2. 中国水产科学研究院黄海水产研究所 农业农村部海洋渔业与可持续发展重点实验室 山东 青岛 266071;
3. 海洋渔业科学与食物产出过程功能实验室 山东 青岛 266071;
4. 邦普种业科技有限公司 山东 潍坊 261000
2. Key Laboratory of Marine Fisheries and Sustainable Development, Ministry of Agriculture and Rural Affairs, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071;
3. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao 266071, China;
4. BLUP Aquabreed Co., Ltd., Weifang 261000, China
凡纳滨对虾(Litopenaeus vannamei),又称南美白对虾、万氏对虾、白脚对虾(林红军等, 2010),隶属于甲壳亚门(Crustacea)、十足目(Decapoda)、对虾科(Palaemonidae)、滨对虾属(Litopenaeus),因其具有耐高温、抗病力强、生长速度快和营养丰富等优越的经济性状(贾文涛, 2018),现已成为我国最重要的养殖对虾品种之一(Du et al, 2015)。
在养殖过程中,经常会发生水中溶解氧(DO)低甚至缺氧(Soñanez-Organis et al, 2010)的现象。特别是在高密度养殖的情况下,短期内急性缺氧就会导致凡纳滨对虾的大量死亡(王梦瑶等, 2022)。开展遗传改良、筛选培育耐低氧的优良品种是促进凡纳滨对虾产业可持续发展的重要措施。目前,国内外凡纳滨对虾育种工作主要针对生长、存活、抗病抗逆等目标性状的遗传改良。王崇懿等(2021)研究表明,凡纳滨对虾的高盐耐受性状遗传力为0.032±0.031,属于低遗传力;生长相关性状遗传力在0.194~0.242之间达到极显著水平;凡纳滨对虾收获体质量和池塘成活率的遗传力分别为0.17~0.24和0.04~0.10 (Gitterle et al, 2005)。有关凡纳滨对虾低氧耐受性状的遗传参数评估、品系筛选的研究仍然较少。张嘉晨等(2016)研究发现,凡纳滨对虾低氧耐受性状的遗传力为0.07~0.15,其采用的DO浓度范围为1~2 mg/L (48~96 h半数死时的DO浓度),为相对长期的持续低氧胁迫实验。研究显示,凡纳滨对虾幼虾的1 h半数死DO浓度为0.2~ 0.3 mg/L (Pérez-Rostro et al, 2004)。凡纳滨对虾在短期高致死DO浓度下耐受性能的遗传参数及关键组织的细胞学特征还未见报道。
本研究以凡纳滨对虾高抗和快大2个品系作为研究对象,通过短期致死DO浓度水平胁迫,比较品系间及不同家系间半致死存活时间和半致死存活率的差异,估计短期高致死DO浓度水平下凡纳滨对虾低氧耐受性状的遗传参数,观察关键组织的细胞学特征,以期为凡纳滨对虾耐低氧性状选育工作提供参考与依据。
1 材料与方法 1.1 实验材料本实验在山东省潍坊市昌邑市邦普种业科技有限公司凡纳滨对虾育种基地开展。实验材料为2021年凡纳滨对虾育种核心群体,包括41个高抗家系(GK)和41个快大家系(K),日龄为P60~P63(仔虾60~63 d)。每个家系随机选取80尾体长为(4.5±0.3) cm、体重为(1.1±0.2) g的大小相近的健康个体,平均分为4组,包括3个平行实验组和1个对照组,每组20尾,共计6 560尾。
1.2 实验方法每组对虾分别置于20 L的塑料白桶中。桶内预先注入经沉淀、消毒的自然海水15 L,盐度为29~30,pH为7.5左右,通过水浴控制桶内温度为28.0 ℃。正式实验前于桶内暂养2 d,暂养期间连续充空气。实验期间,各实验桶的水质控制、日常管理均保持一致。
1.2.1 低氧耐受性实验通过预实验确定凡纳滨对虾P60时期1 h半致死DO浓度为0.3~0.5 mg/L,选择0.3 mg/L作为低氧胁迫实验的DO浓度。通过向海水中充氮气的方法调整水中DO浓度。水面上覆盖塑料薄膜,使用纳米级气石控制充氮气的速率,期间不断将薄膜下的气泡赶出。使用便携式溶氧仪每隔10 min测量、记录DO读数并及时调整氮气和空气的比例,使3个平行实验组的DO水平经过3 h降至预设浓度(0.3±0.1) mg/L。从实验组停止充空气、开始充氮气时开始计时。在实验过程中,每隔15 min观察并记录对虾的死亡数、溶氧含量、死亡时间、家系号、桶号等数据,直至对虾死亡率达到80%时停止实验。对照组正常充氧,控制DO水平为6.6~6.7 mg/L。
1.2.2 组织学观察经低氧胁迫实验后,按照家系平均存活时间排名,选择排名前三和后三分别作为低氧耐受家系和低氧敏感家系,各家系内随机选取4尾实验样品,将鳃、肌肉、完整的肝胰腺组织放入4%的多聚甲醛固定液中固定,24 h后换液一次,进行石蜡切片制作、HE染色,并利用尼康CI-S光学显微镜(日本)观察。
1.3 数据统计与分析 1.3.1 表型统计以个体存活时间和家系半致死存活率为指标,评价家系对低DO的耐受能力。个体存活时间为从胁迫开始到死亡的时间(h),家系存活时间为家系内所有个体的平均存活时间(h),家系半致死存活率为全部测试个体死50%时各个家系的存活率(SS50) (卢霞等, 2018)。利用SPSS 26.0软件对凡纳滨对虾不同家系平均低氧存活时间的描述性参数进行统计及方差分析,利用LSD法对不同家系间的存活时间进行多重比较,P<0.05为达到显著水平。
1.3.2 遗传力评估利用ASReml 4.2软件通过阈值性状动物模型估计凡纳滨对虾不同家系低氧半致死存活率性状的遗传参数,育种分析模型如下:
| $ {y_{ij}} = \left\{ {\begin{array}{*{20}{c}} {0,\;{\eta _{ij}} \leqslant 0} \\ {1,\;{\eta _{ij}} \geqslant 0} \end{array}} \right.;\;\;{\eta _{ij}} = \mu + {\alpha _j} + {e_{ij}} $ | (1) |
式中,
| $ {P_r}{\text{(}}{Y_{ij}}{\text{ = 1) = }}{P_r}({l_{ij}} \gt 0) = \phi {\text{(}}\mu + {\alpha _j} + {e_{ij}}{\text{)}} $ | (2) |
式中,
耐低氧性状的遗传力计算(Trygve, 2005):
| $ {h^2} = \frac{{\sigma _\alpha ^2}}{{\sigma _\alpha ^2 + \sigma _e^2}} $ | (3) |
式中,
存活阈值性状经过转换后在观测值(连续)尺度上的遗传力(
| $ h_p^2 = {h^2}\frac{{{z^2}}}{{p(1 - p)}} $ | (4) |
式中,
遗传力显著性检验利用公式:
| $ {\rm Z} = \frac{{{\chi _i}}}{{{\sigma _i}}} $ | (5) |
式中,
存在极显著差异(Lu et al, 2017)。
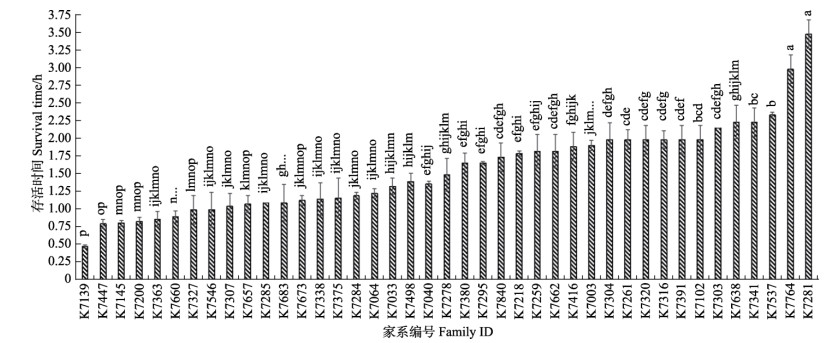
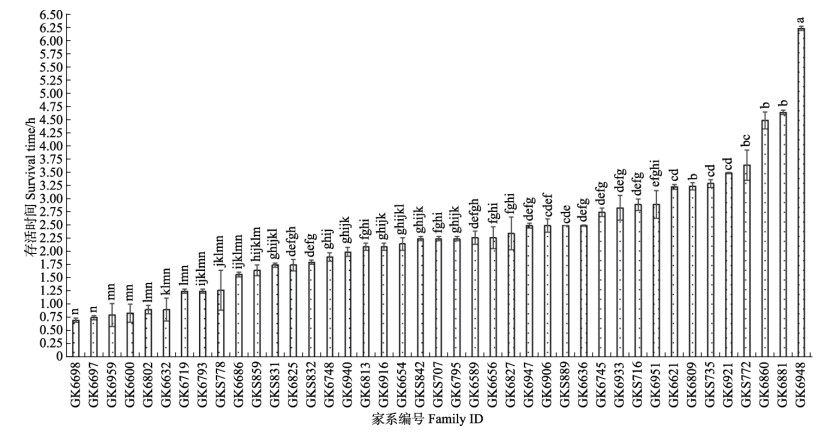
2 结果 2.1 低氧胁迫对不同品系存活性状的影响为探究凡纳滨对虾在极限低氧条件下的耐受性,本实验利用低DO浓度[(0.3±0.1) mg/L]开展对凡纳滨对虾的胁迫测试。结果显示,高抗品系及快大品系各家系平均存活时间存在显著差异(P<0.05)(图 1、图 2):高抗品系1.2 h达到50%死亡率,家系间平均存活时间变异系数为60%;快大品系0.95 h时达到50%死亡率,家系间平均存活时间变异系数为45%。高抗品系2.31 h达到80%死亡率,家系间变异系数为49%;快大品系1.57 h达到80%死亡率,家系间变异系数为40% (表 1)。整体半致死时高抗品系SS50为49.30%,快大品系为42.52%,2个品系间平均存活率存在显著差异(P<0.05) (表 2)。
|
图 1 凡纳滨对虾快大家系间平均低氧存活时间的LSD多重比较 Fig.1 LSD multiple comparison of mean survival time to hypoxia among different K families of L. vannamei 不同字母代表不同家系之间的差异显著(P<0.05)。下同。 Different letters show significant differences between different families (P < 0.05). The same below. |
|
图 2 凡纳滨对虾高抗家系间平均低氧存活时间的LSD多重比较 Fig.2 LSD multiple comparison of mean survival time to hypoxia among different GK families of L. vannamei |
|
|
表 1 凡纳滨对虾不同品系间低氧耐受存活时间的描述性统计 Tab.1 Descriptive statistics of hypoxic tolerance survival time among different strains of L. vannamei |
|
|
表 2 凡纳滨对虾半致死时平均存活率SS50的差异比较 Tab.2 Comparison of average survival rate at half lethal time of L. vannamei |
凡纳滨对虾不同品系间耐低氧性状的遗传力见表 3。通过阈值性状动物模型计算出高抗品系和快大品系的耐低氧性状的遗传力分别为0.345±0.031和0.378±0.029,经过转换后在观测值(连续)尺度上的遗传力分别为0.219±0.031和0.237±0.029,属于中等遗传力性状。Z-score检验表明,2个品系计算的遗传力与0相比均达到极显著水平(P<0.01),且2个品系间的遗传力差异与0相比也达到极显著水平(P<0.01)。由于无法剖分出共同环境效应,计算遗传力时并没有将家系共同环境效应加入模型中。实验时对虾难以辨别性别,模型中也未包含性别效应。
|
|
表 3 凡纳滨对虾不同品系的遗传力 Tab.3 Heritability of different strains of L. vannamei |
与凡纳滨对虾对照组鳃、肌肉和肝胰腺组织相比(图 3A、D、G),耐受家系和敏感家系在胁迫后表现出不同程度的变化,耐受家系鳃上皮细胞呈无规则排列,鳃丝的顶端囊状结构易被破坏(图 3B),肝胰腺组织管腔体积变大,B细胞增多(图 3E),肌肉组织形态无明显差异(图 3I);而敏感家系实验组对虾整个鳃丝鳃腔增大,囊状结构进一步增大,上皮层结构逐渐被破坏甚至解体(图 3C),肝胰腺的空泡化现象严重,管腔变为不规则变形(图 3F),肌肉组织肌束间隔变宽(H),并发生一定程度变形现象。

|
图 3 低氧胁迫下凡纳滨对虾不同组织切片的显微结构 Fig.3 Microstructure of different tissue sections of L. vannamei under hypoxia stress A:对照组鳃组织;B:低氧耐受家系鳃组织;C:低氧敏感家系鳃组织;D:对照组肝胰腺组织;E:低氧耐受家系肝胰腺组织;F:低氧敏感家系肝胰腺组织;G:对照组腹部肌肉组织;H:低氧耐受家系腹部肌肉组织;I:低氧敏感家系腹部肌肉组织。 A: Gill tissue of control group; B: Gill section of a hypoxic tolerant family; C: Gill tissue of a family susceptible to hypoxia; D: Hepatopancreas tissue of control group; E: Hepatopancreas tissue of a hypoxic tolerant family; F: Hepatopancreas tissue of hypoxic sensitive family; G: Abdominal muscle tissue of control group; H: Abdominal muscle tissue of a hypoxic tolerant family; I: Abdominal muscle tissue of a hypoxic-sensitive family. |
理化和生物因素季节性或突发性变动、水质调控不力常会引起水体DO降低甚至缺氧,导致凡纳滨对虾死亡(袁瑞鹏等, 2015)。研究表明,在长途运输过程中,水生动物也经常会出现低氧现象。Vaquer等(2008)根据水生动物DO的半致死浓度和时间实验得出甲壳动物中虾类对低氧胁迫最敏感的结论。本研究前期预实验统计发现,凡纳滨成虾典型浮头DO浓度为0.42 mg/L,而2.5 h半致死浓度(half lethal concentration, LC50)为0.3 mg/L,该范围与其他虾类相关研究结果相近。低氧胁迫8 h后,日本沼虾(Macrobranchium nipponense) LC50为0.85 mg/L (杨明等, 2019),而刀额新对虾(Metapenaeus ensis)的LC50则是0.77 mg/L (Wu et al, 2002);白滨对虾(Litopenaeus setiferus)在低氧环境(1.27 mg/L)下暴露48 h,死亡率达50%;中国对虾(Penaeus chinensis)的窒息点约为0.74 mg/L (Chen et al, 1992);罗氏沼虾(Macrobrachium rosenbergii)相比之下更为敏感,其窒息浓度为0.98 mg/L (陈琴等, 2002)。本研究选择在溶解氧为0.3 mg/L的极限低氧条件下对凡纳滨对虾进行耐受测试,旨在模拟真实生产条件下的低氧情况,以该条件进行测试,探究实际凡纳滨对虾低氧性状的遗传参数以及选育的可能性。
3.2 凡纳滨对虾耐低氧性状的遗传参数目前,已对多个水产动物的耐低氧性状进行了研究。40日龄大黄鱼(Larimichthys crocea)耐低氧遗传参数为0.23,达到显著水平(王晓清等, 2009);Hyuma等(2001)估计的虹鳟(Oncorhynchus mykiss)抗低氧的狭义遗传力为0.54;张嘉晨等(2016)报道凡纳滨对虾在一般低氧水平下耐低氧性状的遗传力范围为(0.07±0.04)~(0.15±0.07)。但在凡纳滨对虾中高致死低溶解氧水平耐受性状的遗传学参数研究还处于空白。本研究通过阈值性状动物模型估算,得出耐低氧性状的遗传力为(0.345±0.031)~(0.378±0.029),经过转换后在观测值尺度上的遗传力为(0.219±0.031)~ (0.237±0.029),属于中等遗传力性状,说明该性状有一定的选育潜力。
本研究中,凡纳滨对虾实验家系均为全同胞家系,家系间亲缘关系较低,各家系在实验前独立养殖引起的共同环境效应难以剖分,可能导致遗传力估计结果偏高(Gallardo et al, 2010; Hoffmann et al, 1999; Winkelman et al, 1994)。McIntyre等(1978)认为,要考察个体对某一阈值性状的抵抗力可用死亡和存活作为判断的标准,但需要较大的样本量来估计相应的发生率,否则会出现很大的偏差。本研究选择了82个家系,每组样本数达到了60个,可较准确地计算累积概率的大小,因此,估计的遗传力可信度较高。Ibarra等(2007)评估凡纳滨对虾幼虾阶段耐低溶氧遗传力为1.15±0.10和1.08±0.11,超出了理论估算范围(h2>1),其原因可能与共同环境效应无法剖分有关。
通过对凡纳滨对虾不同品系(高抗品系和快大品系)比较分析发现,高抗品系对高致死溶解氧浓度的耐受性能明显高于快大品系。这可能与2个品系的选育方法与标准的不同有关。高抗品系育种过程中,经过病毒及弧菌的感染测试,选出抗病家系进行下一代繁育。根据卢霞等(2018)的研究报道,抗病性状与氨氮急性胁迫之间存在正相关性,对氨氮急性胁迫应激耐受力高的群体对病毒的抵抗力也高。而本研究中高抗品系低氧耐受性能明显高于快大品系,推测抗病性状与耐低氧性状之间亦可能存在正相关,其他抗性性状的选育对耐低氧性状具有正选择作用。
3.3 低氧对凡纳滨对虾细胞水平的影响在生物体面临低氧胁迫时,其体内不同组织由于其结构与功能的不同而产生一定的应激反应来应对逆环境(杨明等, 2019)。研究表明,三疣梭子蟹(Portunus tritverruca)的鳃上皮细胞向鳃腔突起形成“隔”,将鳃腔分为许多小通道,通道内含有游离的血细胞(李太武, 1996),血细胞通过吞噬作用或释放糖供鳃细胞利用并保护鳃组织,同时泌氯细胞数目增多且形态由椭圆形转化为圆形,以此增加细胞与水的接触面积来应对逆环境(Bubel, 1976),这与本研究中凡纳滨对虾在低氧胁迫下的鳃组织应激表现一致。且本研究中低氧敏感家系实验组肌束间隙变大,肌纤维排列较为疏松,出现一定弯曲状态。同时,相关虾蟹类胁迫研究中发现,日本沼虾中肝胰腺组织因低氧胁迫导致B细胞数量逐渐增加,胞内运转泡体积逐渐变大(杨明等, 2019);在克氏原螯虾(Procambarus clarkii)中,低pH胁迫导致其肝胰腺的B细胞数量有所增加,且其细胞内运转泡的数量也明显增多(陶易凡等, 2016);三疣梭子蟹在低盐暴露下R细胞减少,B细胞数量增加,胞内运转泡体积增大(韩晓琳等, 2014)。这些现象都与本研究中低氧胁迫期间凡纳滨对虾肝胰腺组织出现的现象类似。
低氧胁迫使对虾的细胞结构、生理特征发生明显的变化,进一步造成生长速率下降、死亡率升高等影响(Sun et al, 2016)。Clark等(1986)分析低氧延长了短沟对虾的(Penaeus semisulcatus)蜕皮周期,进而导致生理胁迫程度加重引起短沟对虾死亡率较高;胁迫条件下加州美对虾(Farfantepenaeus californiensis)自噬现象频发(Ocampo et al, 2002);熊大林等(2020)分析凡纳滨对虾组织切片发现,相比其他组织,鳃更易受到低氧胁迫的影响,呼吸上皮细胞的脱落会使鳃组织获氧能力降低,有氧代谢由于缺氧而减弱,而无氧代谢增强,产生有毒物质进一步破坏内环境渗透压,损伤机体,造成死亡;陶易凡等(2016)报道长期低氧胁迫克氏原螯虾也会破坏其肝胰腺结构,引起肝胰腺代谢功能紊乱,进而导致应激死亡。具体胁迫死亡机制还有待进一步研究。在研究胁迫对凡纳滨对虾的作用机制时,可将鳃、肝胰腺作为必要的目标组织(王伦等, 2022)。
本研究在相同低氧逆环境下,凡纳滨对虾高抗品系中低氧耐受家系和低氧敏感家系的鳃、肌肉和肝胰腺组织均出现不同程度的损伤,但不同的家系间表现出不同程度的抗逆生理特征差异,这可能与高抗不同家系抗逆耐受力强弱有关,说明性状有进一步选育的潜力。
4 结论本研究对比分析了凡纳滨对虾高抗品系和快大品系间及品系内不同家系间的低氧耐受性能,通过阈值性状动物模型估计凡纳滨幼虾不同品系低氧存活性状的方差组分及遗传参数。结果显示,幼虾的低氧耐受性能为中等遗传力水平,并且低氧耐受家系与低氧敏感家系的抗逆生理特征差异明显,说明耐低氧性状具有通过选育提高的可行性。本研究为凡纳滨对虾耐低氧性状选育工作提供了参考与依据。
BUBEL A. Histological and electron microscopical observations on the effects of different salinities and heavy metal ions, on the gills of Jaera nordmanni (Rathke) (Crustacea, Isopoda). Cell and Tissue Research, 1976, 167(1): 65-95 |
CHEN J C, NAN F H. Effects of temperature, salinity and ambient ammonia on lethal dissolved oxygen of Penaeus chinensis juveniles. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 1992, 101(3): 459-461 |
CHEN Q, HUANG J, WANG Y, et al. Determination of the oxygen consumption rate and suffocation point of Macrobrachium rosenbergii larvae. Journal of Hubei Agricultural College, 2002, 22(1): 33-35 [陈琴, 黄景, 王邕. 罗氏沼虾仔虾耗氧率与窒息点的初步测定. 湖北农学院学报, 2002, 22(1): 33-35] |
CLARK J. Inhibition of moulting in Penaeus semisulcatus (De Haan) by long-term hypoxia. Aquaculture, 1986, 52(4): 253-254 DOI:10.1016/0044-8486(86)90367-4 |
DU Z Q, JIN Y H. Molecular characterization and antibacterial activity analysis of two novel penaeid in isoforms from Pacific white shrimp, Litopenaeus vannamei. Applied Biochemistry and Biotechnology, 2015, 177(8): 1607-1620 DOI:10.1007/s12010-015-1840-7 |
GALLARDO J A, LHORENTE J P, NEIRA R. The consequences of including non-additive effects on the genetic evaluation of harvest body weight in Coho salmon (Oncorhynchus kisutch). Genetics Selection Evolution, 2010, 42(1): 19 DOI:10.1186/1297-9686-42-19 |
GITTERLE T, RYE M, SALTE R, et al. Genetic covariation in harvest body weight and survival in Penaeus (Litopenaeus vannamei) under standard commercial conditions. Aquaculture, 2005, 243(1/2/3/4): 83-92 |
HAN X L, GAO B Q, WANG H F, et al. Evaluation of genetic parameters for growth traits and low temperature tolerance of Penaeus chinensis under low temperature stress. Progress in Fishery Science, 2014, 35(1): 104-110 [韩晓琳, 高保全, 王好锋, 等. 低盐胁迫对三疣梭子蟹鳃和肝胰腺显微结构及家系存活的影响. 渔业科学进展, 2014, 35(1): 104-110] |
HOFFMANN A A, MERILA J. Heritable variation and evolution under favourable and unfavourable conditions. Trends in Ecology and Evolution, 1999, 14: 96-101 DOI:10.1016/S0169-5347(99)01595-5 |
HYUMA K, NOBUYUKI I, A KIHIRO K. Estimation of heritability of tolerance to low-salinity by factorial mating system in rainbow trout (Oncorhynchus mykiss). Suisanzoshoku, 2001, 49(2): 253-260 |
IBARRA A M, PÉREZ-ROSTRO C I, RAMÍREZ J L, et al. Genetics of the resistance to hypoxia in postlarvae and juveniles of the Pacific white shrimp Penaeus (Litopenaeus vannamei). Aquaculture Research, 2007, 38(8): 838-846 DOI:10.1111/j.1365-2109.2007.01734.x |
JIA W T. Study on efficient and green culture technology of Penaeus chinensis. Journal of Aquaculture, 2018, 39(9): 32-33 [贾文涛. 南美白对虾高效绿色养殖技术探讨. 水产养殖, 2018, 39(9): 32-33] |
LI T W. Study on liver structure of Portunus tritverruca. Oceanologia et Limnologia Sinica, 1996, 7(5): 471–475, 571–572 [李太武. 三疣梭子蟹肝脏的结构研究. 海洋与湖沼, 1996, 7(5): 471–475, 571–572] |
LIN H J, ZHANG L P, SHEN Q, et al. The comparison of tolerance to fresh water in 10 full-sib families of Litopenaeus vannamei. Transactions of Oceanology and Limnology, 2010, 6(4): 143-148 [林红军, 张吕平, 沈琪, 等. 10个凡纳滨对虾全同胞家系淡水耐受性比较. 海洋湖沼通报, 2010, 6(4): 143-148] |
LU X, LUAN S, CAO B X, et al. Differences in WSSV sensitivity between stress-sensitive and tolerant populations under acute ammonia nitrogen stress in Litopenaeus vannamei. Progress in Fishery Sciences, 2018, 39(1): 83-89 [卢霞, 栾生, 曹宝祥, 等. 凡纳滨对虾氨氮急性胁迫应激敏感群体和耐受群体对WSSV敏感性的差异分析. 渔业科学进展, 2018, 39(1): 83-89] |
LU X, LUAN S, CAO B X, et al. Estimation of genetic parameters and genotype-by-environment interactions related to acute ammonia stress in Pacific white shrimp (Litopenaeus vannamei) juveniles at two different salinity levels. PLoS One, 2017, 12(3): 1-17 |
MCINTYRE J D, AMEND D F. Heritability of tolerance for infectious hematopoietic necrosis in sockeye salmon (Oncorhynchus nerka). Transactions of American Fishery Society, 1978, 107: 305-308 DOI:10.1577/1548-8659(1978)107<305:HOTFIH>2.0.CO;2 |
OCAMPO L, EZQUERRA J M. Digestive protease activity in juvenile Farfantepenaeus californiensis as a function of dissolved oxygen and temperature. Aquaculture Research, 2002, 33(13): 1073-1080 DOI:10.1046/j.1365-2109.2002.00780.x |
ØDEGÅRD J, INGRID O, BJARNE G, et al. Evaluation of statistical models for genetic analysis of challenge test data on furunculosis resistance in Atlantic salmon (Salmo salar): Prediction of field survival. Aquaculture, 2006, 259(1): 116-123 |
PÉREZ-ROSTRO C I, RACOTTA I S, IBARRA A M. Decreased genetic variation in metabolic variables of Litopenaeus vannamei after exposure to acute hypoxia. Journal of Experimental Marine Biology and Ecology, 2004, 302(2): 189-200 DOI:10.1016/j.jembe.2003.10.010 |
SOÑANEZ-ORGANIS J G, RACOTTA I S, YEPIZ-PLASCENCIA G. Silencing of the hypoxia inducible factor 1-HIF-1-obliterates the effects of hypoxia on glucose and lactate concentrations in a tissue-specific manner in the shrimp Litopenaeus vannamei. Journal of Experimental Marine Biology and Ecology, 2010, 393(1/2): 51-58 |
SUN S, XUAN F, FU H, et al. Comparative proteomic study of the response to hypoxia in the muscle of oriental river prawn (Macrobrachium nipponense). Journal of Proteomics, 2016, 138: 115-123 DOI:10.1016/j.jprot.2016.02.023 |
TAO Y F, QIANG J, WANG H, et al. Effects of low pH stress on enzyme activity and tissue structure in gill and hepatopancreas of Procambarus clarkii. Journal of Fishery Sciences of China, 2016, 23(6): 1279-1289 [陶易凡, 强俊, 王辉, 等. 低pH胁迫对克氏原螯虾鳃和肝胰腺酶活力及组织结构的影响. 中国水产科学, 2016, 23(6): 1279-1289] |
TRYGVE G. Selection and breeding programs in aquaculture. Netherlands: Kluwer Academic Pub, 2005, 64-65 |
VAQUER S R, DUARTE C M. Thresholds of hypoxia for marine biodiversity. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(40): 1-6 |
WANG C Y. Estimation of genetic parameters for growth and salinity tolerance of Litopenaeus vannamei and analysis of interaction between genotype and environment. Master´s Thesis of Guangdong Ocean University, 2021, 53(1): 161-167 [王崇懿. 凡纳滨对虾生长和耐高盐性状遗传参数估计及基因型与环境互作效应分析. 广东海洋大学硕士研究生学位论文, 2021, 53(1): 161-167] |
WANG L, WANG C Y, LIU J Y. Evaluation of genetic parameters for growth and comprehensive stress tolerance of Litopenaeus vannamei. South China Fisheries Science, 2022, 18(4): 95-102 [王伦, 王崇懿, 刘建勇. 凡纳滨对虾生长和耐综合胁迫性状的遗传参数评估. 南方水产科学, 2022, 18(4): 95-102] |
WANG M Y, WANG D D, LUO Y C, et al. Effects of acute hypoxia on epigenetic characteristics, related enzyme activities and gene expression of Litopenaeus vannamei in freshwater culture. Journal of Southern Agriculture, 2022, 53(12): 3529-3538 [王梦瑶, 王丹丹, 罗永成, 等. 淡水养殖条件下急性缺氧对凡纳滨对虾表观特征、相关酶活性与基因表达的影响. 南方农业学报, 2022, 53(12): 3529-3538 DOI:10.3969/j.issn.2095-1191.2022.12.024] |
WANG X Q, WANG Z Y, HE X R. Experimental study on environmental resistance and estimation of heritability of Larimichthys crocea. Oceanologia et Limnologia Sinica, 2009, 40(6): 781-785 [王晓清, 王志勇, 何湘蓉. 大黄鱼(Larimichthys crocea)耐环境因子试验及其遗传力的估计. 海洋与湖沼, 2009, 40(6): 781-785] |
WINKELMAN A M, PETERSON R G. Heritabilities, dominance variation, common environmental effects and genotype by environment interactions for weight and length in Chinook salmon. Aquaculture, 1994, 125: 17-30 DOI:10.1016/0044-8486(94)90278-X |
WU R, LAM P, WAN K. Tolerance to, and avoidance of hypoxia by the penaeid shrimp (Metapenaeus ensis). Environmental Pollution, 2002, 118(3): 351-355 DOI:10.1016/S0269-7491(01)00298-6 |
XIONG D L, DUAN Y F, XU J M, et al. Physiological responses of branchial tissues of Litopenaeus vannamei to high temperature and ammonia stress. Journal of Southern Agriculture, 2020, 51(9): 2296-2303 [熊大林, 段亚飞, 徐敬明, 等. 凡纳滨对虾鳃组织对高温和氨氮胁迫的生理响应. 南方农业学报, 2020, 51(9): 2296-2303] |
YANG M, SUN S M, FU H T. Effects of hypoxia and reoxygenation on antioxidant enzyme activity and tissue structure of Macrobrachium nipponense. Journal of Fishery Sciences of China, 2019, 26(3): 493-503 [杨明, 孙盛明, 傅洪拓, 等. 低氧和复氧对日本沼虾抗氧化酶活力及组织结构的影响. 中国水产科学, 2019, 26(3): 493-503] |
YUAN R P, LIU J Y, ZHANG J C, et al. Comparison of F1 tolerance to low dissolved oxygen and high ammonia nitrogen between population hybridization and selfing of Litopenaeus vannamei. Journal of Fishery Sciences of China, 2015, 22(3): 410-417 [袁瑞鹏, 刘建勇, 张嘉晨, 等. 凡纳滨对虾群体杂交与自交F1低溶氧与高氨氮耐受性比较. 中国水产科学, 2015, 22(3): 410-417] |
ZHANG J C, CAO F J, LIU J Y, et al. Estimation of genetic parameters and assessment of genetic acquisition for growth and low soluble oxygen tolerance traits in Litopenaeus vannmei. Oceanologia et Limnologia Sinica, 2016, 47(4): 869-875 [张嘉晨, 曹伏君, 刘建勇, 等. 凡纳滨对虾(Litopenaeus vannmei)生长和耐低溶氧性状的遗传参数估计和遗传获得评估. 海洋与湖沼, 2016, 47(4): 869-875] |



