联免疫检测仪在450 nm处测定光吸收值,可间接反映活细胞数量。使用对数生长期的细胞传代并计数,每毫升20 000个细胞传至96孔板中,每孔200 μL,每个样品5个重复,使实验组和对照细胞密度相同,分别在0、24、48 h检测细胞增殖活性,每孔加10 μL增强型CCK-8检测液,培养箱孵育4 h,使用酶标仪检测450 nm处的吸光值。样品吸光值减去空白吸光值,重复取平均值,使用GraphPad Prism 8软件绘制生长曲线。
1.5 流式细胞术检测活性氧活性氧(ROS)参与细胞的增殖、分化、衰老和凋亡等过程。活性氧检测试剂盒利用二氢乙锭,可自由透过活细胞膜进入细胞内,并被细胞内的ROS氧化,与染色体DNA结合发红色荧光的氧化乙锭。细胞传代并计数后接种至6孔板,相同密度的实验组和对照组贴壁细胞10 ℃低温刺激3 d,收集细胞,200 μL 1×105~1×106 cells/mL的细胞悬液加入2 μL的红色荧光染料原液(5 mmol/L),轻轻混匀,室温避光孵育60 min后,500 g离心5~10 min,去除染色液,用DPBS缓冲液(Sigma)清洗2次,最后用DPBS重悬细胞,BD C6流式细胞仪(BD, 美国)上机FL2通道检测荧光信号,FlowJo软件分析,使用GraphPad Prism 8软件绘制ROS相对表达图。实验组和对照组均设置3个重复。
1.6 细胞凋亡检测使用Hoechst 33342/PI双染试剂盒(Solarbio, 中国)检测凋亡细胞与坏死细胞,细胞传代并计数至96孔板,使实验组和对照组细胞密度相同,适温培养贴壁后,分别于28 ℃和10 ℃培养3 d后,每孔加入5 μL Hoechst染色液和5 μL PI染色液。混匀,冰浴或4 ℃孵育20~30 min。染色后PBS洗涤1次,在荧光显微镜下观察拍照。
1.7 细胞ATP检测通常,ATP水平的下降表示线粒体功能受损,也可代表细胞的凋亡水平,使用试剂盒(Sigma, 美国)对细胞进行ATP检测。将实验组和对照组贴壁细胞放于10 ℃培养箱,低温刺激3 d,取出后吸除培养液,6孔板每孔加入200 μL裂解液,吹打裂解后转移至EP管,4 ℃ 12 000 g离心5 min,取上清液,96孔板每孔加100 μL ATP检测工作液(检测试剂与稀释液1∶4),室温放置5 min后每孔加入20 μL待测样品和标准品,迅速混匀,用酶标仪(Bio-Rad, 美国)检测化学发光。使用Excel软件制作标准曲线,计算样品ATP浓度。
1.8 油红O脂滴染色配制油红储存液(0.15 g油红O粉末及50 mL 100%异丙醇于棕色避光瓶混匀溶解) 4 ℃保存,油红工作液需实验前现用现配,将油红储存液与灭菌去离子水按照3∶2的比例混合过滤后使用。细胞提前转至35 mm培养皿,28 ℃培养箱培养,细胞贴壁后取一组放于10 ℃培养箱低温处理,3 d后取出后,DPBS洗2遍,4%多聚甲醛15 min固定细胞,DPBS洗2遍;每孔加入过滤后的油红工作液,室温静置染色30 min;弃染液,DPBS洗2遍,加入DPBS覆盖细胞,在正置显微镜下观察拍照。
1.9 甘油三酯的检测采用检测试剂盒(南京建成)和GPO-PAP法检测甘油三酯。细胞置于10 cm培养皿,10 ℃处理3 d,取细胞沉淀,DPBS洗2次,加入200~300 μL的DPBS冰浴匀浆1 min,采用Bradford法进行蛋白浓度测定,酶标仪测595 nm吸光值,96孔板每孔加入样品2.5 μL,每个样品3个重复,每孔加检测液250 μL,37 ℃反应10 min,酶标仪检测510 nm吸光值。
1.10 统计分析实验至少进行3次独立重复,实验观察所得数据先使用相应软件处理,再使用SPSS 18软件进行方差分析以及Duncan´s多重比较,GraphPad Prism软件分析作图。P < 0.05为组间差异有统计学意义。
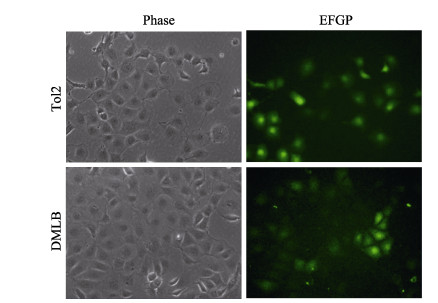
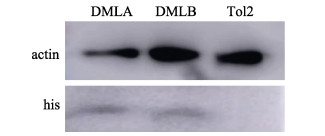
2 结果与分析 2.1 ZFL细胞转染荧光观察与Western-blot检测ZFL细胞转染DMLB质粒和TOL2空载质粒后,经约21 d G418筛选培养后,可见大面积荧光细胞簇(图 2),表示荧光质粒转染成功,培养至转染细胞达60%可进行实验。通过Western blot对阳性对照LepA、LepB和TOL2细胞的总蛋白进行检测,采用HIS标签检测蛋白大小与预测的LepA 16.04 kDa、LepB 14.39 kDa大小一致,空载TOL2细胞未被检测到HIS标签(图 3),表明鳞头犬牙南极鱼LeptinB基因在ZFL细胞中成功过表达。
|
图 2 ZFL细胞转染后的绿色荧光 Fig.2 ZFL cells with green fluorescence after infection with supernatant containing lentivirus particles Phase为明场视野,EGFP为绿色荧光视野。图 4同。 Phase is bright field vision, and EGFP is green fluorescence. The same in Fig. 4. |
|
图 3 Western-blot检测LepA (阳性对照) (6.04 kDa)、LepB (14.39 kDa)和TOL2蛋白 Fig.3 Western blot analysis of LepA (positive control) (6.04 kDa), LepB (14.39 kDa) and TOL2 protein |
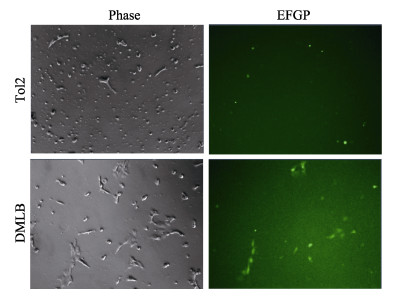
将28 ℃培养箱中生长状态良好的实验组DMLB细胞和对照组TOL2细胞转移至10 ℃培养箱中低温刺激,每日观察生长或死亡状况,至第14天,可见对照组细胞基本死亡,荧光显微镜下除死细胞有假阳性荧光外,对照组无荧光细胞存活;但实验组中有稀疏细胞存活,荧光显微镜下可见细胞多有荧光(图 4)。由此猜测,表达鳞头犬牙南极鱼LepB基因的细胞有耐低温能力。
|
图 4 TOL2细胞与DMLB细胞低温刺激14 d死亡情况 Fig.4 Low temperature stimulated the death of TOL2 cells and DMLB cells for 14 d |
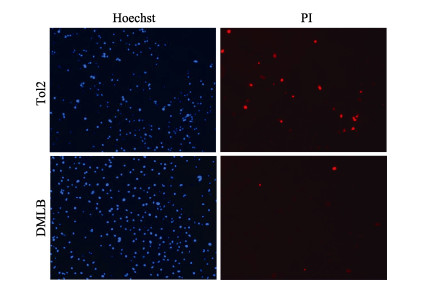
对照组TOL2细胞和实验组DMLB细胞低温刺激3 d后,通过荧光显微镜在Hoechst亮蓝色荧光通道下2组细胞可见蓝色细胞核染色标记,在PI红色荧光通路下,TOL2细胞红色荧光染色数显著多于实验组DMLB细胞,红色荧光细胞数36∶10 (图 5),TOL2细胞在低温刺激下发生较多的细胞凋亡和细胞坏死,这表示南极鱼LepB基因过表达可以减轻低温刺激对细胞的凋亡和坏死作用。
|
图 5 Hoechst/PI染色检测细胞凋亡 Fig.5 Apoptosis was detected by Hoechst/PI staining Hoechst标记细胞核,PI标记凋亡细胞和坏死细胞。 Hoechst labeled the nucleus, and PI labeled apoptotic and necrotic cells. |
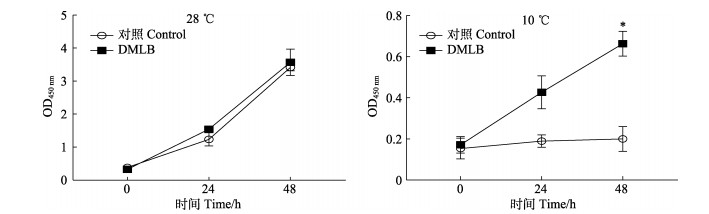
实验组DMLB细胞与对照组TOL2细胞在28 ℃培养箱中均生长状态良好,能快速分裂,培养板细胞密度可达到饱和。将实验组和对照组细胞放至10 ℃培养箱中,经过低温刺激后,对照组细胞生长基本停滞,而实验组却有一定生长,二者差异显著(P < 0.05) (图 6)。由此可知,低温刺激导致对照组细胞活力减弱甚至停滞生长,而鳞头犬牙南极鱼LepB基因过表达可增强细胞低温下的增殖活力。
|
图 6 TOL2细胞与DMLB细胞28 ℃与10 ℃培养细胞增殖情况 Fig.6 Proliferation of TOL2 cells and DMLB cells cultured at 28 ℃ and 10 ℃ *表示差异在0.01 < P < 0.05水平,下同。 * indicates the difference on the level of 0.01 < P < 0.05, the same below. |
细胞本身为绿色荧光,使用试剂盒中的DHE红色荧光探针检测ROS的产生情况,采用流式细胞仪分析实验组和对照组ROS荧光值。结果显示,28 ℃时,ROS含量较少,2组荧光强度无显著差异;而10 ℃时,ROS含量明显增多,对照组平均荧光强度显著高于DMLB实验组(图 7)。由此得出,低温刺激导致细胞内ROS含量显著增加,而鳞头犬牙南极鱼LepB基因过表达能够减轻细胞ROS的产生,减少细胞凋亡。

|
图 7 细胞凋亡分析 Fig.7 Cell apoptosis analysis A:流式细胞仪ROS FlowJo软件分析;B:ROS数据的荧光值用GraphPad Prism 8软件进行分析和绘图 A: Flow cytometer ROS FlowJo software analysis; B: Fluorescence values of ROS data were analyzed and plotted using GraphPad Prism 8 software |
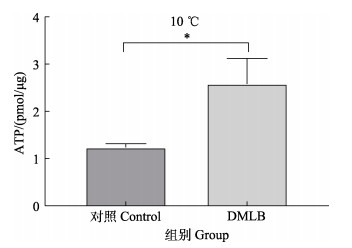
结果显示,经低温处理后,与对照组相比,实验组细胞的ATP水平显著增高(P < 0.05)(图 8)。由此可知,DM-LepB过表达对维持低温刺激下细胞内ATP水平有显著作用,有利于维持细胞线粒体稳态。
|
图 8 低温处理对细胞ATP含量的影响 Fig.8 Effect of low temperature treatment on ATP content in cells |
活性油红将脂滴染为深色,使用正置显微镜明场拍照,脂滴染色拍照图片使用ImageJ软件分析染色面积,由染色面积确定脂滴数量。在正常温度培养时,DMLB组细胞比对照组细胞脂滴略少,而低温刺激后,对照组细胞有部分死亡情况,清洗染色后发现,脂滴显著减少,而DMLB组细胞脂滴数量较多(图 9)。表明鳞头犬牙南极鱼LepB基因能减缓低温刺激时细胞脂质的消耗,减弱细胞的伤害,较多的脂质保留使细胞能更好地抵抗低温损害。

|
图 9 低温处理后细胞脂滴油红O染色 Fig.9 Low temperature treatment of cell lipid drop oil red O staining A:染色显微镜正置明场拍照图片;B:ImageJ软件分析染色面积。**表示差异在P < 0.01水平,***表示差异在P < 0.001水平,下同。 A: The staining microscope is being exposed to take pictures; B: Staining area was analyzed by ImageJ software. ** indicates the difference on the level of P < 0.01, and *** indicates the difference on the level of P < 0.001, the same below. |
甘油三酯是细胞中脂滴构成的主要成分,是体内储存量最大、产能最多的能源物质。低温环境下,甘油三酯作为能源物质对细胞的生长、生存有重要意义。同油红O染色结果一致(图 10),在正常温度培养时,对照组细胞比DMLB组细胞甘油三酯稍多,而低温刺激后,对照组细胞甘油三酯含量显著下降,DMLB组细胞甘油三酯含量变化较对照组稳定。表明鳞头犬牙南极鱼LepB基因对低温刺激下的脂质代谢有减弱作用。

|
图 10 低温处理对细胞甘油三酯含量的影响 Fig.10 Effect of low temperature treatment on cell triglyceride content |
低温胁迫影响着生物的生长发育、能量代谢和繁殖发育等多种过程(汪倩等, 2022)。近年来,关于低温胁迫影响生物体的研究日益增长,其中较多研究集中在分子层面(Wang et al, 2022; 程鹏丽等, 2020)。Leptin作为一种脂肪组织与下丘脑之间的信号传导蛋白(曾沃坦等, 2008),已证实与细胞生长、繁殖、凋亡和代谢密切相关。在哺乳动物中,Leptin的生理功能是通过调节能量代谢来维持能量内稳态(Bakshi et al, 2022),但鱼类作为变温动物与哺乳动物有很大的不同。采用比较法可以更快速和清晰地阐明鱼类leptin的功能机制。Leptin作为调控新陈代谢的关键蛋白产物,暂时缺少其参与冷应激过程调控机制的研究。因此,本研究通过体外实验探究LeptinB在鱼类细胞受到低温胁迫后所起的功能。利用鳞头犬牙南极鱼lepB (DMLB)质粒转染ZFL细胞,并对其在常温及10 ℃低温条件下进行细胞培养;使用增强型CCK-8试剂盒检测了细胞增殖能力和细胞毒性大小,通过流式细胞技术检测DMLB质粒转染ZFL细胞ROS细胞凋亡,探索DMLB基因在调节冷应激过程中发挥的作用及其分子调控机制。
机体内的ATP主要通过有氧代谢来提供,在这个过程中还会产生ROS,即在有氧代谢过程中产生的氧离子、过氧化物和含氧自由基等一系列氧的单电子还原产物(冯锦芝等, 2016)。在正常细胞中,ROS在产生的同时会有很大一部分消耗,当ROS的积累速度远远超出其在机体内的消耗速度,就会对机体造成永久损伤(胡成枫等, 2019)。ROS易与细胞膜上的一些不饱和脂肪酸或胆固醇反应,引发链式反应,这种直接作用于细胞的氧化损伤能导致细胞凋亡(褚启龙等, 2003)。因此,通过流式细胞技术检测细胞内ROS含量,一定程度上可以代表细胞凋亡的程度。28 ℃时,细胞凋亡数较少,而10 ℃时对照组细胞凋亡数显著多于DMLB实验组,说明低温胁迫后DM-LepB基因过表达能够显著降低细胞ROS的产生,从而减少细胞凋亡,进而调节能量的消耗,使动物体内ROS的含量维持在一个相对稳定的水平。同时发现,低温刺激细胞凋亡后,对照组出现了更多的凋亡和坏死,这也反映leptin基因在应对低温应激下的凋亡中发挥了重要作用。
线粒体是ATP产生的主要场所,ATP是细胞直接供能的物质,对细胞的生长代谢、维持细胞稳态有重要作用。通常在细胞处于凋亡、坏死等负面状态下,体内ATP水平会降低(Malhi et al, 2006),这也能指示线粒体功能受损的状态,低温会刺激低温反应相关通路因子,从而激活瘦素的转录,促进线粒体的产生以便提供更多的能量来维持必需的生理活动。本研究中,经低温处理后,DMLB实验组细胞ATP水平出现差异,对照组细胞ATP水平无显著变化,因此,DM-LepB过表达对维持低温刺激下的胞内ATP水平有显著作用,有利于维持细胞线粒体状态。
综上,本研究通过在ZFL细胞中过表达鳞头犬牙南极鱼LeptinB基因,发现DMLB基因对低温应激有维持细胞生长增殖、减少ROS产生、减轻细胞凋亡的作用,有利于维持胞内ATP水平,维持细胞线粒体状态,有效减少细胞的凋亡损伤。另外,鳞头犬牙南极鱼LeptinB基因能减缓低温刺激时细胞脂质的消耗,减弱细胞的伤害,较多的脂质保留使细胞更好地抵抗低温损害,对细胞冷应激起到保护作用。
AHIMA R S, SAPER C B, FLIER J S, et al. Leptin regulation of neuroendocrine systems. Frontiers in Neuroendocrinology, 2000, 21(3): 263-307 DOI:10.1006/frne.2000.0197 |
BAKSHI A, SINGH R, RAI U. Trajectory of leptin and leptin receptor in vertebrates: Structure, function and their regulation. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2022, 257: 110652 DOI:10.1016/j.cbpb.2021.110652 |
CHEN L, LU Y, LI W, et al. The genomic basis for colonizing the freezing Southern Ocean revealed by Antarctic toothfish and Patagonian robalo genomes. GigaScience, 2019, 8(4): giz016 |
CHENG P L, HU R Q, LI G F, et al. Study on stress granules of zebrafish embryo fibroblasts cell (ZF4) under cold stress. Journal of Biology, 2020, 37(4): 40-44 [程鹏丽, 胡瑞芹, 李根芳, 等. 低温胁迫下斑马鱼胚胎成纤维细胞(ZF4)应激颗粒的探究. 生物学杂志, 2020, 37(4): 40-44] |
CHU Q L, YANG K D, WANG A G, et al. Research progress on oxidative stress and apoptosis. Journal of Hygiene Research, 2003, 32(3): 276-279 [褚启龙, 杨克敌, 王爱国. 氧化应激与细胞凋亡关系的研究进展. 卫生研究, 2003, 32(3): 276-279 DOI:10.3969/j.issn.1000-8020.2003.03.030] |
ELMQUIST J K, COPPARI R, BALTHASAR N, et al. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. Journal of Comparative Neurology, 2005, 493(1): 63-71 DOI:10.1002/cne.20786 |
FENG J Z, YAO W Y, LIU J J, et al. Advances in the study of iron metabolic systems affecting the bactericidal action of antibiotics. Chinese Journal of Antibiotics, 2016, 41(8): 561-567 [冯锦芝, 姚文晔, 刘俊坚, 等. 铁代谢体系影响抗生素杀菌作用的研究进展. 中国抗生素杂志, 2016, 41(8): 561-567 DOI:10.13461/j.cnki.cja.005732] |
FOULKES T, WOOD J. Mechanisms of cold pain. Channels, 2007, 1(3): 154-160 DOI:10.4161/chan.4692 |
HANCHET S, DUNN A, PARKER S, et al. The Antarctic toothfish (Dissostichus mawsoni): Biology, ecology, and life history in the Ross Sea region. Hydrobiologia, 2015, 761(1): 397-414 DOI:10.1007/s10750-015-2435-6 |
HU C F, WANG X Y, CHEN L B, et al. Effect of PGC-1a knockdown on zebrafish cells under cold stress. Genomics and Applied Biology, 2019, 38(10): 4422-4429 [胡成枫, 王晓煜, 陈良标. PGC-1α敲降对斑马鱼细胞冷应激过程的影响. 基因组学与应用生物学, 2019, 38(10): 4422-4429 DOI:10.13417/j.gab.038.004422] |
HUANG W, REN C, LI H, et al. Transcriptomic analyses on muscle tissues of Litopenaeus vannamei provide the first profile insight into the response to low temperature stress. PLoS One, 2017, 12(6): e0178604 DOI:10.1371/journal.pone.0178604 |
HUISING M O, KRUISWIJK C P, FLIK G. Phylogeny and evolution of class-I helical cytokines. Journal of Endocrinology, 2006, 189(1): 1-25 DOI:10.1677/joe.1.06591 |
KANDA N, SENO H, KONDA Y, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene, 2004, 23(28): 4921-4929 DOI:10.1038/sj.onc.1207606 |
KHOSROPOUR S, HAMIDI M, FATTAHI A, et al. Leptin and leptin-receptor polymorphisms in fertile and infertile men. Systems Biology in Reproductive Medicine, 2017, 63(1): 7-14 DOI:10.1080/19396368.2016.1258741 |
KUROKAWA T, UJI S, SUZUKI T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Peptides, 2005, 26(5): 745-750 DOI:10.1016/j.peptides.2004.12.017 |
LUO Z, WENG J M, FENG J Q, et al. Research advance on Leptin gene. China Animal Husbandry and Veterinary Medicine, 2018, 45(9): 2524-2530 [罗铮, 翁吉梅, 封竣琪, 等. 瘦素基因的研究进展. 中国畜牧兽医, 2018, 45(9): 2524-2530 DOI:10.16431/j.cnki.1671-7236.2018.09.023] |
MALHI H, GORES G J, LEMASTERS J J. Apoptosis and necrosis in the liver: A tale of two deaths?. Hepatology, 2006, 43(S1): S31-S44 |
MCGAFFIN K R, ZOU B, MCTIERNAN C F, et al. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovascular Research, 2009, 83(2): 313-324 DOI:10.1093/cvr/cvp071 |
NIKOSKELAINEN S, BYLUND G, LILIUS E M. Effect of environmental temperature on rainbow trout (Oncorhynchus mykiss) innate immunity. Developmental and Comparative Immunology, 2004, 28(6): 581-592 DOI:10.1016/j.dci.2003.10.003 |
PÉREZ‐PÉREZ A, TORO A, VILARIÑO‐GARCÍA T, et al. Leptin action in normal and pathological pregnancies. Journal of cellular and molecular medicine, 2018, 22(2): 716-727 DOI:10.1111/jcmm.13369 |
TIEN N. How polar fish survive subzero seas. Chemical and Engineering News of the American Chemical Society, 2018, 96(7): 7-8 |
TORO A R, MAYMÓ J L, IBARBALZ F M, et al. Leptin is an anti-apoptotic effector in placental cells involving p53 downregulation. PloS One, 2014, 9(6): e99187 DOI:10.1371/journal.pone.0099187 |
WANG H, WANG Y, NIU M, et al. Cold acclimation for enhancing the cold tolerance of zebrafish cells. Frontiers in Physiology, 2022, 12: 2532 |
WANG Q, ZHAI W Y, HAN S, et al. Effects of low temperature stress on gut microbial community structure of different strains of obscure puffer Takifugu fasciatus. Journal of Dalian Ocean University, 2022, 37(1): 80-87 [汪倩, 翟万营, 韩爽, 等. 低温胁迫对不同品系暗纹东方鲀肠道微生物群落结构的影响. 大连海洋大学学报, 2022, 37(1): 80-87] |
WANG Y, HU L H, WANG H M, et al. Role of Trematomus bernacchii DUSP1 gene in cell cold resistance. Acta Hydrobiologica Sinica, 2022, 46(8): 1105-1112 [王映, 胡玲红, 王化敏, 等. 伯氏肩孔南极鱼dusp1基因在冷应激中的功能研究. 水生生物学报, 2022, 46(8): 1105-1112] |
WANG Y, WANG H, HU L, et al. Leptin gene protects against cold stress in Antarctic toothfish. Frontiers in Physiology, 2021, 12: 740806 |
YANG J, WANG Z L, ZHAO X Q, et al. Natural selection and adaptive evolution of leptin in the ochotona family driven by the cold environmental stress. PloS One, 2008, 3(1): e1472 |
YU H, LEE H, HERRMANN A, et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nature Reviews Cancer, 2014, 14(11): 736-746 |
ZENG W T, SHAN Y Q, WANG X X, et al. Low temperature autoinducing expression, purification and identification for biological activity of recombined human leptin fusion protein. Military Medical Sciences, 2008, 32(5): 443-446 [曾沃坦, 单永强, 王晓霞, 等. 重组人瘦素融合蛋白在大肠杆菌中的低温自诱导表达、纯化和活性鉴定. 军事医学科学院院刊, 2008, 32(5): 443-446] |
ZHANG Y, CHUA S Jr. Leptin function and regulation. Comprehensive Physiology, 2011, 8(1): 351-369 |
ZHANG Y, PROENCA R, MAFFEI M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature, 1994, 372(6505): 425-432 |
ZOU H F, FENG Y H, LAN Z H, et al. Effect of hypothermia treatment on stress-related genes in the CNSS system of Danio rerio. Jiangsu Agricultural Sciences, 2016, 44(8): 43-46 [邹华锋, 冯宇红, 兰兆辉, 等. 低温处理对斑马鱼CNSS系统应激相关基因的影响. 江苏农业科学, 2016, 44(8): 43-46] |



