2. 浙江万里学院生物与环境学院 浙江省水产种质资源高效利用技术研究重点实验室 浙江 宁波 315000;
3. 上海海洋大学 水产种质资源发掘与利用教育部重点实验室 上海 201306
2. Key Laboratory of Aquatic Germplasm Resource of Zhejiang, College of Biological and Environmental Sciences, Zhejiang Wanli University, Ningbo 315000, China;
3. Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Shanghai Ocean University, Shanghai 201306, China
腺苷三磷酸结合盒转运蛋白(ATP binding cassette transporter, ABC transporter)是一类广泛存在于原核生物和真核生物体内的膜蛋白,对糖类、氨基酸、脂质、外毒素、药物等多种物质具有转运功能,几乎所有真核生物的ABC转运蛋白都是向外转运蛋白(侯文韬等, 2018)。人类ABC转运蛋白基因突变与多种疾病相关,如ABCD1功能紊乱会引起超长链脂肪酸在组织中累积(侯文韬等, 2018);ABCC7是一种离子转运通道蛋白,该蛋白基因的突变会导致囊性纤维化(Zhang et al, 2016);ABCB1、ABCG2和ABCC1能够将抗癌药物转运至胞外,与癌症的耐药性有关(Robey et al, 2018)等。ABC转运蛋白家族特定成员通过介导多型异源物质抗性(multixenobiotic resistance, MXR)机制,将环境毒素和致病病原体等逆浓度运输至胞外,对软体动物具有保护作用(Xu et al, 2011; Jeong et al, 2017),如ABCG2在印度岩牡蛎(Saccostrea forskali)中发挥转运有机污染物三丁基氟化锡的作用(Kingtong et al, 2007);ABCB1和ABCC5在缢蛏感染副溶血弧菌(Vibrio parahaemolyticus)中发挥屏障作用,其功能可能与缢蛏固有免疫的组成有关(Fu et al, 2019)。
根据结构域组成,ABC转运蛋白可分为3类,其中,全转运蛋白(full transporter, FT)由2个核苷酸结合结构域(nucleotide binding domain, NBD)和2个跨膜结构域(transmembrane domain, TMD)组成;半转运蛋白(half transporter, HT)由1个NBD和1个TMD组成,2个半转运蛋白结合为二聚体后才具备转运功能(Ford et al, 2019)。其他ABC转运蛋白只具有NBD或TMD结构域,如ABCE和ABCF亚家族,它们不含TMD,但在一条肽链中有2个NBD,不具备转运功能。NBD结构域序列很保守,功能为结合并水解ATP;TMD结构域决定底物的特异性并构成底物运输的通路,保守性相对NBD较差。
根据序列同源性和结构相似性,ABC转运蛋白分为ABCA~ABCH 8个亚家族。ABCH亚家族目前仅在节肢动物和斑马鱼(Danio rerio)、金鱼(Carassius auratus)等少数硬骨鱼类及部分双壳类软体动物中发现(Dean et al, 2005; Popovic et al, 2010; 刘含梅等, 2020; Wang et al, 2021),在哺乳动物、真菌和植物中没有发现。
在无脊椎动物中,ABC转运蛋白家族鉴定工作在节肢动物中开展较多,如对昆虫纲、蛛形纲及甲壳纲中的家蚕(Bombyx mori)、二点叶螨(Tetranychus urticae)、虎斑猛水蚤(Tigriopus japonicus)等多个物种进行了ABC转运蛋白家族的鉴定分类(Liu et al, 2011; Dermauw et al, 2013; Jeong, 2014)。在软体动物中,目前仅对双壳类的虾夷扇贝(Patinopecten yessoensis)、栉孔扇贝(Chlamys farreri)和长牡蛎(Crassostrea gigas)的ABC转运蛋白家族进行了系统鉴定与分类。缢蛏(Sinonovacula constricta)是软体动物门双壳纲中的重要经济物种,广泛分布于西太平洋沿岸的潮间带和河口水域(Zhao et al, 2017),是我国传统的四大海水养殖贝类之一。研究发现,在铜离子胁迫的福建牡蛎(Crassostrea angulata)中,ABCB1可能发挥保护细胞的作用(Shi et al, 2015);斑马贻贝(Dreissena polymorpha)中,汞可诱导ABCB1与ABCC家族基因表达,参与细胞应激反应(Navarro et al, 2012);在地中海贻贝(Mytilus galloprovincialis)鳃与肝胰腺组织中,ABCB和ABCC亚家族蛋白可能通过外排镉离子发挥保护作用(Torre et al, 2014)。对山东(孙珊等, 2017)与辽东(宋永刚等, 2016)地区海水重金属的检测发现,部分水域毒性重金属超标或存在超标风险,威胁当地贝类的生产养殖工作。ABC转运蛋白通过介导机制发挥排毒解毒功能,对缢蛏ABC转运蛋白功能的研究或有助于缢蛏的健康养殖和优质水产品的供应。对缢蛏ABC转运蛋白家族的鉴定和表达模式分析尚未见报道。本研究对缢蛏ABC转运蛋白基因家族进行系统鉴定、进化树构建和表达模式分析,旨在为理解软体动物ABC转运蛋白基因的进化和缢蛏ABC转运蛋白功能的研究提供基础资料。
1 材料与方法 1.1 缢蛏ABC转运蛋白家族成员鉴定用于鉴定缢蛏ABC转运蛋白的数据来源于已发表的缢蛏基因组和转录组数据(Ran et al, 2019; Dong et al, 2020)。利用人类(Homo sapiens)、二点叶螨(Dermauw et al, 2013)、虾夷扇贝、栉孔扇贝和长牡蛎ABC转运蛋白家族蛋白序列(Wang et al, 2021)作为查询序列,通过本地BLASTP程序在缢蛏基因组蛋白数据库进行搜索比对,E值设置为1×10–5;同时,利用虾夷扇贝、栉孔扇贝和长牡蛎ABC转运蛋白家族蛋白序列作为查询序列,通过本地TBLASTN程序在缢蛏基因组和转录组数据库中进一步搜索比对,E值设置为1×10–5。缢蛏ABC转运蛋白CDS与蛋白序列已上传至Figshare网站(https://figshare.com/articles/dataset/52_razor_clam_ABC/21454542)。
1.2 ABC转运蛋白结构域分析利用SMART网站(http://smart.embl.de/)预测缢蛏ABC转运蛋白结构域,通过Softberry网站(http://www.softberry.com/)的FGENESH+工具(Solovyev, 2007)对缢蛏ABC转运蛋白结构域不完整的序列,以虾夷扇贝蛋白序列为参考进行同源预测来补全序列,并重新利用SMART网站预测蛋白结构域。
1.3 ABC转运蛋白基因在染色体的定位利用NCBI网站下载的缢蛏基因组序列(Dong et al, 2020),通过本地BLASTN程序进行ABC转运蛋白家族基因的染色体定位,使用MapInspect软件完成作图。
1.4 ABC转运蛋白基因结构分析通过本地BLASTN程序将缢蛏ABC转运蛋白CDS序列映射至基因组,得到基因结构信息。利用GSDS网站(http://gsds.gao-lab.org/)(Hu et al, 2015)绘制基因结构示意图。
1.5 缢蛏ABC转运蛋白家族系统进化树构建选取虾夷扇贝、栉孔扇贝、长牡蛎和缢蛏的ABC转运蛋白序列,通过ClustalW (Thompson et al, 1997)程序进行多序列比对,然后利用MEGA X (Kumar et al, 2018)软件的最大似然法(maximum likelihood, ML)构建系统进化树,选择JTT矩阵模型(Jones et al, 1992),自展值(bootstrap)设置为1 000。
1.6 不同物种间ABC转运蛋白家族种类与数量的比较选取6种脊椎动物,即人类(Dean et al, 2001)、小鼠(Mus musculus)、非洲爪蟾(Xenopus laevis)、金鱼(刘含梅等, 2020)、斑马鱼(Annilo et al, 2006)和海七鳃鳗(Petromyzon marinus)(Ren et al, 2015)和7种无脊椎动物,即柑橘木虱(Diaphorina citri)(Wang et al, 2019)、二点叶螨(Dermauw et al, 2013)、瓜实蝇(Zeugodacus cucurbitae)(Xu et al, 2021)、虾夷扇贝、栉孔扇贝、长牡蛎(Wang et al, 2021)和缢蛏,进行ABC转运蛋白亚家族种类与数量比较分析。小鼠与非洲爪蟾ABC转运蛋白家族信息检索自Ensembl数据库(http://www.ensembl.org/index.html)。
1.7 ABC转运蛋白基因表达模式分析利用已发表的缢蛏不同发育时期和成体不同组织的转录组数据(Dong et al, 2020),使用软件Mev 4.90绘制缢蛏鳃、足、闭壳肌、肝胰脏、外套膜、水管、卵巢与精巢8个成体不同组织和卵子、4细胞期、囊胚、原肠胚、担轮幼虫、D形幼虫、壳顶幼虫和稚贝8个不同发育时期的ABC转运蛋白基因表达热图。
2 结果 2.1 缢蛏ABC转运蛋白家族成员鉴定与特征描述利用缢蛏基因组和转录组数据经过序列同源比对分析,在全基因组水平上鉴定出52个ABC转运蛋白基因,分布在8个亚家族(A~H),其中,7个ABCA、10个ABCB、13个ABCC、3个ABCD、1个ABCE、3个ABCF、14个ABCG和1个ABCH (表 1)。根据蛋白结构域,分为24个全转运蛋白、24个半转运蛋白以及4个非转运蛋白。ABC转运蛋白编码基因长度区间为1 588~7 224 bp,预测蛋白序列长度跨度为382~2 408 aa。
|
|
表 1 缢蛏52个ABC转运蛋白信息 Tab.1 Information of 52 ABC transporters in razor clam |
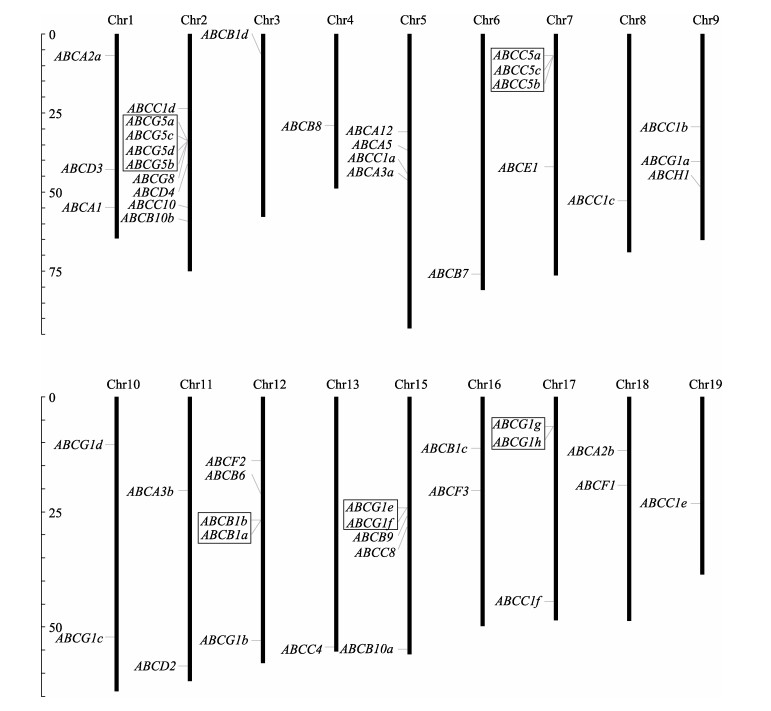
对ABC转运蛋白基因在染色体的定位研究显示(图 1),来自ABCB、ABCC与ABCG亚家族的部分基因可能在进化过程中发生过串联复制。对串联重复基因的结构进一步研究发现,发生串联复制的基因编码的蛋白同源性很高,进化树分支支持率在95%以上,且蛋白长度相近;每组串联复制基因长度存在一定差异,但外显子数目接近,最多相差4个。
|
图 1 缢蛏ABC转运蛋白基因染色体位置图示 Fig.1 Graphical representation of ScABCs locations on chromosomes 方框内标注的基因可能发生过串联复制,最左侧为染色体相对长度(Mb)示意标尺。 Genes within the rectangle box are putatively tandem duplicated, the scale (in megabase) on the left depicts the relative lengths of the different chromosomes in razor clam. |
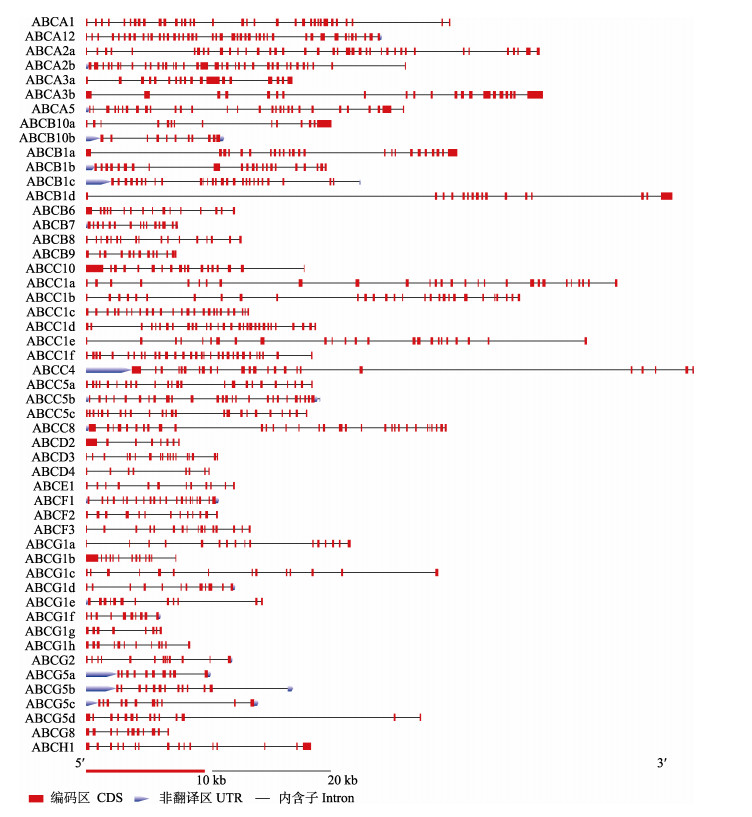
ABC转运蛋白基因外显子数目为8~44个,全转运蛋白编码基因的外显子数目总体多于半转运蛋白编码基因,不同基因间UTR长度差距明显,如ABCC4的5′UTR长度明显长于其他基因(图 2)。
|
图 2 缢蛏ABC转运蛋白基因结构 Fig.2 Gene structure of ScABCs |
A亚家族包括1个ABCA1、2个ABCA2、2个ABCA3、1个ABCA5和1个ABCA12,共7个成员,均为全转运蛋白。B亚家族包括4个全转运蛋白,即ABCB1a~ABCB1d,以及6个半转运蛋白,即ABCB6~ABCB9和2个ABCB10,共10个成员。C亚家族包括7个ABCC1、1个ABCC4、3个ABCC5、1个ABCC8和1个ABCC10,共13个成员,均为全转运蛋白。
D亚家族包括ABCD2~ABCD4,共3个成员,该家族成员结构域全部为TMD-NBD,属于半转运蛋白。E亚家族和F亚家族为非转运蛋白,均由2个NBD构成,不包含TMD,E亚家族包含1个ABCE1,F亚家族包括ABCF1~ABCF3,共3个成员。G亚家族包括8个ABCG1、1个ABCG2、4个ABCG5和1个ABCG8,共14个成员;ABCH亚家族共鉴定出1个成员ABCH1,ABCG与ABCH家族成员全部为半转运蛋白,其结构域均为反向结构域,即NBD-TMD。
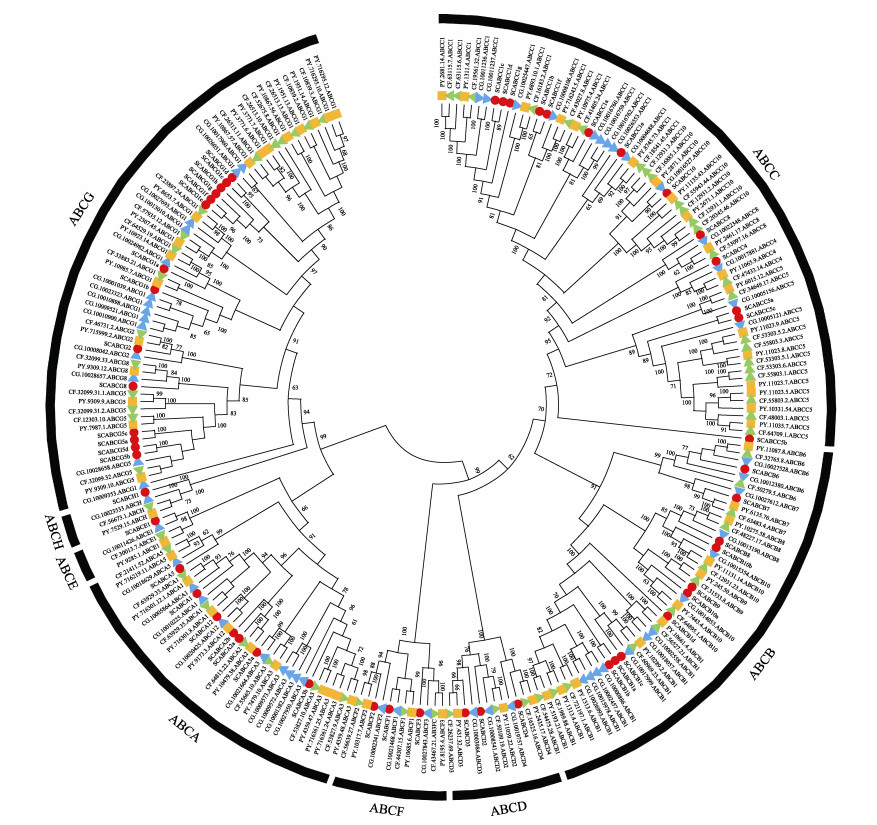
2.2 贝类ABC转运蛋白亚家族成员进化分析依据虾夷扇贝、栉孔扇贝、长牡蛎与缢蛏ABC转运蛋白序列的同源性,绘制4种双壳类ABC转运蛋白家族系统进化树(图 3)。双壳类物种ABC转运蛋白各亚族成员聚合良好,虾夷扇贝与栉孔扇贝的ABC转运蛋白往往先以很高的支持率聚于分支最内侧,再与缢蛏或长牡蛎的序列相聚。
|
图 3 4种双壳类ABC转运蛋白家族系统进化树 Fig.3 Phylogenetic tree of ABC transporters from four bivalves 物种及简写:PY:虾夷扇贝(橙色正方形);CF:栉孔扇贝(绿色正三角形);CG:长牡蛎(蓝色倒三角形);SC:缢蛏(红色圆点) Species and abbreviations: PY: Patinopecten yessoensis (orange squares); CF: Chlamys farreri (green triangles); CG: Crassostrea gigas (blue inverted triangles); SC: Sinonovacula constricta (red dots) |
对双壳类物种ABC转运蛋白成员数目差异进行比较,发现虾夷扇贝ABC转运蛋白数量为67,栉孔扇贝为64,长牡蛎为58,而缢蛏为52。ABCA亚家族中,除栉孔扇贝外,其他贝类均含有1个ABCA12;B亚家族中,缢蛏、2种扇贝、长牡蛎各具有不同的ABCB1数量,栉孔扇贝与长牡蛎ABCB6数量为2,缢蛏与虾夷扇贝ABCB6数量为1;C亚家族中,ABCC1和ABCC5在4种贝类中均有多个拷贝,而ABCC10仅在2种扇贝体内为多拷贝;4种贝类D、E、F和H亚家族成员种类与数量一致;在G亚家族中,ABCG1为多拷贝基因,ABCG5在长牡蛎中为单拷贝基因,在其他贝类中存在3~4个拷贝。不同贝类间基因数目的差异,提示双壳类生物进化过程中可能发生基因增加或丢失事件。
2.3 不同物种间ABC转运蛋白家族成员比较研究选取6种脊椎动物和7种无脊椎动物,脊椎动物包括哺乳动物人类与小鼠,两栖动物非洲爪蟾,鱼类的金鱼和斑马鱼,无颌类的海七鳃鳗;无脊椎动物包括节肢动物柑橘木虱、二点叶螨和瓜实蝇,软体动物长牡蛎、虾夷扇贝、栉孔扇贝与缢蛏,对各个物种的ABC转运蛋白基因家族成员的数量进行了比较分析(表 2)。结果显示,ABC转运蛋白家族总数量在脊椎动物中较为保守,在节肢动物中数量变化很大,部分物种ABC转运蛋白基因数量多达一百以上。人类ABC转运蛋白家族中A与C亚家族含有的基因数量最多,小鼠和非洲爪蟾的A与B亚家族含有基因数量最多,金鱼、斑马鱼和海七鳃鳗的B与C亚家族含有最多基因,而二点叶螨、瓜实蝇、长牡蛎、虾夷扇贝、栉孔扇贝以及缢蛏基因数目最高的亚家族为C和G。与脊椎动物相比,节肢动物和软体动物的ABCG基因家族成员数量更多;鱼类和软体动物只含有1个ABCH,节肢动物含有多个ABCH;无脊椎动物中柑橘木虱含有4个ABCF家族基因,其他动物ABCF家族基因数目为3个。
|
|
表 2 13个物种ABC转运蛋白基因家族比较 Tab.2 Comparison of ABC transporter gene family among 13 species |
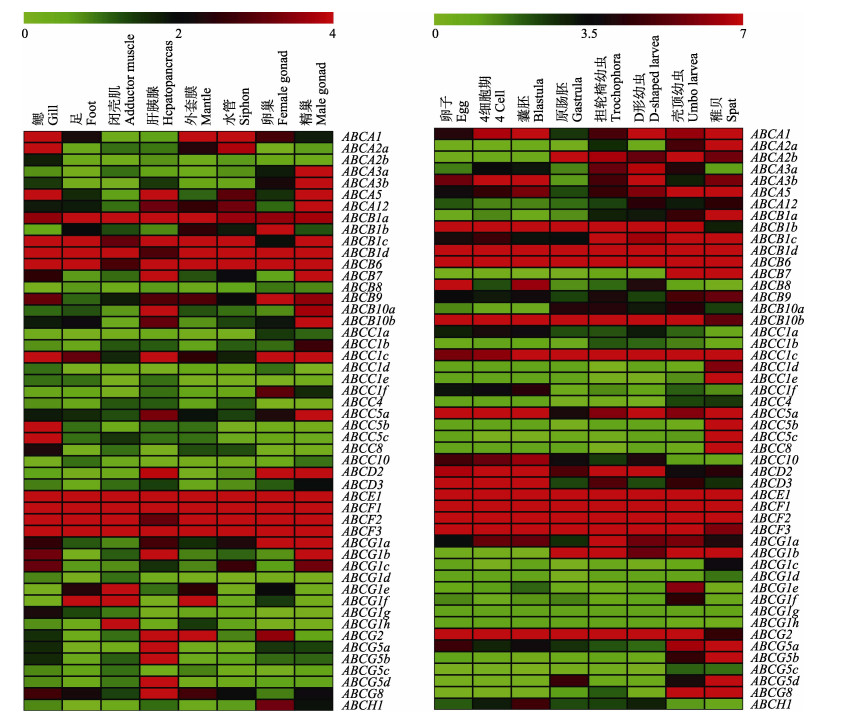
缢蛏成体不同组织基因表达热图显示(图 4A),转运蛋白基因在鳃、肝胰腺、水管与精巢中表达种类较多,ABCD2仅在肝胰腺与性腺中出现高表达量;ABCG5、ABCB8在缢蛏肝胰腺中特异性高表达;缢蛏鳃与精巢中较多ABCA亚家族成员出现较高表达量,ABCB亚家族多个成员和ABCE-F亚家族成员在8个组织中普遍高表达。Luckenbach等(2008)发现,ABCB基因在加州贻贝(Mytilus californianus)鳃部细胞发挥外排环境毒素以保护细胞的生理功能,Fu等(2019)研究发现,缢蛏ABCB1与ABCC5在缢蛏鳃部发挥对病原菌感染的屏障作用。在缢蛏鳃组织中观察到ABCB1、ABCB6、ABCB9与ABCC5基因的高表达,鳃作为持续性暴露于海水和进行呼吸代谢的器官,推测上述基因的高表达对缢蛏具有重要免疫保护意义。
|
图 4 缢蛏成体不同组织(A)和不同发育时期(B) ABC转运蛋白基因表达谱 Fig.4 Expression profiles of ABC transporter genes in different tissues (A) and during development stages (B) of razor clam |
缢蛏不同发育时期基因表达热图显示(图 4B),部分ABCC1、ABCC5和ABCC8等多个基因表达量在缢蛏稚贝时期达到最高值,而ABCC10表达量随缢蛏发育而下降;ABCG亚家族多个成员在壳顶幼虫期出现最高表达量;缢蛏由卵子发育至囊胚期间,除ABCB8外,绝大部分基因表达量无明显变化;进入原肠胚时期后,ABCA2b、ABCB10a和ABCG1b基因表达量升高,ABCA1、ABCA3b、ABCA5和ABCB8等基因表达量下降明显;ABCH1在各个发育时期均维持较低表达量;A、B、C、G亚家族部分成员和E、F亚家族全部成员在8个发育阶段中普遍维持高表达量。
Wang等(2021)对ABC转运蛋白基因在虾夷扇贝与栉孔扇贝成体组织中的表达进行了研究,比较发现,ABCE与ABCF亚家族基因在2种扇贝的成体组织中同样维持高表达量,提示E、F亚家族基因发挥管家基因作用;3种贝类的外套膜中均发现高表达量的ABCG1基因,鳃中均存在高表达量的ABCB1基因,ABCG5和ABCG8基因在3种贝类肝胰腺中特异性高表达;ABCB亚家族多个成员在缢蛏及2种扇贝性腺中有较高表达量,不同的是,大部分ABCA亚家族成员在缢蛏精巢中出现高表达量,在2种扇贝体内表达量较低;2种扇贝鳃部ABCC家族基因表达量普遍低,而缢蛏鳃中观察到ABCC1与ABCC5的较高表达量。
3 讨论利用缢蛏2个基因组蛋白数据库结合全长转录组数据共鉴定出52个ABC转运蛋白,分属A~H 8个亚家族。通过对缢蛏ABC转运蛋白基因进行系统分析,及基因在染色体上的定位分析和不同物种ABC转运蛋白家族数量比较,发现软体动物的多个ABC转运蛋白基因为串联重复基因。部分基因序列通过本地BLASTN程序未在缢蛏染色体上搜索出对应序列,因此缺乏染色体定位或外显子数量信息(ABCC1g和ABCG2);由于基因组注释不够完整,一部分基因缺少3'UTR或5'UTR信息;进行成体不同组织和不同发育时期的表达谱分析时,由于发现基因组未注释到ABCC1g和ABCD4基因,因此未提供转录表达数据。
对虾夷扇贝、栉孔扇贝、长牡蛎与缢蛏ABC转运蛋白家族数量进行比较发现,虾夷扇贝ABC转运蛋白数量为64个,栉孔扇贝67个,长牡蛎58个,而缢蛏只鉴定出52个蛋白。虾夷扇贝、栉孔扇贝与缢蛏染色体数目均为2n=38 (赵洋等, 2006; Ran et al, 2019),长牡蛎为2n=20 (Qi et al, 1999),造成不同双壳类物种间基因成员数目差异的可能原因如下:1)进化地位不同,导致基因在进化过程中发生增加或缺失;虾夷扇贝与栉孔扇贝同属扇贝科,亲缘关系近,基因数目相近;长牡蛎属牡蛎科,与扇贝科更近;缢蛏属竹蛏科,与扇贝、牡蛎关系较远;2)缢蛏基因组组装不完整,尚存在未鉴定到的基因。
脊椎动物含有较为保守的ABC转运蛋白家族总数量,除金鱼和斑马鱼各含有1个ABCH外,其他物种不含有ABCH;节肢动物中ABC转运蛋白家族数量变化很大,ABCH亚家族均含有多个成员,推测与ABCH运送脂质至外表皮形成防水蜡质层的功能有关(Broehan et al, 2013; Dermauw et al, 2013);与脊椎动物相比,无脊椎动物的ABCG亚家族成员数量更高。双壳类中ABC转运蛋白基因家族部分成员存在多个拷贝,如ABCB1、ABCG1等均为至少2个拷贝,这可能与基因的起源有关,这些基因可能是由串联复制(tandem duplication)产生的。软体动物多种参与环境响应和免疫防御的基因均发生过独立扩增(Takeuchi et al, 2016),研究发现,缢蛏ABCB1和ABCC5对病原感染发挥屏障作用(Fu et al, 2019),而这2个基因在缢蛏、虾夷扇贝、栉孔扇贝和长牡蛎中均存在多个拷贝,故推测软体动物的基因串联复制增加了部分ABC转运蛋白的拷贝数量,且这对于海洋双壳类对病原生物的屏障作用和毒素的排出具有积极作用。
对缢蛏ABC转运蛋白基因在成体不同组织和不同发育时期的表达谱进行分析,发现ABC转运蛋白基因在缢蛏不同组织存在差异表达,在肝胰脏和鳃表达量较高,在足和闭壳肌中表达量低,可能与ABC转运蛋白在缢蛏体内对毒素等异物的外排生理功能有关;多个ABC转运蛋白基因表达量随缢蛏发育而增加,在稚贝时期达到最高,这可能有助于缢蛏适应不同发育时期的生活环境改变。
4 结论综上,本研究对缢蛏ABC转运蛋白进行了鉴定、结构域预测和进化分析,通过对比13种动物的ABC转运蛋白亚家族种类与数量,探讨其在物种进化过程中的变化,并对ABC转运蛋白基因在缢蛏不同组织和不同发育时期的差异表达进行分析,为缢蛏ABC转运蛋白在生理学、毒理学、环境适应与进化方向的研究提供了基础资料。
ANNILO T, CHEN Z Q, SHULENIN S, et al. Evolution of the vertebrate ABC gene family: Analysis of gene birth and death. Genomics, 2006, 88(1): 1-11 DOI:10.1016/j.ygeno.2006.03.001 |
BROEHAN G, KROEGER T, LORENZEN M, et al. Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum. BMC Genomics, 2013, 14: 6 DOI:10.1186/1471-2164-14-6 |
DEAN M, ANNILO T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annual Review of Genomics and Human Genetics, 2005, 6: 123-142 DOI:10.1146/annurev.genom.6.080604.162122 |
DEAN M, RZHETSKY A, ALLIKMETS R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Research, 2001, 11(7): 1156-1166 DOI:10.1101/gr.184901 |
DERMAUW W, OSBORNE E J, CLARK R M, et al. A burst of ABC genes in the genome of the polyphagous spider mite Tetranychus urticae. BMC Genomics, 2013, 14: 317 DOI:10.1186/1471-2164-14-317 |
DONG Y H, ZENG Q F, REN J F, et al. The chromosome-level genome assembly and comprehensive transcriptomes of the razor clam (Sinonovacula constricta). Frontiers in Genetics, 2020, 11: 664 DOI:10.3389/fgene.2020.00664 |
FORD R C, BEIS K. Learning the ABCs one at a time: Structure and mechanism of ABC transporters. Biochemical Society Transactions, 2019, 47(1): 23-36 DOI:10.1042/BST20180147 |
FU J, ZHAO X, SHI Y, et al. Functional characterization of two ABC transporters in Sinonovacula constricta gills and their barrier action in response to pathogen infection. International Journal of Biological Macromolecules, 2019, 121: 443-453 DOI:10.1016/j.ijbiomac.2018.10.047 |
HOU W T, WANG L, XU D, et al. ABC transporters and human diseases. Journal of University of Science and Technology of China, 2018, 48(10): 853-861 [侯文韬, 王亮, 徐达, 等. ABC转运蛋白与人类疾病. 中国科学技术大学学报, 2018, 48(10): 853-861] |
HU B, JIN J P, GUO Y A, et al. GSDS 2. 0:An upgraded gene feature visualization server. Bioinformatics, 2015, 31(8): 1296-1297 |
JEONG C B, KIM B M, LEE J S, et al. Genome-wide identification of whole ATP-binding cassette (ABC) transporters in the intertidal copepod Tigriopus japonicus. BMC Genomics, 2014, 15(1): 651 DOI:10.1186/1471-2164-15-651 |
JEONG C B, KIM H S, KANG H M, et al. ATP-binding cassette (ABC) proteins in aquatic invertebrates: Evolutionary significance and application in marine ecotoxicology. Aquatic Toxicology, 2017, 185: 29-39 DOI:10.1016/j.aquatox.2017.01.013 |
JONES D T, TAYLOR W R, THORNTON J M. The rapid generation of mutation data matrices from protein sequences. Computer Applications in the Biosciences, 1992, 8(3): 275-282 |
KINGTONG S, CHITRAMVONG Y, JANVILISRI T. ATP-binding cassette multidrug transporters in Indian-rock oyster Saccostrea forskali and their role in the export of an environmental organic pollutant tributyltin. Aquatic Toxicology, 2007, 85(2): 124-132 DOI:10.1016/j.aquatox.2007.08.006 |
KUMAR S, STECHER G, LI M, et al. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 2018, 35(6): 1547-1549 DOI:10.1093/molbev/msy096 |
LIU H M, CUI W Y, SONG S Y, et al. Identification and analysis of ABC transporter gene family in goldfish (Carassius auratus var.). Genomics and Applied Biology, 2020, 39(1): 1-10 [刘含梅, 崔文耀, 宋诗颖, 等. 金鱼ABC转运蛋白基因家族成员鉴定及分析. 基因组学与应用生物学, 2020, 39(1): 1-10] |
LIU S, ZHOU S, TIAN L, et al. Genome-wide identification and characterization of ATP-binding cassette transporters in the silkworm, Bombyx mori. BMC Genomics, 2011, 12: 491 DOI:10.1186/1471-2164-12-491 |
LUCKENBACH T, EPEL D. ABCB- and ABCC- type transporters confer multixenobiotic resistance and form an environment-tissue barrier in bivalve gills. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 2008, 294(6): R1919-1929 DOI:10.1152/ajpregu.00563.2007 |
NAVARRO A, WEIßBACH S, FARIA M, et al. Abcb and Abcc transporter homologs are expressed and active in larvae and adults of zebra mussel and induced by chemical stress. Aquatic Toxicology, 2012, 122/123: 144-152 DOI:10.1016/j.aquatox.2012.06.008 |
POPOVIC M, ZAJA R, LONCAR J, et al. A novel ABC transporter: The first insight into zebrafish (Danio rerio) ABCH1. Marine Environmental Research, 2010, 69(Suppl): S11-13 |
QI H Y, LIU M X, ZHU H X, et al. Karyotype and meiosis of oyster Crassostrea gigas (Bivalvia, Ostreidae). Journal of Liaocheng University (Natural Science), 1999(1): 51-55 |
RAN Z, LI Z, YAN X, et al. Chromosome-level genome assembly of the razor clam Sinonovacula constricta (Lamarck, 1818). Molecular Ecology Resources, 2019, 19(6): 1647-1658 DOI:10.1111/1755-0998.13086 |
REN J F, CHUNG-DAVIDSON Y W, YEH C Y, et al. Genome-wide analysis of the ATP-binding cassette (ABC) transporter gene family in sea lamprey and Japanese lamprey. BMC Genomics, 2015, 16(1): 436 DOI:10.1186/s12864-015-1677-z |
ROBEY R W, PLUCHINO K M, HALL M D, et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nature Reviews Cancer, 2018, 18(7): 452-464 DOI:10.1038/s41568-018-0005-8 |
SHI B, XIANG X, KE Y Z, et al. Abcb1 gene expression pattern and function of copper detoxification in Fujian oyster, Crassostrea angulata. Comparative Biochemistry and Physiology, Part B: Biochemistry and Molecular Biology, 2015, 190: 8-15 DOI:10.1016/j.cbpb.2015.08.007 |
SOLOVYEV V V. Statistical approaches in eukaryotic gene prediction in handbook of statistical genetics (eds. BALDING D, CANNINGS C, AND BISHOP M). Wiley-Interscience, 2007, 3rd edition, 1616
|
SONG Y G, WU J H, SHAO Z W, et al. Evaluation of heavy metal pollution in the offshore surface seawater of the Liaodong Bay. Progress in Fishery Sciences, 2016, 37(3): 14-19 [宋永刚, 吴金浩, 邵泽伟, 等. 辽东湾近岸表层海水重金属污染分析与评价. 渔业科学进展, 2016, 37(3): 14-19] |
SUN S, ZHAO Y T, WANG L M, et al. Status of heavy metal pollution in the shellfish culture area of Shangdong Province and the risk analysis of heavy metal elements in the shellfish. Progress in Fishery Sciences, 2017, 38(4): 118-125 [孙珊, 赵玉庭, 王立明, 等. 山东省主要贝类养殖区重金属环境状况及贝类安全风险分析. 渔业科学进展, 2017, 38(4): 118-125] |
TORRE D C, BOCCI E, FOCARDI S E, et al. Differential ABCB and ABCC gene expression and efflux activities in gills and hemocytes of Mytilus galloprovincialis and their involvement in cadmium response. Marine Environmental Research, 2014, 93: 56-63 DOI:10.1016/j.marenvres.2013.06.005 |
TAKEUCHI T, KOYANAGI R, GYOJA F, et al. Bivalve-specific gene expansion in the pearl oyster genome: Implications of adaptation to a sessile lifestyle. Zoological Letters, 2016, 2: 3 DOI:10.1186/s40851-016-0039-2 |
THOMPSON J D, GIBSON T J, PLEWNIAK F, et al. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 1997, 25(24): 4876-4882 DOI:10.1093/nar/25.24.4876 |
WANG H, LIU S, XUN X, et al. Toxin- and species-dependent regulation of ATP-binding cassette (ABC) transporters in scallops after exposure to paralytic shellfish toxin-producing dinoflagellates. Aquatic Toxicology, 2021, 230: 105697 DOI:10.1016/j.aquatox.2020.105697 |
WANG Z, TIAN F, CAI L, et al. Identification of candidate ATP-binding cassette transporter gene family members in Diaphorina citri (Hemiptera: Psyllidae) via adult tissues transcriptome analysis. Scientific Reports, 2019, 9(1): 15842 DOI:10.1038/s41598-019-52402-3 |
XU H Q, MA M, MA Y P, et al. Identification and expression characterization of ATP-binding cassette (ABC) transporter genes in melon fly. Insects, 2021, 12(3): 270 DOI:10.3390/insects12030270 |
XU W F, QIAO K, HUANG S P, et al. Quantitative gene expression and in situ localization of scygonadin potentially associated with reproductive immunity in tissues of male and female mud crabs, Scylla paramamosain. Fish and Shellfish Immunology, 2011, 31(2): 243-251 DOI:10.1016/j.fsi.2011.05.009 |
ZHANG Z, CHEN J. Atomic structure of the cystic fibrosis transmembrane conductance regulator. Cell, 2016, 167(6): 1586-1597 DOI:10.1016/j.cell.2016.11.014 |
ZHAO X, DUAN X, WANG Z, et al. Comparative transcriptome analysis of Sinonovacula constricta in gills and hepatopancreas in response to Vibrio parahaemolyticus infection. Fish and Shellfish Immunology, 2017, 67: 523-535 DOI:10.1016/j.fsi.2017.06.040 |
ZHAO Y, BAO Z M, BI K, et al. Karyotypes of hybrid scallop (hybridizing cross the female Patinopecten yessoensis with the male Chlamys farreri) and their parents. Haiyang Xuebao, 2006(1): 100-105 [赵洋, 包振民, 毕克, 等. 雌性虾夷扇贝与雄性栉孔扇贝杂交子代及其亲本的核型研究. 海洋学报(中文版), 2006(1): 100-105] |



