2. 中国水产科学研究院黄海水产研究所 海洋渔业科学与食物产出过程功能实验室 农业农村部海水养殖病害防治重点实验室 青岛市海水养殖流行病学与生物安保重点实验室 山东 青岛 266071
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences; Laboratory for Marine Fisheries Science and Food Production Processes; Key Laboratory of Maricultural Organism Disease Control, Ministry of Agriculture and Rural Affairs; Qingdao Key Laboratory of Mariculture Epidemiology and Biosecurity, Qingdao 266071, China
海水贝类养殖是我国海水养殖产业的重要组成部分,养殖品类涵盖牡蛎、扇贝、贻贝和鲍鱼等8大类,约48种;形成了滩涂养殖、浅海养殖、池塘养殖和工厂化养殖等多种养殖模式(王波等, 2017)。2020年我国贝类海水养殖规模达到120万hm2,产量达1 480万t,经济总产值220多亿美元(王如才等, 2004; 农业农村部渔业渔政管理局等, 2020a)。流行性疫病对贝类养殖产业造成巨大经济损失,其病原种类繁多,分布广泛,防治困难(刘婷, 2019),一直是制约贝类养殖产业健康发展的主要制约性因素之一(宋林生, 2020)。根据近年来的流行病学调查,多个病害引起的贝类大规模死亡事件都与细菌性病原的感染有关。弧菌(Vibrio)作为海洋环境中最常见的细菌类群,是海洋微生物区系的主要组成部分,同时也是常见的条件性致病菌。由弧菌引起的贝类病害一般具有流行面积广、发病率高、对养殖业造成的损失比较严重等特点。
溶藻弧菌(Vibrio alginolyticus)隶属于弧菌科(Vibrionaceae)、弧菌属(Vibrio),是一种常见的无芽孢、无荚膜的嗜盐、嗜温的厌氧性革兰氏阴性海洋细菌,广泛分布于海水、底泥和水生生物中(陈强等, 2006)。流行病学调查结果显示,溶藻弧菌是近年引起水产养殖动物育苗期和养成期病害和死亡的主要病原之一(韩风杰, 2016; 方皓, 2019; 张瑞卿, 2021; Zhang et al, 2022)。研究发现,溶藻弧菌可引起大黄鱼(Pseudosciaena crocea)、黑鲷(Sparus macrocephlus)、凡纳滨对虾(Litopenaeus vannamei)、日本对虾(Penaeus japonicus)等多种水生生物发病和大规模死亡(Lee et al, 1996a、b; 鄢庆枇等, 2001; 黄志坚等, 2002; 丁燏等, 2004)。贝类如长牡蛎(Crassostrea gigas)、近江牡蛎(Crassostrea ariakensis)和紫贻贝(Mytilus edulis)等的成体和幼体也有被溶藻弧菌感染、导致大规模死亡的案例报道(郑国兴等, 1991; Riquelme et al, 1996; Sainz et al, 1998; 林永添, 2007; 张占会等, 2008; Wang et al, 2021; Yang et al, 2021)。
溶藻弧菌的致病力与其毒力因子密切相关。毒力基因是产生各种毒力因子的遗传学基础,携带不同毒力基因的溶藻弧菌,表现出不同的生物学特征与致病性(梅冰等, 2015)。多位点序列分型(multilocus sequence typing, MLST)是基于测定核酸序列的一种细菌分型方法,通过比较序列型(sequence type, ST)可以反应不同菌株之间的亲缘关系。密切相关的菌株具有相同的ST或仅有极个别基因位点有不同的ST,而不相关菌株的ST至少有3个基因位点不同(Maiden et al, 1998)。经过多年发展和完善,MLST已成为一种成熟的细菌分型技术,具有分辨力高、结果重复性强等优点,既适用于细菌流行病学调查,也可用于菌株的遗传变异等研究(Martin, 2006)。目前,对不同贝类养殖环境中,与溶藻弧菌生物学功能相似的副溶血弧菌(Vibrio parahaemolyticus)已经进行了广泛研究(李翠苹等, 2020),而溶藻弧菌的遗传分型和生物学特性的研究相对较少。本研究通过探究不同区域内不同来源的溶藻弧菌遗传变异及耐药性差异,旨在为贝源溶藻弧菌有效防控提供一定的理论参考。
1 材料与方法 1.1 菌株分离与纯化研究所用溶藻弧菌从山东省青岛、威海、烟台和潍坊周边4个贝类养殖地区的贝类组织和养殖水体中分离、鉴定得到,–80 ℃低温储存(20%甘油)。使用2216E培养基将甘油冻存菌株复苏后,用TCBS选择性平板进行划线纯培养,观察菌落形态并挑取丰度高的单菌落接种于2216E液体培养基,28 ℃摇床过夜培养,次日将菌液置于4 ℃冰箱备用。每个养殖海区/育苗场均选取3株溶藻弧菌作为代表菌株,具体采样信息见表 1。
|
|
表 1 溶藻弧菌采样信息 Tab.1 Sampling information of strains of V. alginolyticus |
菌液DNA提取:将菌液12 000 r/min,离心30 s,弃上清液,加入500 μL无菌水,95 ℃,10 min,得到菌液DNA。利用16S rRNA通用引物(见表 2),按如下PCR反应条件对提取的菌液DNA进行PCR扩增。PCR反应体系(25 μL):DNA模板2 μL;上下游引物F/R(10 μmol/l)各1 μL;Premix TaqTM 12.5 μL;ddH2O 8.5 μL。PCR反应程序:94 ℃,5 min;94 ℃,30 s;55 ℃,30 s;72 ℃,30 s;72 ℃,7 min;35个循环;4 ℃,保存。扩增产物在120 V条件下,经1.5%琼脂糖凝胶电泳30 min,选取条带明亮的PCR产物送至生工生物工程(上海)股份有限公司进行Sanger测序。对测序峰图进行人工校验无误后,将本研究测序得到的12株弧菌16S rRNA序列与NCBI数据库进行BLAST同源序列比对,初步鉴定菌株分类学地位。
|
|
表 2 本研究所用全部引物序列及反应条件 Tab.2 Primer sequences and reaction conditions used in this study |
根据PubMLST (http://pubmlst.org/Vibriospp./)数据库所列弧菌属的4个管家基因和引物(见表 2)进行多位点序列分型,实验方法参考张小丽等(2021)。PCR反应体系和反应程序同1.2,退火温度同表 2。扩增产物在120 V条件下,1.5%琼脂糖凝胶电泳30 min,选取条带明亮的PCR产物送至生工生物工程(上海)股份有限公司测序。对测序丰度和序列进行人工校验后,将所获取的溶藻弧菌各变异株4个管家基因序列在MLST数据库中进行查询比对,得到相应的等位基因编码。按照gyrB、pyrH、recA、atpA的排列顺序,确定菌株的ST型。串联每株溶藻弧菌4个管家基因序列,使用MEGA 11.0软件的MUSCLE功能模块儿进行多重序列比对,删除比对后序列矩阵两端不能比对到其他同源序列的片段。使用邻接法(NJ法),构建MLST分型系统发育树。采用Bootstrap法重复抽取1 000次样本,对所得进化树的拓扑结构的准确性进行检验和验证。
1.4 毒力基因检测选取已知弧菌毒力因子基因(魏霜, 2013; 李启蒙, 2017; 张瑞卿, 2021),包括合成相关溶血素基因(tdh、tlh、trh)、毒力调控基因(toxS、toxR)、铁调节蛋白基因(fur)等共13种与致病力相关的编码基因。根据文献获取各毒力因子基因的引物(见表 2)。以1.2中溶藻弧菌的菌液DNA为模板,PCR反应体系及反应程序同1.2,退火温度如表 2所示。扩增产物在120 V条件下,1.5%琼脂糖凝胶电泳30 min,凝胶成像系统下观察扩增条带,从而判断该变异珠是否携带毒力因子的编码基因。
1.5 耐药性分析根据美国国家临床实验室标准化委员会(NCCLS)推荐的标准(李雅军等, 2006),采用Kirby-Bauer法对12株溶藻弧菌分离株进行药敏试验。使用酶标仪将菌液浓度调至OD600 nm=1 (1.0×108 CFU/mL),在超净台中取100 μL菌液均匀涂布于2216E平板上,用无菌镊子将10种抗生素药敏纸片贴附于培养基表面,28 ℃恒温培养24 h,观察并测量记录药敏纸片抑菌圈直径大小,作为判定敏感度高低的标准。
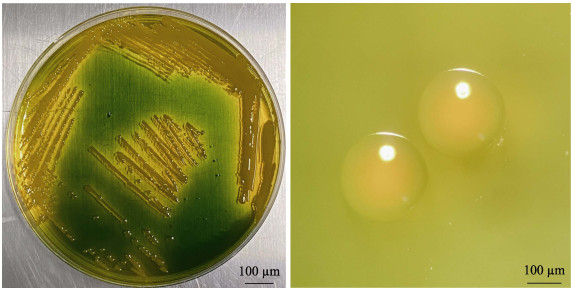
2 结果 2.1 溶藻弧菌的分离鉴定使用TCBS选择性培养基,对采集自4个海区的溶藻弧菌进行复苏、活化和划线培养。所有弧菌菌落外部颜色均呈现黄色或淡黄色,菌落形态为圆形透明、中央凸起、边缘光滑,直径为2~3 mm,菌落湿润粘稠且不易挑取(见图 1)。菌落PCR和16S测序峰图波峰与波谷清晰,峰与峰之间的距离均匀,说明菌落单一未受到污染。经NCBI-Blast对16S测序结果进行比对,结果显示,与溶藻弧菌同源性在99%以上,初步鉴定为溶藻弧菌,与最初鉴定结果相吻合。
|
图 1 溶藻弧菌在TCBS平板上的生长情况 Fig.1 Growth of V. alginolyticus on TCBS plate |
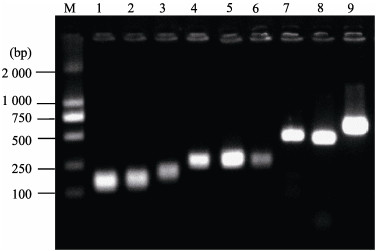
根据弧菌属MLST数据库提供的4个管家基因(gyrB、pyrH、recA、atpA)和引物序列进行多位点分型。12株溶藻弧菌的PCR扩增产物经1.5%的琼脂糖凝胶电泳,均检测到单一明亮条带(见图 2)。4个管家基因PCR产物测序结果经PubMLST数据库比对,确认为相应管家基因序列,并得到各管家基因相应的等位基因编码,按照gyrB、pyrH、recA和atpA基因排序确定ST型(见表 3)。检测发现,12株溶藻弧菌的ST型互不相同,其中,7株为数据库中已经收录的ST型,5株为新ST型。7株已收录ST型中除贝源溶藻弧菌(D2),其他6株均为水源溶藻弧菌;5株新ST型中有4株贝源溶藻弧菌(A1、B1、C1、D1)和1株水源溶藻弧菌(C2)。5株新ST型溶藻弧菌与数据库中收录的部分ST具有较近的亲缘关系:青岛分离株(A1)与数据库中ST型为38、131、134、46、56的溶藻弧菌更相近;威海分离株(C1、C2)与数据库中ST为111、322、61的溶藻弧菌更相近;烟台分离株(D1)与数据库中ST型为268、275、341、344、351、358的溶藻弧菌更相近,详情见表 3。
|
|
表 3 12株溶藻弧菌各管家基因等位基因编码及ST型结果 Tab.3 Encoding and ST type of alleles of each steward gene of 12 V. alginolyticus strains |
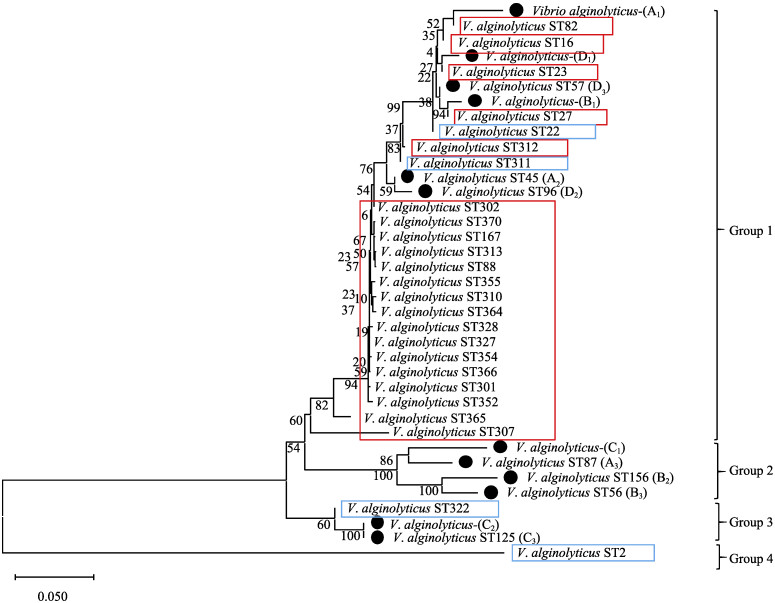
4个管家基因序列串联后,对12株溶藻弧菌ST型的系统发育分析结果显示,系统发育树主要分为4个明显的分支:组1、组2、组3和组4 (见图 3)。12株溶藻弧菌中4株贝源溶藻弧菌(A1、B1、D1、D2)和2株水源溶藻弧菌(A1、D3)聚类于组1中,且组1对照组中ST型大多来自海洋动物组织;3株水源溶藻弧菌(A3、B2、B3)和1株贝源溶藻弧菌(C1)聚类于组2;2株水源溶藻弧菌(C2、C3)聚类于组3;组4为对照组副溶血弧菌。贝源溶藻弧菌主要分布在组1中,表明溶藻弧菌间进化关系上更加接近。不同地区的同一来源溶藻弧菌在进化关系上可能更加亲近;组2和组3中的分离株主要来自海水环境,并且同一地区如潍坊地区和威海地区的水源溶藻弧菌和贝源溶藻弧菌在进化关系上差异较大,而青岛暂养池和烟台育苗场中水源溶藻弧菌和贝源溶藻弧菌在进化关系上差异较小。

|
图 2 溶藻弧菌管家基因检测凝胶电泳示例 Fig.2 Example of gel electrophoresis results for V. alginolyticus housekeeping genes M:标准分子量Marker;1~4:管家基因,依次为gyrB、recA、atpA和pyrH。 M: Standard molecular weight marker; 1~4: Housekeeping genes, in order of gyrB, recA, atpA, and pyrH. |
|
图 3 基于4个管家基因串联12株溶藻弧菌ST型系统发育树 Fig.3 A phylogenetic tree of 4 housekeeping genes of 12 strains of V. alginolyticus 红色方框内溶藻弧菌来自海洋动物组织;蓝色方框内溶藻弧菌来自环境或海水。 V. alginolyticus in the red box are from marine animal tissues; V. alginolyticus in the blue box are from the environment or seawater. |
|
图 4 溶藻弧菌9对毒力基因检测凝胶电泳示例 Fig.4 Example of gel electrophoresis results for 9 pairs of virulence genes of V. alginolyticus M:标准分子量Marker;1~9:毒力基因依次为tlh、fur、AspA、toxS、FlaA、ompw、UreR、vscB和collagenase。 M: Standard molecular weight Marker; 1~9: Virulence genes, in order of tlh, fur, AspA, toxS, FlaA, ompw, UreR, vscB, and collagenase. |
通过分子水平对13种主要毒力基因进行检测(见图 4)。结果显示(表 4),12株溶藻弧菌均携带tlh、fur、collagenase基因;VscB、Ompw、FlaA、toxS等毒力基因在大多数菌株存在;UreB、AspA等毒力基因出现在少数菌株中;而均未检测到tdh、trh、toxR和tcpA基因。不同地区或同一地区不同来源(贝类组织或海水)的溶藻弧菌携带毒力基因的种类和数量均存在差异,如威海和青岛地区的水源溶藻弧菌携带毒力基因种类和数量均多于潍坊和烟台地区水源溶藻弧菌。同一地区水源溶藻弧菌携带毒力基因的种类和数量,普遍多于贝源溶藻弧菌,如青岛和烟台地区中水源溶藻弧菌携带毒力基因种类和数量普遍多于贝源溶藻弧菌。
|
|
表 4 不同地区的溶藻弧菌毒力基因携带 Tab.4 Virulence gene distribution of V. alginolyticus in different regions |
12株溶藻弧菌对10种抗生素的耐药性结果见表 5。本次分离的溶藻弧菌均至少对2种及以上抗生素多重耐药,所有溶藻弧菌对氨苄西林和青霉素表现出耐药性,而对复方新诺明和氯霉素表现出高度敏感性。大部分溶藻弧菌对丁胺卡那、庆大霉素、红霉素、诺氟沙星、环丙沙星等抗生素产生中介敏感或高度敏感。
|
|
表 5 12株溶藻弧菌药敏试验结果 Tab.5 Drug susceptibility test results of 12 strains of V. alginolyticus |
在所有溶藻弧菌中,对头孢唑林耐药1株,中介敏感5株,高度敏感6株;对丁胺卡那、庆大霉素、红霉素中介敏感均为5株,高度敏感均为6株;对诺氟沙星中介敏感为2株,高度敏感均为10株;对环丙沙星中介敏感为4株,高度敏感均为8株;不同地区的溶藻弧菌对抗生素的耐药性均存在差异。同一地区同一取样来源的溶藻弧菌耐药谱更加接近。
3 讨论溶藻弧菌感染包括贝类在内的各种海水养殖动物的报道已有很多,但对贝源溶藻弧菌致病力、遗传变异等流行病学特征数据的报道却很少(韩风杰等, 2016)。本研究挑选从不同地区海水和贝类组织中分离的具代表性溶藻弧菌,经TCBS培养基划线培养后得到的所有疑似溶藻弧菌菌落均为黄色、光滑透明、湿润、中间凸起、粘稠的圆形菌落,在外部形态上差异不显著。这一结果与高璐等(2015)报道的溶藻弧菌形态特征结果基本一致,结果均表明,在不同环境条件下,溶藻弧菌的菌落外部形态结构并未发生显著改变(龙云映等, 2014)。不同溶藻弧菌变异株表现出致病力等表型差异,可能是由菌体内部其他遗传物质变异引起的。
MLST方法通过PCR扩增多个管家基因位点核酸序列,各位点核酸序列的变异就代表一个新的基因型,根据各基因型发现时间顺序赋予每个基因型一个等位编号,并将各基因位点按照指定规定的顺序排列形成序列型。该方法广泛应用于病原菌流行病学调查及不同菌株进化关系分析,为不同病原菌变异株的鉴定溯源和病害防控提供了有力的工具(Martin, 2006)。本研究选取的不同地区来源的溶藻弧菌在进化关系上差异较大,同一地区的溶藻弧菌在进化关系上更加接近,这一结果符合由于地理隔离限制了地理种群间的交流融合,从而呈现较大的遗传变异的群体遗传规律。之前的研究也得到类似的研究结论,即同一地区及周边环境分离株和组织分离株在ST型上存在较高的相似性(魏大伟, 2018; 向勇等, 2022)。研究发现,5种新ST型均为数据库中已收录ST型部分管家基因编码发生改变而形成,类似格局在方苹等(2017)和杨昆明等(2019)对鲁氏耶尔森菌(Yersinia ruckeri)进行ST分型时也有报道。史秀杰等(2012)研究认为,外部环境变异(不同年份、不同地域、不同来源)和生物作用(宿主、微生物)或菌体自身对外部环境的主动应答,可能导致菌体内部遗传成分发生改变,从而在进化过程中表现出较大的遗传多样性。
毒力基因种类和数量是决定弧菌在宿主体内的定植能力、致病力和环境适应性的关键因素。同时,弧菌携带毒力基因的种类和数量可能随时间推移而变化,这种变化一方面可能是受到外部环境压力而产生的适应性进化(范腾飞, 2013),另一方面也可能是毒力基因在菌株间发生的随机水平转移引起(谢珍玉等, 2005)。邬长祥(2012)和张晶等(2016)研究表明,从水生动物和环境中分离出的不同变异株间携带毒力基因的种类和数量存在较大差异。不耐热溶血素(tlh)、铁调蛋白基因(fur)、胶原酶基因(collagenase)在不同溶藻弧菌变异株中普遍存在。Taniguchi等(1985)和Ellison等(2001)研究指出,临床和环境分离弧菌中都含有tlh基因,并具有种属特异性,在毒力评估中具有较高的实用性,但目前对溶藻弧菌tlh的功能及其致病性机理仍不清楚(朱雪兰等, 2007)。铁摄取系统在溶藻弧菌的生存和致病性方面都有重要的作用,通过从宿主血红蛋白中夺取铁离子,从而导致宿主缺氧贫血,甚至死亡(Balebona et al, 1998);在限制铁含量条件下,溶藻弧菌生长受到抑制,当补加铁时可以消除这种抑制作用(王蓬勃等, 2006)。Collagenase具有很强的特异性,其降解胶原的能力对动物细胞外基质的整体降解起着关键作用,表明该酶有可能在水产动物组织溃疡等病症发展发生过程中发挥着作用(王学川等, 2021)。以上3种毒力基因在溶藻弧菌生长、定殖过程中必不可少,这可能是它们普遍存在于本研究所选取菌株中的重要原因。耐热直接溶血素(tdh)和相关溶血素(trh)、毒力调节因子(toxR)和毒素共调菌毛基因(tcpA)作为弧菌属的主要毒力因子均未在本研究中检测到。据报道,携带这些毒力基因的菌株常引起贝类软体部消瘦、闭壳肌松弛、外套膜萎缩、组织溃烂发白、黏液分泌减少等症状(张瑞卿, 2021)。研究还表明,该类基因在动物源分离株中存在几率要高于水源分离株(张晶等, 2016)。目前普遍认为,tdh和trh基因是副溶血弧菌的关键毒力因子,且2种基因的核酸序列高度同源,它们可能由共同的祖先序列分化而来(Shirai et al, 1990)。毒力基因toxR作为调控因子在弧菌中广泛分布,toxR通过不同的调控机制,能够增强弧菌主要毒力基因和外膜蛋白的表达,其中包括对tdh和trh基因表达的调控(Lin et al, 1993; Lee et al, 2000; Machado et al, 2015)。本研究分离的贝源和水源溶藻弧菌中均未检测到tdh、trh和toxR基因,三者在这些溶藻弧菌中共同缺失可能是潜在相互作用的结果。tcpA常见于霍乱弧菌(V. cholerae)和拟态弧菌(V. mimicus)等弧菌,具有组织黏附和参与致病性等作用,目前在溶藻弧菌中尚未见报道(陆吉虎等, 2007)。
从水体和贝类组织中分离出的溶藻弧菌对不同的抗生素耐药性不同,且均具有多重耐药性的特点。本研究与相关研究均表明,不同时间、不同来源和不同环境中分离出的溶藻弧菌耐药谱具有很大差异(Zorrilla et al, 2003; Oh et al, 2011)。尽管研究中使用的如红霉素、环丙沙星和氯霉素等多种抗生素在抗菌杀菌等方面效果显著,但其中多属于水生动物禁用抗生素(农业农村部渔业渔政管理局等, 2020b)。此外,抗生素类药物滥用现象在水产养殖业中已经显而易见,增强细菌耐药性、破坏微生态平衡、污染水质和水产品等副作用已不可忽视(肖倩, 2020)。因此,在防控水产细菌性病害和维护水生生物健康的工作中,首先应明确药物种类、最低有效治疗浓度以及病原菌的耐药性,并通过合理使用药物或微生态制剂等手段才能实现水产养殖行业可持续发展。
毒力基因、耐药性以及ST型等影响水产动物病害的流行病学数据是水生动物病害防治的重要基础;本研究结果对了解贝源溶藻弧菌的致病机理和贝类育苗和养成期病害的科学防控具有重要参考价值。但综合本研究MLST分型结果和耐药、毒力基因结果,暂未发现三者之间存在显著相关性。近来,针对鲟源鲁氏耶尔森菌和不动杆菌(Acinetobacter sp.)等菌株进行分型鉴定的研究,也未明确ST分型和毒力因子以及耐药性三者之间存在显著相关性(李岱霞, 2019; 翟盼盼, 2020; 张小丽等, 2021)。本研究中选用菌株数量较少、菌株采集时间、地域差异和分型方法等可能对三者之间相关性的解析造成不利影响。在后续的研究中,可通过扩大样本量、引入新分型方法和减少外部环境影响等方式,从不同层面进一步解析它们之间的内在关联。
BALEBONA M C, ANDREU M J, BORDAS M A, et al. Pathogenicity of Vibrio alginolyticus for cultured gilt-head sea bream (Sparus aurata L. ). Applied and Environmental Microbiology, 1998, 64(11): 4269-4275 DOI:10.1128/AEM.64.11.4269-4275.1998 |
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, Chinese Academy of Fishery Sciences, National Fisheries Technology Extension Center. Aquaculture drugs clear paper 2020 No. 1 and 2. Beijing: China Agriculture Press, 2020a [农业农村部渔业渔政管理局, 中国水产科学研究院, 全国水产技术推广总站. 水产养殖用药明白纸2020年1号、2号. 北京: 中国农业出版社, 2020a]
|
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2020. Beijing: China Agriculture Press, 2020b [农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2020中国渔业统计年鉴. 北京: 中国农业出版社, 2020b]
|
CHEN Q, YAN Q P, MA S. Progress on pathogenicity research of Vibrio alginolyticus. Marine Sciences, 2006, 30(8): 83-89 [陈强, 鄢庆枇, 马甡. 溶藻弧菌致病性研究进展. 海洋科学, 2006, 30(8): 83-89] |
DING Y, XU G. The study of pathogenic bacteria and whole cell vaccine on Sparus latus vibrio disease. Microbiology China, 2004, 31(2): 1-5 [丁燏, 徐刚. 黄鳍鲷弧菌病病原特性及其全菌苗的研究. 微生物学通报, 2004, 31(2): 1-5] |
ELLISON R K, MALNATI E, DEPAOLA A, et al. Populations of Vibrio parahaemolyticus in retail oysters from Florida using two methods. Journal of Food Protection, 2001, 64(5): 682-686 DOI:10.4315/0362-028X-64.5.682 |
FAN T F. Detection of virulence genes in Aeromonas strains and the influence of environmental factors on virulence genes expression. Master´s Thesis of Hefei University of Technology, 2013 [范腾飞. 气单胞菌的毒力基因检测及环境因子对毒力基因表达的影响. 合肥工业大学硕士研究生学位论文, 2013]
|
FANG H. Epidemiological investigation and virulence gene detection of shellfish Vibrio in Shandong Province. Master´s Thesis of Shandong Agricultural University, 2019 [方皓. 山东省贝类弧菌流行病学调查及毒力基因检测. 山东农业大学硕士研究生学位论文, 2019]
|
FANG P, DING C X, WU Y F, et al. Isolatioan identification and phylogenetic analysis of Yersinia ruckeri from silver carp and bighead. Journal of Huaihai Ocean University (Natural Science Edition), 2017, 26(2): 84-87 [方苹, 丁彩霞, 吴亚锋, 等. 鲢鱼、鳙鱼源鲁氏耶尔森氏菌的分离鉴定及系统发育分析. 淮海工学院学报(自然科学版), 2017, 26(2): 84-87] |
GAO L, TAO X Y, CHEN J S, et al. Distribution of Vibrio alginolyticus in aquatic products in Jiangsu Province. Chinese Journal of Microbiology, 2015, 35(6): 82-85 [高璐, 陶晓雅, 陈峻琛, 等. 江苏省水产品中溶藻弧菌分布情况. 微生物学杂志, 2015, 35(6): 82-85] |
HAN F J, XU W N, LIU M, et al. Isolation and identification of Vibrio alginolyticus and susceptibility test in Anadara uropygimelana. Shandong Animal Husbandry and Veterinary Medicine, 2016, 37(5): 13-14 [韩风杰, 徐文娜, 刘敏, 等. 魁蚶溶藻弧菌分离鉴定与药敏试验. 山东畜牧兽医, 2016, 37(5): 13-14] |
HAN F J. The investigation of shellfish Vibrio disease epidemiology in Shandong Province and establishment of a rapid detection method about Vibrio parahaemolyticus. Master´s Thesis of Shandong Agricultural University, 2016 [韩风杰. 山东省贝类弧菌病流行病学调查及副溶血弧菌快速检测方法的建立. 山东农业大学硕士研究生学位论文, 2016]
|
HUANG Z J, HE J G. Isolation, identification and pathogenicity of the bacterial pathogen in Epinephlus fario. Journal of Sun Yat-sen University (Natural Science Edition), 2002(5): 64-67 [黄志坚, 何建国. 鲑点石斑鱼细菌病原的分离鉴定和致病性. 中山大学学报(自然科学版), 2002(5): 64-67] |
LEE K K, YU S R, CHEN F R, et al. Virulence of Vibrio alginolyticus isolated from diseased tiger prawn, Penaeus monodon. Current Microbiology, 1996a, 32(4): 229-231 DOI:10.1007/s002849900041 |
LEE K K, YU S R, YANG T I, et al. Isolation and characterization of Vibrio alginolyticus isolated from diseased kuruma prawn, Penaeus Japonicus. Letters in Applied Microbiology, 1996b, 22(2): 111-114 DOI:10.1111/j.1472-765X.1996.tb01121.x |
LEE S E, SHIN S H, KIM S Y, et al. Vibrio vulnificus has the transmembrane transcription activator ToxRS stimulating the expression of the hemolysin gene vvhA. Journal of Bacteriology, 2000, 182(12): 3405-3415 DOI:10.1128/JB.182.12.3405-3415.2000 |
LI C P, ZHAI Q Q, WANG X, et al. lsolation and identification of Vibrio parahaemolyticus from shrimp culture ponds and analysis of its drug resistance and virulence genes. Progress in Fishery Sciences, 2020, 41(6): 174-180 [李翠苹, 翟倩倩, 王想, 等. 对虾养殖池副溶血弧菌的分离鉴定及其耐药特征、毒力基因分析. 渔业科学进展, 2020, 41(6): 174-180] |
LI D X. Study on virulence genes, drug resistance and multi-locus sequence typing of Enterococcus faecalis isolated from pigs and chickens of some farms in Hunan. Master´s Thesis of Hunan Agricultural University, 2019 [李岱霞. 湖南部分养殖场猪、鸡源粪肠球菌毒力基因、耐药性及多位点序列分型研究. 湖南农业大学硕士研究生学位论文, 2019]
|
LI Q M. Epidemiology, antibiotic susceptibility test and virulence gene detection of Vibrios islotated from shelfish in Shandong Province. Master´s Thesis of Shandong Agricultural University, 2017 [李启蒙. 山东省贝类弧菌流行病学调查、药敏试验及毒力基因检测. 山东农业大学硕士研究生学位论文, 2017]
|
LI Y J, ZHANG B, WANG H. 2006 CLSI/NCCLS susceptibility standard update (CLSI/NCCLS M100-S16 instead of M100-S15). Jiangxi Medical Laboratory, 2006(4): 355-356 [李雅军, 张斌, 王华. 2006年CLSI/NCCLS药敏标准的更新(CLSI/NCCLS M100-S16代替M100-S15). 江西医学检验, 2006(4): 355-356] |
LIN Y T. Preliminary study on death cause of a mass of Mytilus edulis Linnaeu. Modern Fishery Information, 2007(3): 26-28 [林永添. 紫贻贝大量死亡原因的初步研究. 现代渔业信息, 2007(3): 26-28] |
LIN Z, KUMAGAI K, BABA K, et al. Vibrio parahaemolyticus has a homolog of the Vibrio cholerae ToxRS operon that mediates environmentally induced regulation of the thermostable direct hemolysin gene. Journal of Bacteriology, 1993, 175(12) |
LIU T. The main characteristics and trends of aquaculture diseases in China. Agricultural Knowledge, 2019(24): 32-33 [刘婷. 我国水产养殖病害发生的主要特点及趋势. 农业知识, 2019(24): 32-33] |
LONG Y Y, ZHANG L P, WEI L, et al. Viability analysis and cell morphology observation of Vibrio alginolyticus under long-term starvation. Chinese Journal of Tropical Biology, 2014, 5(3): 199-207 [龙云映, 张吕平, 韦露, 等. 溶藻弧菌在海水长期饥饿胁迫下的活性分析及菌落相变特征. 热带生物学报, 2014, 5(3): 199-207] |
LU J H, LI J N, ZHNAG C L. Advance in toxin coregulated pilus of pathogenic Vibrio. Advances in Veterinary Medicine, 2007(8): 55-58 [陆吉虎, 李槿年, 张传亮. 病原弧菌毒素共调菌毛研究进展. 动物医学进展, 2007(8): 55-58] |
MACHADO H, GRAM L. The fur gene as a new phylogenetic marker for Vibrionaceae species identification. Applied and Environmental Microbiology, 2015, 81(8): 2745-2752 DOI:10.1128/AEM.00058-15 |
MAIDEN M C, BYGRAVES J A, FEIL E, et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(6): 3140-3145 |
MARTIN C J. Multilocus sequence typing of bacteria. Annual Review of Microbiology, 2006, 60(1) |
MEI B, LU X, WANG L N, et al. Progress on virulence factor and virulence gene research of Vibrio alginolyticus. Liaoning Agricultural Sciences, 2015(5): 58-60 [梅冰, 陆翔, 王丽娜, 等. 溶藻弧菌的毒力因子与相关基因的研究进展. 辽宁农业科学, 2015(5): 58-60] |
OH E G, SON K T, YU H, et al. Antimicrobial resistance of Vibrio parahaemolyticus and Vibrio alginolyticus strains isolated from farmed fish in Korea from 2005 through 2007. Journal of Food Protection, 2011, 74(3): 380-386 DOI:10.4315/0362-028X.JFP-10-307 |
RIQUELME C, TORANZO A E, BARJA J L, et al. Association of Aeromonas hydrophila and Vibrio alginolyticus with larval mortalities of scallop (Argopecten purpuratus). Journal of Invertebrate Pathology, 1996, 67(3): 213-218 DOI:10.1006/jipa.1996.0035 |
SAINZ J C, MAEDA-MARTÍNEZ A N, ASCENCIO F. Experimental Vibriosis induction with Vibrio alginolyticus of Larvae of the Catarina Scallop (Argopecten ventricosus =circularis) (Sowerby Ⅱ, 1842). Microbial Ecology, 1998, 35(2): 188-192 DOI:10.1007/s002489900073 |
SHI X J, ZHENG X C, RUAN Z X, et al. Molecular subtyping studies of Vibrio parahaemolyticus isolated from aquatic animals. China Animal Quarantine, 2012, 29(6): 47-53 [史秀杰, 郑晓聪, 阮周曦, 等. 水生动物中分离的副溶血性弧菌菌株分子分型研究. 中国动物检疫, 2012, 29(6): 47-53] |
SHIRAI H, ITO H, HIRAYAMA T, et al. Molecular epidemiologic evidence for association of thermostable direct hemolysin (TDH) and TDH-related hemolysin of Vibrio parahaemolyticus with gastroenteritis. Infection and Immunity, 1990, 58(11): 3568-3573 DOI:10.1128/iai.58.11.3568-3573.1990 |
SONG L S. An early warning system for diseases during mollusc mariculture: Exploration and utilization. Journal of Dalian Ocean University, 2020, 35(1): 1-9 [宋林生. 海水养殖贝类病害预警预报技术及其应用. 大连海洋大学学报, 2020, 35(1): 1-9] |
TANIGUCHI H, OHTA H, OGAWA M, et al. Cloning and expression in Escherichia coli of Vibrio parahaemolyticus thermostable direct hemolysin and thermolabile hemolysin genes. Journal of Bacteriology, 1985, 162(2): 510-515 DOI:10.1128/jb.162.2.510-515.1985 |
WANG B, HAN L M. A study on the basic situation and pattern of shellfish breeding in China. Journal of Ocean University of China (Social Sciences Edition), 2017(3): 5-12 [王波, 韩立民. 我国贝类养殖发展的基本态势与模式研究. 中国海洋大学学报(社会科学版), 2017(3): 5-12] |
WANG H, YANG B, LI X, et al. Screening of bacterial pathogens associated with mass summer mortality of the Pacific oyster, Crassostrea gigas, in China. Aquaculture Reports, 2021, 20 |
WANG P B, MA Y, LIU Q, et al. Growth, siderophore production and outer membrane protein expression of Vibrio alginolyticus by iron regulation. Chinese Journal of Microbiology, 2006(2): 48-53 [王蓬勃, 马悦, 刘琴, 等. 溶藻弧菌铁载体合成及外膜蛋白表达的研究. 微生物学通报, 2006(2): 48-53] |
WANG R C, ZHENG X D. Progress of marine shellfishes culture in China and its prospect. Periodical of Ocean University of China, 2004, 34(5): 775-780 [王如才, 郑小东. 我国海产贝类养殖进展及发展前景. 中国海洋大学学报(自然科学版), 2004, 34(5): 775-780] |
WANG X C, WANG X Y, YANG S Q, et al. A revieuc: Micobial coliagenase. Leather Science and Engineering, 2021, 31(2): 16-22 [王学川, 王雪莹, 杨淑琴, 等. 微生物胶原酶研究进展. 皮革科学与工程, 2021, 31(2): 16-22] |
WEI D W. Epidemiological investigation and genetic diversity of Vibrio parahaemolyticus isolated from coastal areas of China. Master´s Thesis of Northwest A & F University, 2018 [魏大伟. 中国沿海地区副溶血弧菌流行病学调查及遗传多样性分析. 西北农林科技大学硕士研究生学位论文, 2018]
|
WEI S. Detection of Vibrio alginolyticus and its virulence- related genes. Master´s Thesis of Jinan University, 2013 [魏霜. 溶藻弧菌及其毒力相关基因的检测. 暨南大学硕士研究生学位论文, 2013]
|
WU C X. The genetic diversity and distribution of virulent genes in Vibrio alginolyticus isolated from south China. Master´s Thesis of Guangdong Ocean University, 2012 [邬长祥. 我国南方养殖海域溶藻弧菌的遗传多样性及部分毒力基因的分布特征研究. 广东海洋大学硕士研究生学位论文, 2012]
|
XIANG Y, LI Q B, LIU P, et al. Analyses of drug resistance, multi-locus sequence typing and genetic evolution of Peudomonas aeruginosa in Pheasant farms. Journal of South China Agricultural University, 2022(2): 1-10 [向勇, 李庆钵, 刘鹏, 等. 七彩山鸡养殖场铜绿假单胞菌的耐药性、MLST分型及遗传进化分析. 华南农业大学学报, 2022(2): 1-10] |
XIAO Q. Research on antibiotic abuse in aquaculture. Journal of Aquaculture and Feedstuffs, 2020, 19(10): 46-48 [肖倩. 水产养殖中抗生素滥用问题研究. 养殖与饲料, 2020, 19(10): 46-48] |
XIE Z Y, HU C Q. Studing progress in horizontal transfer and evolution of Vibrio virulence gene. Journal of Tropical Oceanography, 2005(3): 86-95 [谢珍玉, 胡超群. 弧菌毒力基因水平转移与进化的研究进展. 热带海洋学报, 2005(3): 86-95] |
YAN Q P, WANG J, SU Y Q, et al. Studies on Vibriosis in caged-cultured Pseudosciaena crocea (Richardson). Journal of Jimei University (Natural Science Edition), 2001(3): 191-196 [鄢庆枇, 王军, 苏永全, 等. 网箱养殖大黄鱼弧菌病研究. 集美大学学报(自然科学版), 2001(3): 191-196] |
YANG B, ZHAI S, LI X, et al. Identification of Vibrio alginolyticus as a causative pathogen associated with mass summer mortality of the Pacific oyster (Crassostrea gigas) in China. Aquaculture, 2021, 535: 736363 |
YANG K M, ZHANG W R, MA J X, et al. Isolation, identification and antibiotic sensitivity of pathogenic bacterium Yersinia ruckeri from Siberian Sturgeon Acipenser Baerii. Fisheries Science, 2019, 38(1): 48-54 [杨昆明, 张文润, 马江霞, 等. 鲟源致病性鲁氏耶尔森菌的分离、鉴定及药敏研究. 水产科学, 2019, 38(1): 48-54] |
ZHAI P P. Study on the relationship between the whole genome sequencing identification, typing and pathogenicity of clinical isolates of Acinetobacter. Master´s Thesis of University of South China, 2020 [翟盼盼. 不动杆菌全基因组测序鉴定分型及其致病相关性研究. 南华大学硕士研究生学位论文, 2020]
|
ZHANG J, CHEN J X, WU Y J, et al. Analysis of the contamination status and virulence related genes of Vibrio alginolyticus in aquatic products and external environmental water in Guangzhou. Chinese Journal of Health Laboratory Technology, 2016, 26(4): 539-541 [张晶, 陈佳璇, 伍业健, 等. 广州地区水产品及外环境水体溶藻弧菌污染状况与毒力相关基因分析. 中国卫生检验杂志, 2016, 26(4): 539-541] |
ZHANG R Q. Epidemiological investigation of Vibrio from shellfish in Shandong Province and detection of virulence genes. Master´s Thesis of Shandong Agricultural University, 2021 [张瑞卿. 山东省贝源弧菌流行病学调查及其毒力基因检测. 山东农业大学硕士研究生学位论文, 2021]
|
ZHANG X L, MA Q S, JIAO S Z, et al. Multilocus sequence typing and resistance range determination of Yersinia ruckeri from Sturgeon Hybrid. Fisheries Science, 2021, 40(3): 394-402 [张小丽, 麻强生, 焦世璋, 等. 鲟源鲁氏耶尔森菌多位点序列分型及耐药谱测定. 水产科学, 2021, 40(3): 394-402] |
ZHANG X, HUANG B W, ZHENG Y D, et al. Identification and characterization of infectious pathogens associated with mass mortalities of Pacific oyster (Crassostrea gigas) cultured in northern China. Biology, 2023, 12(6): 759 |
ZHANG Z H, ZHANG Q Z, LI Y C, et al. Isolation and identification of pathogenic vibrios of oyster Crassostrea ariakensis. Journal of Tropical Oceanography, 2008, 27(6): 49-56 [张占会, 张其中, 李春勇, 等. 养殖近江牡蛎致病弧菌的分离与鉴定. 热带海洋学报, 2008, 27(6): 49-56] |
ZHENG G X, LI H, HUANG N Y, et al. Characteristics of Vibrio alginolyticus isolated from diseased clam (Meretrix meretrix) and histopathological observations on diseased clam by electron microscope. Journal of Fisheriesof China, 1991, 15(2): 85-95 [郑国兴, 李何, 黄宁宇, 等. 文蛤病原菌(溶藻弧菌)的分离与性状及病文蛤组织的电镜观察. 水产学报, 1991, 15(2): 85-95] |
ZHU X L, CHEN Y, LIU X M, et al. Research progress on the hemolytic gene of Vibrio parahaemolyticus and its detection. Foreign Medical Sciences Section Hygiene), 2007, 34(4): 233-237 [朱雪兰, 陈艳, 刘秀梅, 等. 副溶血性弧菌溶血素基因及其检测的研究进展. 国外医学卫生学分册, 2007, 34(4): 233-237] |
ZORRILLA I, MORINIGO M A, CASTRO D, et al. Intraspecific characterization of Vibrio alginolyticus isolates recovered from cultured fish in Spain. Journal of Applied Microbiology, 2003, 95(5): 1106-1116 |



