2. 海洋渔业科学与食物产出过程功能实验室 山东 青岛 266071;
3. 辽宁省淡水水产科学研究院 辽宁 辽阳 111000;
4. 莱州明波水产有限公司 山东 莱州 261418;
5. 福建省逸有水产科技有限公司 福建 漳州 363402
2. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao 266071, China;
3. Freshwater Fisheries Science Academy of Liaoyang 111000, China;
4. Laizhou Mingbo Aquatic Products Co., Ltd, Laizhou 261418, China;
5. Fujian Yiyou Aquatic Science and Technology Co. Ltd, Zhangzhou 363402, China
莱氏拟乌贼(Sepioteuthis lessoniana)是枪乌贼科(Loliginidae)中在印度–西太平洋中分布最广的品种之一。莱氏拟乌贼在中国的广东和闽南近海常有渔获,年产量约200 t,具有体型大(最大体重达5.6 kg)、肉厚、可食部分较高、肉质细嫩鲜美、干制后为海味佳品等特点,具有极高的食用、营养和经济价值(董正之, 2001; 王峥等, 2020)。
头足类雌性生殖系统由卵巢、输卵管和附属腺组成,其中,附属腺包括缠卵腺、副缠卵腺和输卵管腺(林东明等, 2013)。不同种类头足类的输卵管腺具有较大差异,如乌贼目(Sepiida)和枪形目(Teuthoidea)等头足类的输卵管腺不具备精子储存功能,腺体分为两部分,第1部分膨大成喇叭状,连接输卵管;第2部分呈圆柱状(刘必林等, 2010)。八腕目(Octopoda)的输卵管腺区别于其他头足类,腺体位于输卵管中部,膨胀成球形,内部分为中央腔、纳精囊和输卵管腔,其中,纳精囊具备储存精子的功能。目前,对于输卵管腺的研究主要集中在真蛸(Octopus vulgaris)输卵管腺的结构和功能(Froesch et al, 1975)、太平洋褶柔鱼(Todarodes pacificus)输卵管腺诱导绒毛膜扩张的因素(Adachi et al, 2016)、章鱼(Octopus mimus)输卵管腺中神经肽的分泌与作用(Cristo et al, 2002)等,但未见莱氏拟乌贼输卵管腺的超显微结构和功能的研究。本研究采用组织切片和透射电镜技术,揭示了莱氏拟乌贼输卵管腺的组织类型和细胞构造,探讨了腺体在头足类生殖活动中的作用,以丰富头足类繁殖生物学内容。
1 材料与方法 1.1 材料野生莱氏拟乌贼雌性样品于2020年8月采自福建海域(117°73′~118°02′E、23°26′~23°48′N),采集工具为底层拖网。本研究共采用5只实验样本,样本体表健康无破损,平均胴长为(17.5±6.4) cm,平均体重为(392.0±76.0) g。
1.2 方法 1.2.1 解剖结构观察将莱氏拟乌贼的腹面朝上并放置于解剖盘中,用镊子夹紧外套膜,再使用解剖剪刀将外套膜肌肉沿中线剪开,记录输卵管腺的位置、形态、大小和颜色等特征。
1.2.2 石蜡切片样品的制备解剖完成后,使用解剖刀将输卵管腺样品切割成小块,长、宽、高分别为0.5 cm左右,置于Bouin’s固定液中保存固定24 h,然后更换70%的酒精溶液保存。按照组织学研究方法,对样品进行梯度乙醇溶液脱水、二甲苯透明、石蜡包埋。
石蜡包埋样品后,使用KD-2508型轮转式切片机连续切片,切片厚度约为5 μm。石蜡切片经脱蜡干燥、HE染色、中性树胶封片后,置于Nikon 80i(日本)显微镜下观察并拍照。
1.2.3 透射电镜样品的制备切取体积约为0.5~1 mm3的输卵管腺实验样品,采用2.5%戊二醛固定4 h,PBS缓冲液冲洗3次,每次冲洗10 min,1%的锇酸4 ℃固定2 h。随后,经H3PO4缓冲液漂洗,梯度乙醇溶液脱水,Epon812环氧树脂包埋,37 ℃、45 ℃和65 ℃温箱固化,每级温度固化24 h。
固化样品使用Ultracut E超薄切片机半薄切片,切片厚度约70 nm。切片经醋酸铀和柠檬酸双重染色,置于JEM-1200EX的透射电镜下观察,拍照电压为80 kV。
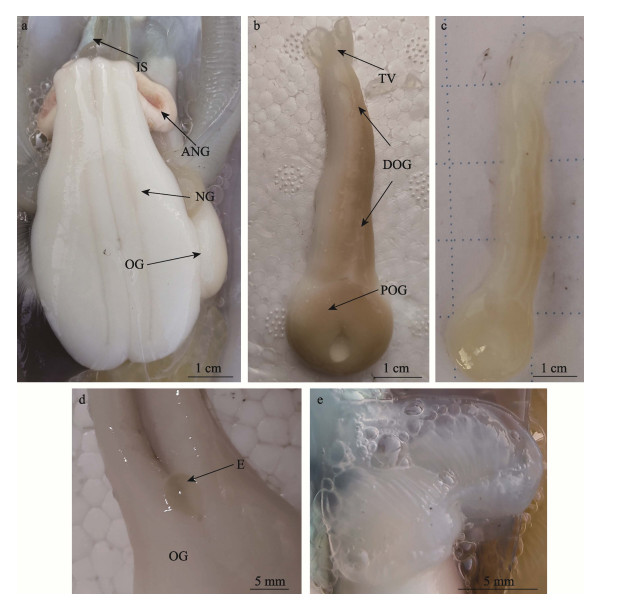
2 结果 2.1 输卵管腺的解剖结构莱氏拟乌贼具输卵管腺1个,位于腹腔右侧(腹面观),分别与左侧的墨囊、副缠卵腺、缠卵腺相邻,右侧与鳃相邻,腺体中部位于鳃和鳃心下侧(图 1a)。性成熟后的腺体整体呈乳白色,腺体全长约为7.5 cm,重约7.2 g,靠近内壳侧的腺体有色素沉着,呈棕黄色。输卵管腺由透明薄膜包裹,可见规则分布的缝隙(图 1b)。未性成熟的腺体颜色较浅,整体呈白色,腺体上有少量浅黄色的色素沉着(图 1c)。腺体由近端输卵管腺、远端输卵管腺和透明瓣膜构成。近端输卵管腺与透明的输卵管相连,输卵管延展到腺体内部,腺体的管道开口呈圆形,直径约1.8 cm,呈漏斗状,输卵管中的成熟卵子单个排列由开口进入输卵管腺。远端输卵管腺呈圆柱状,逐渐变细,剪开后的腺体中可见单个的成熟卵子(图 1d)。远端输卵管腺末端为透明瓣膜,又称阀门,由两片瓣膜构成,瓣膜外表光滑接近透明,内侧具有条纹状突起,自然状态下处于闭合状态(图 1e)。
|
图 1 莱氏拟乌贼输卵管腺 Fig.1 The oviducal gland (OG) of S. lessoniana a:莱氏拟乌贼内部构造;b:已性成熟的输卵管腺;c:未性成熟的输卵管腺;d:输卵管腺中的卵子;e:输卵管腺的透明瓣膜;ANG:副缠卵腺;DOG:远端输卵管腺;E:卵子;IS:墨囊;NG:缠卵腺;OG:输卵管腺;POG:近端输卵管腺;TV:透明瓣膜。 a: The internal structure of S. lessoniana; b: A sexually mature oviducal gland; c: A sexually immature oviducal gland; d: An egg in the oviducal gland; e: The transparent valve of the oviducal gland; ANG: Accessory nidamental gland; DOG: Distal oviducal gland; E: egg; IS: Ink sac; NG: Nidamental gland; OG: Oviducal gland; POG: Proximal oviducal gland; TV: Transparent value. |
输卵管腺由腺壁组织、分泌叶瓣和结缔组织构成。腺壁组织由外膜层和结缔组织构成,外膜层由复层柱状上皮细胞构成,细胞核呈椭圆形(图 2a);结缔组织与外膜层紧密相连,二者之间分界明显,主要是疏松结缔组织,为220~400 μm (图 2a、b);疏松结缔组织中分布少量肌肉组织,主要是平滑肌,结缔组织中分布少量血管和导管(图 2a、c)。透明瓣膜表层由单层柱状上皮细胞构成,细胞核呈圆形或椭圆形,染色较深,皆呈蓝色(图 2d);腺体外膜层和透明瓣膜表层的上皮细胞间还分布少量杯状细胞,细胞呈上大下小的形态,形状类似水滴,呈深蓝色(图 2d、e、f);透明瓣膜由肌肉组织构成,主要是平滑肌,内表层的上皮细胞外连续着生大量纤毛(图 2e);结缔组织分布在外膜层与分泌叶瓣的交界处、分泌叶瓣的基底处(图 2a、b、c、g)。

|
图 2 莱氏拟乌贼输卵管腺的显微结构 Fig.2 Microstructure of the oviducal gland (OG) of S. lessoniana a:输卵管腺横切面,示腺壁组织、分泌叶瓣和结缔组织;b:分泌叶瓣横切面,示结缔组织;c:腺壁组织横切面,示肌肉组织和血管;d:透明瓣膜横切面,示柱状上皮细胞和杯状细胞;e:腺壁组织横切面,示肌肉组织和纤毛;f:透明瓣膜横切面,示杯状细胞;g:分泌叶瓣横切面,示结缔组织;h:分泌叶瓣横切面,示分泌叶瓣和叶瓣间隙;i:分泌叶瓣横切面,示叶瓣间隙和上皮细胞;j:分泌叶瓣横切面,示分泌叶瓣、结缔组织和粘液性腺泡;k:分泌叶瓣横切面,示结缔组织和蛋白颗粒;l:分泌叶瓣横切面,示结缔组织和蛋白颗粒;BV:血管;CL:纤毛;CT:结缔组织;EC:上皮细胞;GC:杯状细胞;GP:蛋白颗粒;ILS:叶瓣间隙;LA:分泌叶瓣;ML:肌肉层;OL:腺壁组织。 a: Transverse of the oviducal gland, showing the outer layer, lamellar and connective tissue; b: Transverse of the lamellar, showing connective tissue; c: Transverse of the outer layer, show muscular layer and blood vessel; d: Transverse of the transparent value, showing epithelial cell and goblet cell; e: Transverse of the outer layer, show muscular layer and cilia; f: Transverse of the transparent valve, showing goblet cell; g: Transverse of the lamellar, showing connective tissue; h: Transverse of the lamellar, showing lamellar and lamellar space; i: Transverse of the lamellar, showing lamellar space and epithelial cell; j: Transverse of the lamellar, showing lamellar, connective tissue and glycoprotein particle; k: Transverse of the lamellar, showing connective tissue and glycoprotein particle; l: Transverse of the lamellar, showing connective tissue and glycoprotein particle; BV: Blood vessel; CL: Cilia; CT: Connective tissue; EC: Epithelial cell; GC: Goblet cell; GP: Glycoprotein particle; ILS: Lamellar space; LA: Lamella; ML: Muscular layer; OL: Outer layer. |
分泌叶瓣在腺体呈层状分布,依附于腺壁组织(图 2a、b、h)。分泌叶瓣由单层上皮细胞组成,包括核染色较浅的纤毛柱状上皮和核染色较深的支持细胞(图 2g)。输卵管腺处于不同的发育时期,分泌叶瓣的细胞类型、瓣内区间大小均不同。腺体尚未发育成熟时,分泌叶瓣形状规则,分泌细胞中间的结缔组织清晰可见,数量较多,两侧以单层柱状上皮为主(图 2g、i);腺体即将发育成熟时,叶瓣中间的结缔组织数量增多,两侧的柱状上皮细胞数量减少,有大量黏液性腺泡生成(图 2j);腺体发育成熟且到产卵后期,腺体叶瓣中的结缔组织减少,柱状上皮细胞消失,黏液性腺泡破裂,腺泡中的分泌颗粒充满瓣内区间,此时,只能见到染色较深的支持细胞(图 2k、l)。
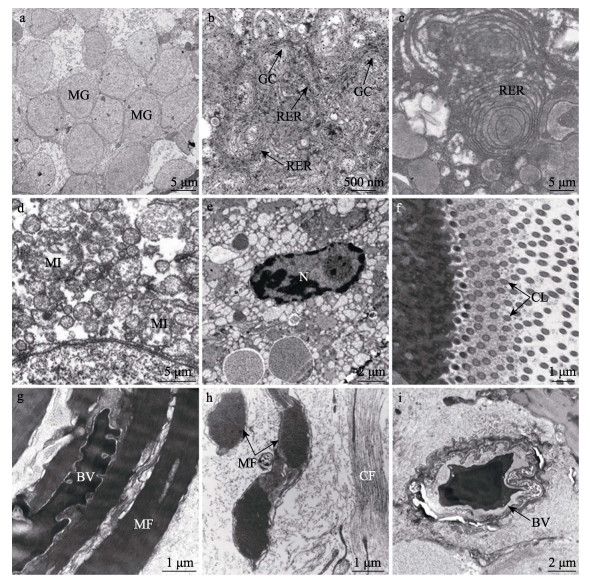
2.3 输卵管腺超微结构莱氏拟乌贼成体输卵管腺的分泌细胞个体较大,主要以低电子密度的黏液颗粒为主,颗粒呈圆形或椭圆形,直径约为4~7 μm,颗粒间排列紧密(图 3a)。分泌细胞的细胞核呈椭圆形,细胞质中还分布内质网、线粒体和高尔基体等细胞器。其中,内质网包括粗面和滑面内质网,线粒体大多呈椭圆形,高尔基复合体周围分布大量合成后的分泌小泡(图 3b~f)。分泌细胞外侧连续分布纤毛,轴丝具有典型的“9+2”结构(图 3f)。
|
图 3 莱氏拟乌贼输卵管腺的超微结构 Fig.3 Ultrastructure of the oviducal gland (OG) of S. lessoniana a:黏液颗粒横切面;b:上皮细胞细胞质,示粗面内质网和高尔基复合体;c:粗面内质网;d:线粒体;e:细胞核;f:纤毛;g:透明瓣膜横切面,示肌纤维和血管;h:透明瓣膜纵切面,示肌纤维和胶原纤维;i:血管横切面;BV:血管;CF:胶原纤维;CL:纤毛;GC:高尔基复合体;MF:肌纤维;MG:黏液颗粒;MI:线粒体;N:细胞核;RER:粗面内质网。 a: Transverse of the mucous granule; b: The cytoplasm of epithelial cell, showing rough endoplasmic reticulum and golgi complex; c: Rough endoplasmic reticulum; d: Mitochondria; e: Nucleus; f: Cilia; g: Transverse of the transparent valve, showing muscle fiber and blood vessel; i: Transverse of the blood vessel; BV: Blood vessel; CF: Collage fiber; CL: Cilia; GC: Goligo complex; MF: Muscle fiber; MG: Mucous granule; MI: Mitochondria; N: Nucleus; RER: Rough endoplasmic reticulum. |
透明瓣膜由肌细胞构成,肌肉细胞与上皮细胞相邻,可见横切和纵切的肌丝(图 3g、h),细胞内分布大量胶原纤维,在肌肉组织中分布血管(图 3i)。
3 讨论 3.1 关于输卵管腺的形态特征输卵管腺的形状、长度以及是否有色素沉着,是区分头足类不同种类的标志之一(林东明等, 2013),如莱氏拟乌贼成体包括类似漏斗状的近端输卵管腺和圆柱状的远端输卵管腺,腺体整体呈乳白色,近端和远端输卵管腺上均有少量棕黄色的色素沉积。拟目乌贼(Sepia lycidas)的输卵管腺黏附于输卵管上,呈淡黄色,弥散状(罗江等, 2014);菱鳍乌贼(Thysanoteuthis rhombus)具有1对输卵管腺,性成熟后的近端输卵管腺类似椭圆形的蘑菇伞,远端输卵管腺很大,类似长矛(Nigmatullin et al, 1991);福氏枪乌贼(Loligo forbesi)的输卵管腺呈梨形,白色,背靠着缠卵腺(Lum-Kong,1991);八腕目等头足类的输卵管腺区别于枪形目和乌贼目,输卵管腺呈球形,输卵管由腺体中间贯穿,输卵管腺由中央腺和周边腺组成,如嘉庚蛸(Octopus tankahkeei)性成熟时,其输卵管腺的腺管呈两级淡白色,其余部分为棕褐色(焦海峰, 2005)。
3.2 关于输卵管腺的结构特征随着个体的发育成熟,输卵管腺会发生组织结构和细胞类型的变化。章鱼的输卵管腺是典型代表,发育期间,输卵管腺的腺泡由假复层柱状上皮构成,到达产卵期则变成柱状上皮,并且在细胞质中还会出现大量嗜碱性颗粒(Olivares et al, 2017)。未成熟的福氏枪乌贼输卵管腺,分泌上皮由纤毛柱状上皮和支持细胞组成,达性成熟后,分泌细胞破裂,分泌物质充满腺体(Lum-Kong, 1992)。莱氏拟乌贼输卵管腺的变化情况与福氏枪乌贼接近,产卵前,腺体内分泌叶瓣上的上皮细胞会逐渐变成柱状上皮细胞,分泌叶瓣上出现大量黏液腺泡,进入产卵期后,分泌叶瓣增大且腺泡破裂,叶瓣间隙充满分泌物质。
在虎斑乌贼(Sepia pharaonis)输卵管腺的组织学研究中发现,部分上皮细胞具有糖蛋白染色的特性,随着腺体的发育,这些糖蛋白类物质集中到腺体管腔内(江姿吟, 2016),皮氏枪乌贼的输卵管腺同样能分泌蛋白类物质(Peterson, 1959)。这与莱氏拟乌贼的研究结果接近,输卵管腺分泌叶瓣及叶瓣间隙分布大量呈深蓝色的蛋白颗粒,推测可能是头足类输卵管腺分泌物质的主要成分为糖蛋白类物质。
3.3 关于输卵管腺的功能随着莱氏拟乌贼输卵管腺的发育,分泌上皮逐渐形成,分泌细胞内的内质网和高尔基复合体有利于糖蛋白等物质的合成,随着上皮细胞破裂并将分泌物释放到腺体的叶瓣间隙中,真蛸(Froesch et al, 1975)、皮氏枪乌贼(Loligo pealei)和太平洋褶柔鱼(Adachi et al, 2016)等头足类的输卵管腺也出现相同变化,表明输卵管腺具备分泌功能。头足类输卵管腺的上皮细胞在产卵时进行分泌活动,其中,章鱼输卵管腺分泌的凝胶状物质将卵固着在水底。皮氏枪乌贼的输卵管腺能分泌形成卵囊的粘蛋白和内层卵鞘(Peterson,1959),但八腕目输卵管腺的功能与乌贼目和枪形目存在明显区别,即章鱼的输卵管腺具备储存精子的功能,精子附着于纳精囊壁上,当成熟卵子进入近端输卵管时,精子被释放到输卵管腺腔中,卵子随即受精,而乌贼目和枪形目等头足类进行交配时,精子被储存在雌体口膜下方的纳精囊中(Naud et al, 2005)。此外,Atkinson (1973)研究发现,福氏枪乌贼的卵经过纳精囊时已受精,但此时卵已被输卵管腺的分泌物包裹,推测认为这些分泌物对精子鞭毛有聚集作用。
头足类输卵管腺的分泌物形成受精卵的内层卵鞘(Lum-Kong, 1992),外层卵鞘由缠卵腺和副缠卵腺的分泌物形成。Hamabe (1962)研究表明,头足类受精卵的孵化率与绒毛膜的扩张有关,在太平洋褶柔鱼绒毛膜扩张的活性成分分析中,表明输卵管腺分泌的盐溶级分泌物质导致了绒毛膜的扩张和卵黄间隙的形成(Adachi et al, 2016; 江姿吟, 2016)。此外,枪乌贼科头足类输卵管腺分泌的胶状物质还为胚胎在正常发育过程中调节受精卵与环境海水调节渗透压(Ikeda et al, 1993; Adachi et al, 2016)。本研究表明,输卵管腺在生殖活动中主要发挥分泌功能,腺体的分泌物质能形成莱氏拟乌贼等十腕目头足类的内层卵鞘,保证受精卵的正常孵化,并在八腕目头足类中起到储存精子和固着受精卵的功能。
3.4 关于输卵管腺的调控因素由莱氏拟乌贼输卵管腺的石蜡切片可以看出,在输卵管腺的生长发育过程中,腺体的组织结构会出现明显变化。在其他头足类的输卵管腺中也出现类似变化,在真蛸生殖周期中观察输卵管腺的形态变化时发现,17b-雌二醇和孕酮的靶器官是输卵管腺,这2种性激素含量的波动情况与真蛸生殖系统中输卵管腺的形态变化一致,二者具备协同作用,前者促进上皮细胞的增殖分化,后者在蛋白分泌和卵壳形成中发挥作用,表明该物种的输卵管腺受到激素的调控(Cristo et al, 2010、2007)。Young(1967)研究表明,输卵管腺的腺上皮和纳精囊具有丰富神经网络,神经丛具备2种功能:控制腺体中细胞的分泌活动,以及在产卵过程中协调卵子产出和精子释放。在欧洲横纹乌贼(Sepia officinalis)中还发现1种神经肽(Gly-Trp-NH2),它参与输卵管腺中卵和分泌物质运输,但尚未明确是直接还是间接的控制(Henry, 1997)。综合分析表明,莱氏拟乌贼等头足类输卵管腺的生长发育和生殖活动受到体内激素、蛋白类物质以及神经系统等多种因素调控,进而保障了输卵管腺的正常发育以及腺体功能的正常发挥。
4 结论本研究首次对莱氏拟乌贼的输卵管腺进行超显微结构观察,描述了腺体的结构特征,分析并总结腺体的结构和功能。研究表明,输卵管腺是头足类雌性生殖系统中重要的腺体之一,该腺体具备分泌功能,其分泌物质形成受精卵的内层卵鞘,起到保护受精卵正常孵化的作用。
ADACHI K, YABUMOTO M, YOO H, et al. Salt soluble without jelly-like component from the oviducal gland induces chorionic expansion in the ova of the Japanese common squid Todarodes pacificus. Invertebrate Reproduction and Development, 2016, 60(4): 281-289 DOI:10.1080/07924259.2016.1229234 |
ATKINSON B G. Squid nidamental gland extract: Isolation of a factor inhibiting ciliary activity. Journal of Experimental Zoology, 1973, 184(3): 335-340 DOI:10.1002/jez.1401840306 |
COSMO D A, CRISTO D C. Neuropeptidergic control of the optic gland of Octopus vulgaris: FMRF-amide and GnRH immunoreactivity. Journal of Comparative Neurology, 1998, 398(1): 1-12 DOI:10.1002/(SICI)1096-9861(19980817)398:1<1::AID-CNE1>3.0.CO;2-5 |
CRISTO C D, COSMO A D. Neuropeptidergic control of Octopus oviducal gland. Peptides, 2007, 28(1): 163-168 DOI:10.1016/j.peptides.2006.09.016 |
CRISTO C D, PAOLUCCI M, JOSÈ I, et al. Presence of two neuropeptides in the fusiform ganglion and reproductive ducts of Octopus vulgaris: FMRFamide and gonadotropin- releasing hormone (GnRH). Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 2010, 292(3): 267-276 |
DONG Z Z. Fauna sinica-phylum mollusca-class cephalopoda. Beijing: Science Press, 2001: 104-106 [董正之. 中国动物志: 软体动物门头足纲. 北京: 科学出版社, 2001: 104-106]
|
FROESCH D, MARTHY H J. The structure and function of the oviducal gland in octopods (Cephalopoda). Proceedings of the Royal Society of London, 1975, 188(1090): 95-101 |
HAMABE M. Embryological studies on the common squid, Ummastrephes sloani pacificus Steenstrup, in the southwestern waters of the sea of Japan. Bulletin of the Japanese Society of Scientific Fisheries, 1962, 10: 1-45 |
HENRY J, FAVREL P, BOUCAUD-CAMOU E. Isolation and identification of a novel Ala-ProGly-Trp-amide-Related peptide inhibiting the motility of the mature oviduct in the cuttlefish, Sepia officinalis. Peptides, 1997, 18(10): 1469-1474 DOI:10.1016/S0196-9781(97)00241-6 |
IKEDA Y, SAKURAI Y, SHIMAZAKI K. Fertilizing capacity of squid (Todarodes pacofocus) spermatozoa collected from various sperm storage sites, with special reference to the role of gelatinous substance from oviducal gland in fertilization and embryonic development. Invertebrate Reproduction and Development, 1993, 23(1): 39-44 DOI:10.1080/07924259.1993.9672291 |
JIANG Z Y. Ontogenetic development of the ovary and the reproductive accessory glands of the female pharaoh cuttlefish, Sepia pharaonic. Master´s Thesis of Penghu University of Science and Technology, 2016 [江姿吟. 雌性虎斑乌贼(Sepia pharaonis)生殖腺与生殖附属腺体发育过程中组织结构变化. 澎湖科技大学硕士研究生学位论文, 2016]
|
JIAO H F. Morphology of reproductive system and spermiogenesis in Octopus tankahkeei. Master´s Thesis of Ningbo University, 2005 [焦海峰. 嘉庚蛸生殖系统结构和配子发生. 宁波大学硕士研究生学位论文, 2005]
|
LIU B L, CHEN X J. Cephalopods reproductive system and its application to taxonomy. Journal of Fisheries of China, 2010, 34(8): 1219-1226 [刘必林, 陈新军. 头足类生殖系统及其在分类学上的应用. 水产学报, 2010, 34(8): 1219-1226] |
LIN D M, CHEN X J. Research on histological structure of reproductive system in cephalopod. Journal of Shanghai Ocean University, 2003, 22(3): 410-418 [林东明, 陈新军. 头足类生殖系统组织结构研究进展. 上海海洋大学学报, 2003, 22(3): 410-418] |
LUM-KONG A. A histological study of the accessory reproductive organs of female Loligo forbesi (Cephalopoda: Loliginidae). Journal of Zoology, 1992, 226(3): 469-490 DOI:10.1111/j.1469-7998.1992.tb07493.x |
LUO J, JIANG X M, PENG R B, et al. Histology of reproductive system in Sepial lycidas. Journal of Fisheries of China, 2014, 38(7): 946-955 [罗江, 蒋霞敏, 彭瑞冰, 等. 拟目乌贼生殖系统的组织学研究. 水产学报, 2014, 38(7): 946-955] |
NAUD M J, SHAW P W, HANLON R T, et al. Evidence for biased use of sperm sources in wild female giant cuttlefish (Sepia apama). Proceedings of the Royal Society of London B: Biological Sciences, 2005, 272(1567): 1047-1051 DOI:10.1098/rspb.2004.3031 |
NIGMATULLIN C M, ARKHIPKIN A I, SABIROV R M. Structure of the reproductive system of the squid Thysanoteuthis rhombus (Cephalopoda: Oegopsida). Journal of Zoology, 1991, 224(2): 271-283 DOI:10.1111/j.1469-7998.1991.tb04804.x |
OLIVARES A, AVILA-POVEDA O H, LEYTON V, et al. Oviducal glands throughout the gonad development stages: A case study of Octopus mimus (Cephalopoda). Molluscan Research, 2017, 37(4): 229-241 DOI:10.1080/13235818.2017.1334275 |
PETERSON R P. The anatomy and histology of the reproductive systems of Octopus bimaculoides. Journal of Morphology, 1959, 104: 61-87 DOI:10.1002/jmor.1051040103 |
WANG Z, LIU C L, ZHAI J M, et al. Analysis and evaluation of muscle nutrition of Sepioteuthis lessoniana. Progress in Fishery Sciences, 2020, 41(4): 102-109 [王峥, 刘长琳, 翟介明, 等. 莱氏拟乌贼肌肉营养成分分析及评价. 渔业科学进展, 2020, 41(4): 102-109] |
YOUNG J Z. The visceral nerves of Octopus. Philosophical Transactions of the Royal Society of London, 1967, 253(782): 1-22 |



