2. 江苏省淡水水产研究所 农业农村部淡水虾蟹遗传育种与养殖重点实验室 江苏 南京 210017;
3. 江苏海洋大学海洋科学与水产学院 江苏 连云港 222005
2. Key Laboratory of Genetic Breeding and Cultivation for Freshwater Crustacean, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Institute of Jiangsu Province, Nanjing 210017, China;
3. College of Marine Science and Fisheries, Jiangsu Ocean University, Lianyungang 222005, China
克氏原螯虾(Procambarus clarkii)俗称小龙虾,原产于北美洲,是我国重要的淡水养殖甲壳动物。2021年全国克氏原螯虾养殖面积为17 3333 hm2,养殖产量为263.36万t (于秀娟等, 2022)。克氏原螯虾养殖过程中水体溶解氧变化剧烈,低氧胁迫会导致克氏原螯虾壳色变红,蜕壳次数减少,性腺提早发育成熟,抗病力下降,甚至造成大面积死亡现象(芦超等, 2020; Wang et al, 2016)。短期轻度缺氧条件下,水生甲壳类动物通过提高氧结合蛋白的浓度和亲和力,增强氧气摄入量来适应低氧环境(孙盛明等, 2020)。长期缺氧条件下,水生甲壳类动物通过降低蛋白合成速率和自身代谢需求来减少需氧量,同时诱导糖酵解、脂质代谢等厌氧途径来临时补充能量,补偿有氧代谢不足(殷丽坤等, 2023; 曹梅等, 2021)。但厌氧代谢会抑制线粒体中电子与氧气结合,引起机体自由基稳衡态失衡,降低机体呼吸代谢和抗氧化代谢水平,造成细胞氧化应激损伤(陆逸等, 2020)。
虾青蛋白(Crustacyanin, CRCN)是甲壳动物所特有的一类载脂蛋白,属ApoD家族,主要由A和C两大亚族构成。虾青蛋白可与虾青素聚合形成虾青结合蛋白复合体,引起虾青素末端质子重置,从而调控甲壳动物壳色(Begum et al, 2015; Chayen et al, 2005)。除了调控壳色功能外,该家族还在脂类代谢及低氧应激等方面具有重要的生物学功能(王磊等, 2008; Goessling et al, 2000)。虾青蛋白同系物Glaz功能缺失性突变,会导致果蝇(Drosophila melanogaster)氧化应激损伤和饥饿耐受力降低,体重减少,寿命缩短;重启Glaz表达后,果蝇脂肪储存恢复,机体抗氧化能力和饥饿敏感性增加;过表达Glaz时,果蝇平均寿命延长,饥饿耐受力增强,低氧胁迫下的运动爬行能力提高(Sanchez et al, 2006; Walker et al, 2006)。除此之外,虾青蛋白在蜕皮生长、性腺和神经系统发育等过程中均发挥了重要的作用(Wang et al, 2007; Li, 2019; Gao et al, 2021; Yang et al, 2011)。
本研究基于课题组前期所获取的克氏原螯虾全基因组信息(Xu et al, 2021),采用同源比对与分子克隆相结合的策略获取了类虾青蛋白基因(PcCRCN-L)的cDNA序列,并研究其基因结构与进化模式,分析该基因在不同组织与性腺不同发育时期的表达特征,比较PcCRCN-L基因在低氧–复氧胁迫下的表达模式,旨在为深入研究该基因在克氏原螯虾性腺发育与低氧应激调控中的作用提供参考。
1 材料与方法 1.1 实验材料本实验所用克氏原螯虾采集自江苏省淡水水产研究所浦口遗传育种基地,体重为(25.3±0.8) g,于实验室中流水暂养(所有样本均为雌虾)。随机取3只健康的克氏原螯虾(性腺发育Ⅰ期),分别取肝胰腺、肌肉、鳃、眼柄、后肠、表皮、心脏、胃、卵巢等9个组织用于组织表达特征分析。分别取3只处于性腺不同发育时期的雌虾,取肝胰腺和卵巢组织,分析PcCRCN-L基因在性腺不同发育时期的表达特征。根据卵粒形态特征判断性腺发育时期:卵巢发育初期(Ⅰ期)呈白色透明状,未见卵粒;Ⅱ期、Ⅲ期卵巢颜色逐步变黄加深,卵粒明显增大;发育至Ⅳ~Ⅴ期,卵黄沉积后的卵粒呈深褐色(徐增洪等, 2010)。
低氧胁迫实验参照文献报道的研究方案进行(Xu et al, 2022)。将克氏原螯虾随机分为2组,常氧对照组溶解氧为(6.8±0.2) mg/L,低氧–复氧胁迫组初始溶解氧为(1.0±0.1) mg/L。每组分别设置3个重复水族箱,每箱养殖15只克氏原螯虾。于低氧胁迫1 h和6 h、复氧1 h和24 h [溶解氧为(6.8±0.2) mg/L],从各箱中分别随机取3只克氏原螯虾样本,采集肝胰腺组织,置于RNA Keeper Tissue (Vazyme, 南京)中,4 ℃放置1 h,液氮速冻后–80 ℃保存备用。
1.2 RNA提取与cDNA的合成参照RNAisoPlus试剂盒使用说明书(TaKaRa, 大连),提取克氏原螯虾不同组织总RNA。采用微量分光光度计TGemPro (北京天根生化科技有限公司)检测RNA浓度,琼脂糖凝胶电泳检测其完整性,使用反转录试剂盒PrimeScriptTM RT reagent kit with gDNA Eraser (TaKaRa, 大连)获取cDNA。
1.3 PcCRCN-L cDNA序列的克隆基于克氏原螯虾基因组信息(JAIWQB 000000000.1)类虾青蛋白PcCRCN-L基因序列,设计引物(PcCRCN-LF: AGCCGGTCCGTGTATAGTTG,PcCRCN-LR: GTACGTCTGAGCAGCAGCAG)用于扩增PcCRCN-L的完整开放读码框(ORF)区域。PCR扩增体系:95 ℃预变性5 min,95 ℃变性30 s,55 ℃退火60 s/120 s,72 ℃延伸1 min,40个循环;最后72 ℃延伸10 min。PCR产物纯化回收(TaKaRa, 大连)后连接到Peasy-T3 cloning Vector (全式金, 北京),并转化到Trans-T1感受态细胞(全式金, 北京),挑取阳性克隆进行测序。
1.4 生物信息学分析应用EditSeq程序进行开放阅读框(ORF)分析并将其推导为相应的氨基酸序列;使用NCBI (http://www.ncbi.nlm.nih.gov) BLAST进行蛋白质序列同源性检索分析;使用ExPASy (https://web.expasy.org/protparam/)在线工具分析蛋白质分子量、等电点及基本性质;使用SMART (http://smart.embl-heidelberg.de/)预测蛋白功能域;使用Prosite (https://prosite.expasy.org/)预测蛋白氨基酸结构功能位点;使用ClustalW2软件将PcCRCN-L序列与同源氨基酸序列对比分析;应用MEGA 6软件,采用Neighbor-Joining法构建系统进化树;使用AnimalTFDB3 (http://bioinfo.life.hust.edu.cn/AnimalTFDB/#!/)预测目的基因上游2 000 bp转录因子。
1.5 PcCRCN-L mRNA实时荧光定量PCR检测根据获得的PcCRCN-L基因cDNA序列信息,设计引物用于实时荧光定量PCR (RT-qPCR)检测,RT-PcCRCN-LF:CCACCATCACCATCATCAT,RT-PcCRCN-LR:TCCACATCTTCAACCATCAA。内参引物为RT-GAPDHF:GATGCCCCAATGTTCGTC TG,RT-GAPDHR:CGTCATCCTCAGTGTAACC CA)。每个样品的目的基因和内参基因分别进行3次重复。RT-qPCR条件:95 ℃预变性5 min;95 ℃变性30 s,60 ℃退火延伸30 s,40个循环;72 ℃延伸10 min。PcCRCN-L基因在不同组织、性腺不同发育时期和低氧胁迫下的相对表达情况采用2–ΔΔCt法进行分析(Livak et al, 2001)。使用单因素方差分析(one-way ANOVA)对表达结果进行统计,采用LSD法进行多重比较(P<0.05)。
2 结果 2.1 PcCRCN-L序列分析克氏原螯虾类虾青蛋白基因(PcCRCN-L)的cDNA序列长2 700 bp (GenBank登录号:OP235978),位于第12号染色体上,开放阅读框(ORF)为1 587 bp,编码528个氨基酸残基(图 1)。其理论等电点为5.64,相对分子量为57.75 kDa,属于亲水性蛋白。PcCRCN-L DNA序列长6 130 bp,包含5个外显子和4个内含子,5个外显子大小从88 bp到1 047 bp不等,4个内含子大小从195 bp到1 966 bp不等。与内含子相比,外显子相对较小,内含子/外显子剪接方式符合GT-AG规则(表 1)。在转录起始位点上游2 000 bp内预测到有2个低氧诱导因子HIF-1结合位点5´-RCGTG-3´ (–814 ~ –831 bp和–244 ~ –255 bp) (图 1)。
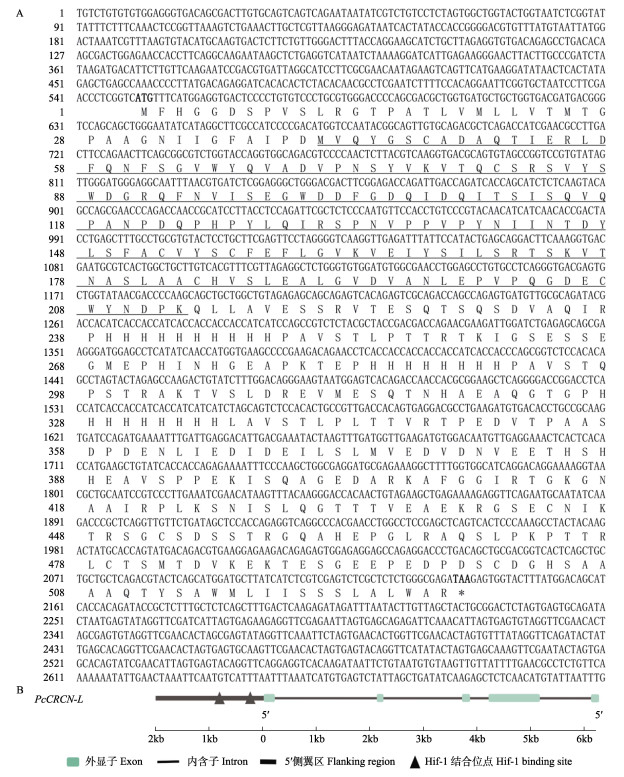
|
图 1 PcCRCN-L基因的cDNA序列及其氨基酸和基因结构 Fig.1 cDNA sequence and its amino acid and gene structure of PcCRCN-L A:粗体ATG和TAA分别代表起始密码子和终止密码子,下划线部分代表催化结构域;B:基因结构 A: Bold ATG and TAA represent the start and stop codons, respectively, and the underlined part represents the catalytic domain; B: The structure of the PcCRCN-L gene |
|
|
表 1 PcCRCN-L基因的外显子–内含子剪接 Tab.1 Exon-intron splice junctions of PcCRCN-L |
对氨基酸序列所含结构域进行预测,PcCRCN-L具有一个完整的lipocalin结构域,包含典型的序列保守区Ⅰ(SCR1)序列G-X-W、保守区Ⅱ(SCR2)序列T-D-Y和保守区Ⅲ(SCR3)序列-R。同源比对结果显示,PcCRCN-L与美洲螯龙虾(Homarus americanus)同源性最高(68.09%),与中国对虾(Penaeus chinensis)同源性为55.24%,与日本对虾(Penaeus japonicus)同源性为53.85%,与凡纳滨对虾(Litopenaeus vannamei)同源性56.64% (图 2)。系统进化树分析结果显示,PcCRCN-L与美洲螯龙虾、中国对虾、凡纳滨对虾和日本对虾聚为一支,独立于传统虾青蛋白A与C,与甲壳动物ApoD和昆虫ApoD形成独立分支,与传统CRCN分类方法结果相一致(图 3)。
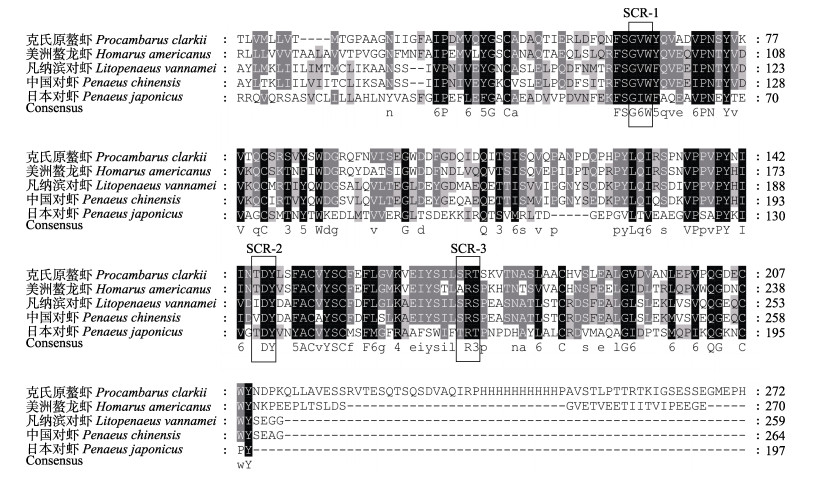
|
图 2 PcCRCN-L氨基酸序列同源比对分析 Fig.2 Alignment of amino acid sequences of PcCRCN-L and other related sequences 图中黑色代表相同水平100%,灰色代表相同水平≥50%。序列内黑色实线方框表示3个Lipocalin家族特征保守区域(G-X-W、T-D-Y和-R)。 Black denotes the homology level is 100%, and gray denotes the homology level is ≥50%. Black solid line box in the sequence represents three characteristic conservative regions of Lipocalin family (G-X-W, T-D-Y, and -R). |
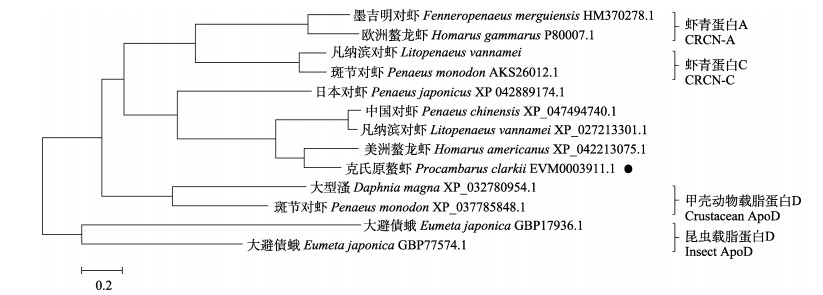
|
图 3 基于PcCRCN-L氨基酸序列构建的系统进化树 Fig.3 Phylogenetic relationship between PcCRCN-L and other related species based on amino acid sequences |
PcCRCN-L基因在克氏原螯虾9个组织(鳃、眼柄、胃、表皮、心脏、肌肉、卵巢、肠道和肝胰腺)中的表达结果显示,PcCRCN-L基因在所检测的各个组织中均有表达,其中肝胰腺中的基因表达量显著高于其他组织(P<0.05)。PcCRCN-L基因在肠道、肌肉、心脏、胃、表皮、鳃、卵巢以及眼柄等组织中的表达量较低,但是各组织间的表达量无显著性差异(P>0.05) (图 4)。
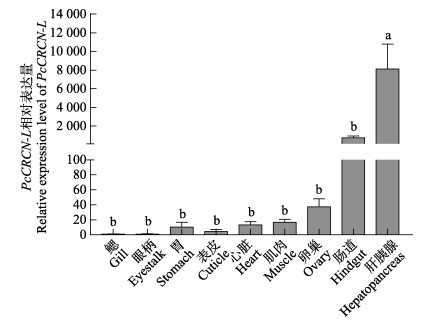
|
图 4 PcCRCN-L基因在不同组织中的表达 Fig.4 Expression of PcCRCN-L in different tissues 不同字母代表不同实验组之间具有显著性差异(P<0.05),图中误差线为标准差,下同。 Different letters represent significant differences in different experimental groups (P < 0.05). The error lines in the figure are standard deviations. The same below. |
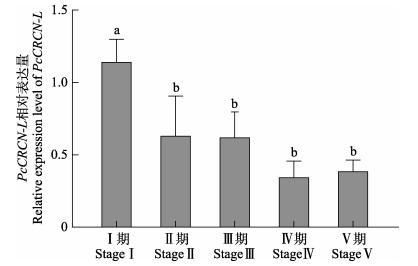
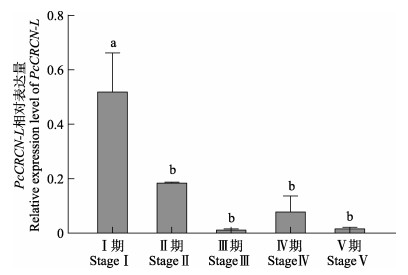
PcCRCN-L基因在克氏原螯虾性腺不同发育时期的表达变化分析结果显示,PcCRCN-L基因在卵巢与肝胰腺组织中的表达趋势类似。性腺发育Ⅰ~Ⅱ期,卵巢与肝胰腺组织中的PcCRCN-L基因表达水平显著降低(P<0.05);性腺发育Ⅱ~Ⅴ期,PcCRCN-L基因表达水平较低,但各发育时期间无显著差异(P>0.05) (图 5和图 6)。
|
图 5 PcCRCN-L基因在性腺不同发育时期的表达(肝胰腺组织) Fig.5 Expression of PcCRCN-L in hepatopancreas at different developmental stages of gonad |
|
图 6 PcCRCN-L基因在性腺不同发育时期的表达(卵巢组织) Fig.6 Expression of PcCRCN-L in ovary at different developmental stages of gonad |
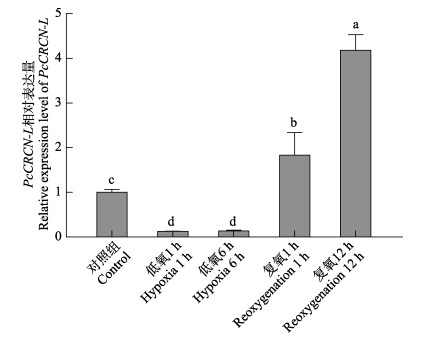
低氧胁迫1 h时,克氏原螯虾肝胰腺组织中PcCRCN-L基因表达量显著降低(P<0.05),但低氧胁迫1 h和低氧胁迫6 h之间无显著差异;复氧1 h后,PcCRCN-L基因表达量显著上调(P<0.05),复氧12 h后,PcCRCN-L表达量呈显著性增加(P<0.05) (图 7)。
|
图 7 PcCRCN-L基因在低氧–复氧胁迫下的表达特征 Fig.7 Expression of PcCRCN-L in hepatopancreas under hypoxia and reoxygenation stress |
虾青蛋白(CRCN)是脂质运载蛋白ApoD家族的重要成员,该家族主要负责运输类胡萝卜素、胆固醇、视黄醇和磷脂等疏水脂类,参与甲壳动物的壳色与应激反应调控(Flower, 1996; Sanchez et al, 2021)。Wang等在罗氏沼虾(Macrobrachium rosenbergii)中分离出一种虾青蛋白MrLC,发现其参与罗氏沼虾的蜕皮生殖进程,MrLC基因表达受到抑制后导致罗氏沼虾壳色变红(Wang et al, 2007; Yang et al, 2011)。Gao等(2021)在脊尾白虾(Exopalaemon carinicauda)中获得了A1、A2、C1和C2等4种虾青蛋白,A1和A2主要在皮下脂肪组织分布,C1和C2分别在眼柄和腹索神经高表达,RNA干扰其表达后脊尾白虾皮下脂肪组织中的蓝色素细胞消失,壳色由透明变为淡红色。本研究通过前期所获取的克氏原螯虾基因组信息结合分子克隆从克氏原螯虾肝胰腺组织中克隆获取了类虾青蛋白PcCRCN-L基因的cDNA序列,发现PcCRCN-L具有虾青蛋白典型的催化功能域,包含3个结构保守区(SCR),形成以8个反向平行的β折叠组成的桶装结构并伴有1个α螺旋(Chayen et al, 2005)。与典型虾青蛋白序列结构有所不同,克氏原螯虾PcCRCN-L预测氨基酸序列C端含有一个富含脯氨酸的结构域,推测通过该结构域内嵌形成的空腔体能够与虾青素、类固醇、磷脂等配体结合,从而具有不同的生理功能。
肝胰腺是甲壳动物脂类存储和代谢的中心(Mourente, 1996)。中华绒螯蟹(Eriocheir sinensis)虾青蛋白CRCN-C2在肝胰腺中表达量最高,虾青蛋白CRCN-C1主要分布在肝胰腺和卵巢组织(李清清, 2019)。斑节对虾(Penaeus monodon)类虾青蛋白PmCLL在肝胰腺和血淋巴中高表达,干扰PmCLL表达后,表皮组织中的虾青素含量显著降低;PmCLL过表达时,表皮中虾青素含量显著增加(Zhao et al, 2021)。虾青蛋白同系物载脂蛋白D在调节哺乳动物肝脏组织脂质稳态中发挥重要作用,缺乏载脂蛋白D会改变脂质构成,引发脂质代谢失衡,导致脂肪细胞退化(Charkoftaki et al, 2019; Sanchez et al, 2021)。本研究发现,PcCRCN-L基因在肝胰腺中高表达,推测肝胰腺作为PcCRCN-L的合成场所,在克氏原螯虾脂类存储与代谢过程中发挥着重要作用。性腺发育与脂类代谢密切相关(Teshima et al, 1983)。磷脂是甲壳类动物肝胰腺组织的主要脂质成分,在甲壳类动物的能量代谢和性腺发育中发挥着重要作用(Mourente et al, 1994)。成永旭等(2000)研究发现,不同发育阶段卵巢中性脂和磷脂占比不同,其中磷脂比例随着卵巢发育逐步降低,而中性脂比例不断升高。PcCRCN-L基因在卵巢中的表达量随着性腺发育逐步下调,提示PcCRCN-L可能与克氏原螯虾性腺发育过程中的脂类代谢密切相关。
甲壳动物可通过降低蛋白合成速率和诱导厌氧途径来应对低氧环境(孙盛明等, 2020)。在小鼠(Mus musculus)中发现,低氧胁迫可显著抑制载脂蛋白表达,降低生理代谢速率,甚至导致发育迟缓;复氧后载脂蛋白含量显著上升,生理代谢速率升高(程港丽等, 2022; 刘玲燕等, 2018)。低氧胁迫下,PcCRCN-L基因表达量显著下降,复氧后PcCRCN-L基因表达量显著提高,与长牡蛎(Crassostrea gigas)在缺氧期间蛋白质合成受到抑制,在复氧期间重新激活的趋势相一致(Sokolov et al, 2019)。低氧诱导因子HIF-1对于维持动物机体内部的氧稳态至关重要,HIF-1可通过调控下游载脂蛋白基因的表达,从而引起一系列的生理、生化反应(肖武汉, 2014)。PcCRCN-L基因启动子区域包含2个HIF-1结合位点,推测在低氧条件下HIF-1可与PcCRCN-L基因的低氧反应元件结合,参与了低氧胁迫下PcCRCN-L的表达调控。
总之,本研究首次克隆了克氏原螯虾类虾青蛋白PcCRCN-L基因序列,分析了PcCRCN-L基因在性腺不同发育时期以及低氧–复氧胁迫下的表达特征,推测PcCRCN-L可能参与了克氏原螯虾的性腺发育和低氧胁迫应答,为深入研究PcCRCN-L基因在克氏原螯虾繁殖以及氧化应激中的作用奠定了基础。
BEGUM S, CIANCI M, DURBEEJ B, et al. On the origin and variation of colors in lobster carapace. Physical Chemistry Chemical Physics, 2015, 17(26): 16723-16732 DOI:10.1039/C4CP06124A |
CAO M, WANG X Q, QIN C X, et al. Transcriptome analysis of Palaemon carincauda subjected to hypoxic stress. Progress in Fishery Sciences, 2021, 42(2): 112-123 [曹梅, 王兴强, 秦传新, 等. 脊尾白虾对低氧响应的转录组学分析. 渔业科学进展, 2021, 42(2): 112-123] |
CHARKOFTAKI G, WANG Y, MCANDREWS M, et al. Update on the human and mouse lipocalin (LCN) gene family, including evidence the mouse Mup cluster is result of an "evolutionary bloom". Human Genomics, 2019, 13(1): 1-14 DOI:10.1186/s40246-018-0187-x |
CHAYEN N E, CIANCI M, GROSSMANN J G, et al. Unravelling the structural chemistry of the colouration mechanism in lobster shell. Acta Crystallographica, Section D, Biological Crystallography, 2005, 61(9): 1312 DOI:10.1107/S0907444905019621 |
CHENG G L, YAO S, SHI Y P, et al. Expression difference and role of apolipoprotein M in hypoxia/reoxygenation-induced cardiomyocyte injury. Journal of Clinical and Pathological Research, 2022, 42(4): 771-778 [程港丽, 姚霜, 施媛萍, 等. 载脂蛋白M在心肌细胞缺氧/复氧损伤中的表达差异及其作用. 临床与病理杂志, 2022, 42(4): 771-778] |
CHENG Y X, DU N S, LAI W. The lipid accumulations during the stages of the ovarian fast maturation and their effect on the spawning of Eriocheir sinensis. Journal of Fisheries of China, 2000, 24(2): 113-118 [成永旭, 堵南山, 赖伟. 中华绒螯蟹卵巢快速发育期内脂类积累以及对抱卵的影响. 水产学报, 2000, 24(2): 113-118] |
FLOWER D R. The lipocalin protein family: Structure and function. Biochemical Journal, 1996, 318(1): 1-14 DOI:10.1042/bj3180001 |
GAO H, MA H K, SUN J Q, et al. Expression and function analysis of crustacyanin gene family involved in resistance to heavy metal stress and body color formation in Exopalaemon carinicauda. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 2021, 336(4): 352-363 DOI:10.1002/jez.b.23025 |
GOESSLING W, ZUCKER S D. Role of apolipoprotein D in the transport of bilirubin in plasma. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2000, 279(2): G356-365 DOI:10.1152/ajpgi.2000.279.2.G356 |
LI Q Q. Estimates of genetic parameters for colour traits and colour formation mechanism in Eriocheir sinensis. Doctoral Dissertation of Shanghai Ocean University, 2019 [李清清. 中华绒螯蟹色泽遗传参数评估及色泽形成机制研究. 上海海洋大学博士研究生学位论文, 2019]
|
LIU L Y, HUANG T P, HAO J L, et al. Changes of apoE protein expression in lung of mice with hypoxic pulmonary arterial hypertension. Chinese Journal of Applied Physiology, 2018, 34(6): 492-495 [刘玲燕, 黄天鹏, 郝家乐, 等. 低氧性肺动脉高压小鼠肺组织apoE蛋白表达的变化及其意义. 中国应用生理学杂志, 2018, 34(6): 492-495] |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Method, 2001, 25(4): 402-408 DOI:10.1006/meth.2001.1262 |
LU C, DING D, CHEN B. Adaptation to environmental hypoxia in insects: Progresses in physiological and molecular mechanisms. Journal of Environmental Entomology, 2020, 42(1): 82-93 [芦超, 丁玎, 陈兵. 昆虫对环境低氧的适应: 生理和分子机制研究进展. 环境昆虫学报, 2020, 42(1): 82-93] |
LU Y, CAI J, WANG D M, et al. Hypoxic stress and mitochondrial function on mechanism in animals. Chinese Journal of Animal Nutrition, 2020, 32(11): 5046-5052 [陆逸, 蔡杰, 王迪铭, 等. 动物低氧应激与线粒体功能机制. 动物营养学报, 2020, 32(11): 5046-5052] |
MOURENTE G, MEDINA A, GONZALEZ S, et al. Changes in lipid class and fatty acid contents in the ovary and midgut gland of the female fiddler crab Uca tangeri (Decapoda, Ocypodiadae) during maturation. Marine Biology, 1994, 121(1): 187-197 DOI:10.1007/BF00349488 |
MOURENTE G. In vitro metabolism of 14C-polyunsaturated fatty acids in midgut gland and ovary cells from Penaeus kerathurus Forskl at the beginning of sexual maturation. Comparative Biochemistry and Physiology Part B Biochemistry and Molecular Biology, 1996, 115(2): 255-266 DOI:10.1016/0305-0491(96)00111-3 |
SANCHEZ D, B LÓPEZ-ARIAS, TORROJA L, et al. Loss of glial lazarillo, a homolog of apolipoprotein D, reduces lifespan and stress resistance in Drosophila. Current Biology, 2006, 16(7): 680-686 DOI:10.1016/j.cub.2006.03.024 |
SANCHEZ D, GANFORNINA M D. The lipocalin apolipoprotein D functional portrait: A systematic review. Frontiers in Physiology, 2021(12): 738991 |
SOKOLOV E P, MARKERT S, HINZKE T, et al. Effects of hypoxia-reoxygenation stress on mitochondrial proteome and bioenergetics of the hypoxia-tolerant marine bivalve Crassostrea gigas. Journal of Proteomics, 2019(194): 99-111 |
SUN S M, ZHU M R, PAN F Y, et al. Research progress on the effects of hypoxia stress on crustacean and its molecular regulation. Journal of Fisheries of China, 2020, 44(4): 690-704 [孙盛明, 祝孟茹, 潘方艳, 等. 低氧对甲壳动物的影响及其分子调控研究进展. 水产学报, 2020, 44(4): 690-704] |
TESHIMA S I, KANAZAWA A. Variation in lipid compositions during the ovarian maturation of the prawn. Bulletin of the Japanese Society of Scientific Fisheries, 1983, 49(6): 957-962 DOI:10.2331/suisan.49.957 |
WALKER D W, MUFFAT J, RUNDEL C, et al. Overexpression of a Drosophila homolog of apolipoprotein D leads to increased stress resistance and extended lifespan. Current Biology, 2006, 16(7): 674-679 DOI:10.1016/j.cub.2006.01.057 |
WANG L, SU H Y, MING F Y, et al. Structure, function and clinical application of the apolipoprotein D. Chinese Journal of Cell Biology, 2008, 30(1): 50-54 [王磊, 宿红艳, 明永飞, 等. 载脂蛋白D的结构、功能及临床应用. 细胞生物学杂志, 2008, 30(1): 50-54] |
WANG M R, ZHU X J, YANG J S, et al. Prawn lipocalin: Characteristics and expressional pattern in subepidermal adipose tissue during reproductive molting cycle. Comparative Biochemistry and Physiology Part B, Biochemistry and Molecular Biology, 2007, 147(2): 222-229 DOI:10.1016/j.cbpb.2007.01.003 |
WANG S Y, LAU K, LAI K P, et al. Hypoxia causes transgenerational impairments in reproduction of fish. Nature Communications, 2016, 7: 12114 DOI:10.1038/ncomms12114 |
XIAO W H. Hypoxia signaling pathway and hypoxic adaptation of fishes. Scientia Sinica Vitae, 2014, 44(12): 1227-1235 [肖武汉. 低氧信号传导途径与鱼类低氧适应. 中国科学: 生命科学, 2014, 44(12): 1227-1235] |
XU Y, LIN H, YAN W, et al. Full-length transcriptome of red swamp crayfish hepatopancreas reveals candidate genes in Hif-1 and antioxidant pathways in response to hypoxia-reoxygenation. Marine Biotechnology (NY), 2022, 24(1): 55-67 DOI:10.1007/s10126-021-10086-9 |
XU Z, GAO T, XU Y, et al. A chromosome-level reference genome of red swamp crayfish Procambarus clarkii provides insights into the gene families regarding growth or development in crustaceans. Genomics, 2021, 113(5): 3274-3284 DOI:10.1016/j.ygeno.2021.07.017 |
XU Z H, BING X W, ZHAO C Y, et al. Observation on ovary growth and spawning cycle of crayfish. Jiangsu Journal of Agricultural Sciences, 2010, 26(6): 1304-1307 [徐增洪, 邴旭文, 赵朝阳, 等. 克氏原螯虾卵巢发育与产卵周期变化的观察. 江苏农业学报, 2010, 26(6): 1304-1307] |
YANG F, WANG M R, MA Y G, et al. Prawn lipocalin: Characterization of a color shift induced by gene knockdown and ligand binding assay. Journal of Experimental Zoology, Part A, Ecological Genetics and Physiology, 2011, 315(9): 562-571 |
YIN L K, HUANG K, YU K, et al. Research progress on the effects of hypoxia stress on crustacean and its molecular regulation. Progress in Fishery Sciences, 2023, 44(4): 167-178 [殷丽坤, 黄凯, 于凯, 等. 低氧胁迫下中华圆田螺的肝脏转录组学分析. 渔业科学进展, 2023, 44(4): 167-178] |
YU X J, HE X J, DANG Z Q, et al. Development report of Procambarus clarkii industry in China (2022). China Fisheries, 2022(6): 47-54 [于秀娟, 郝向举, 党子乔, 等. 中国小龙虾产业发展报告(2022). 中国水产, 2022(6): 47-54] |
ZHAO C, PENG C, FAN S, et al. Identification of a novel crustacyanin-like lipocalin in Penaeus monodon: Molecular cloning, tissue distribution and its functional studies in astaxanthin accumulation. Aquaculture, 2021, 539: 736615 |



